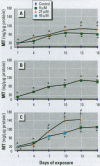
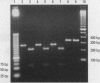
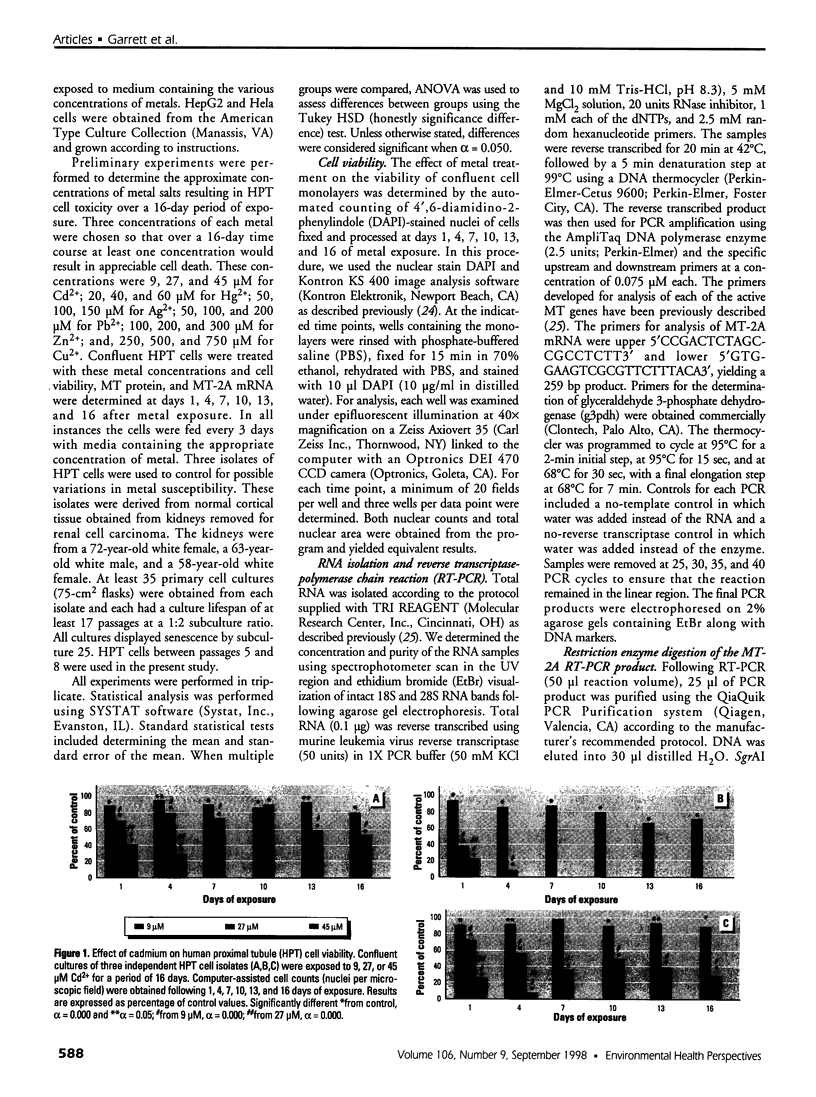
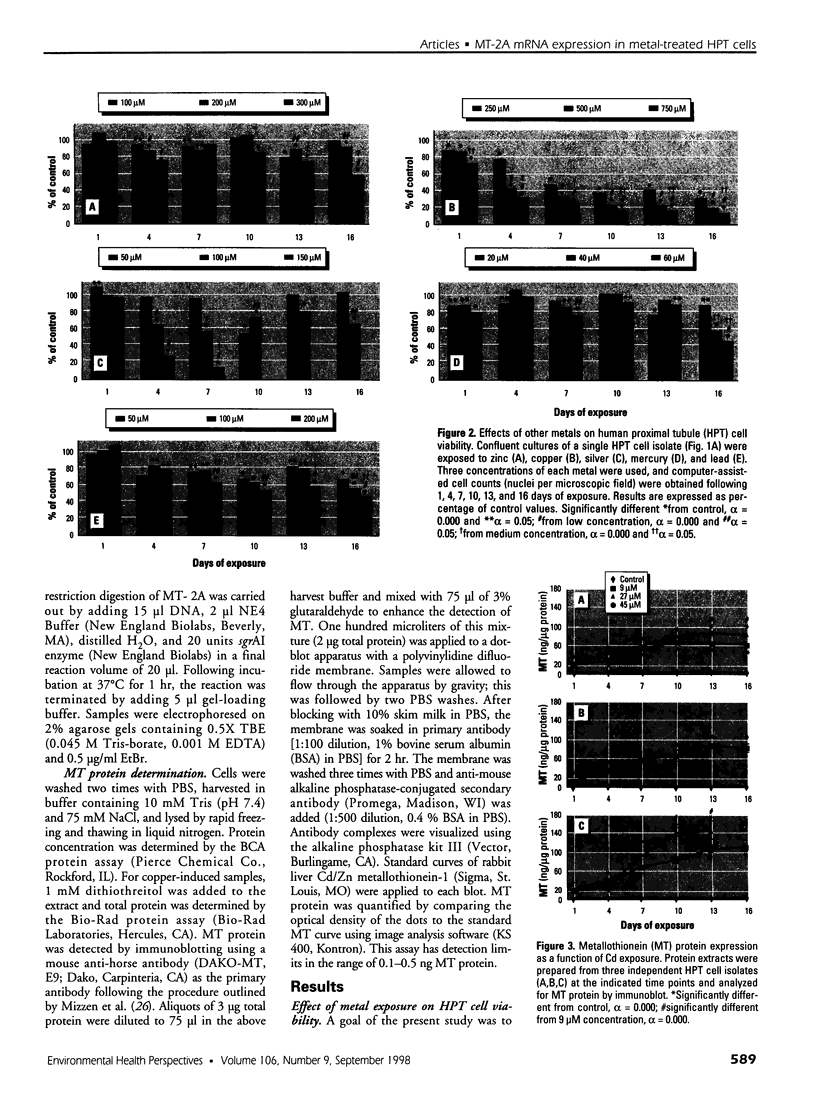
Abstract
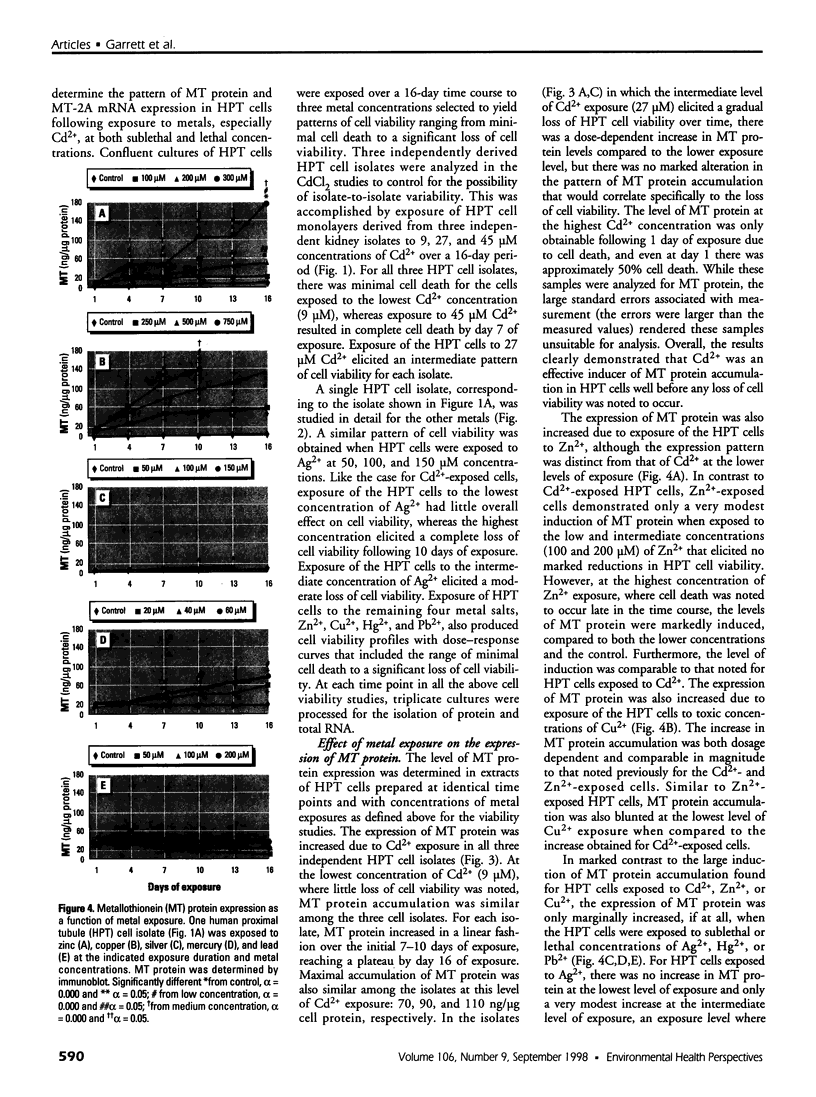
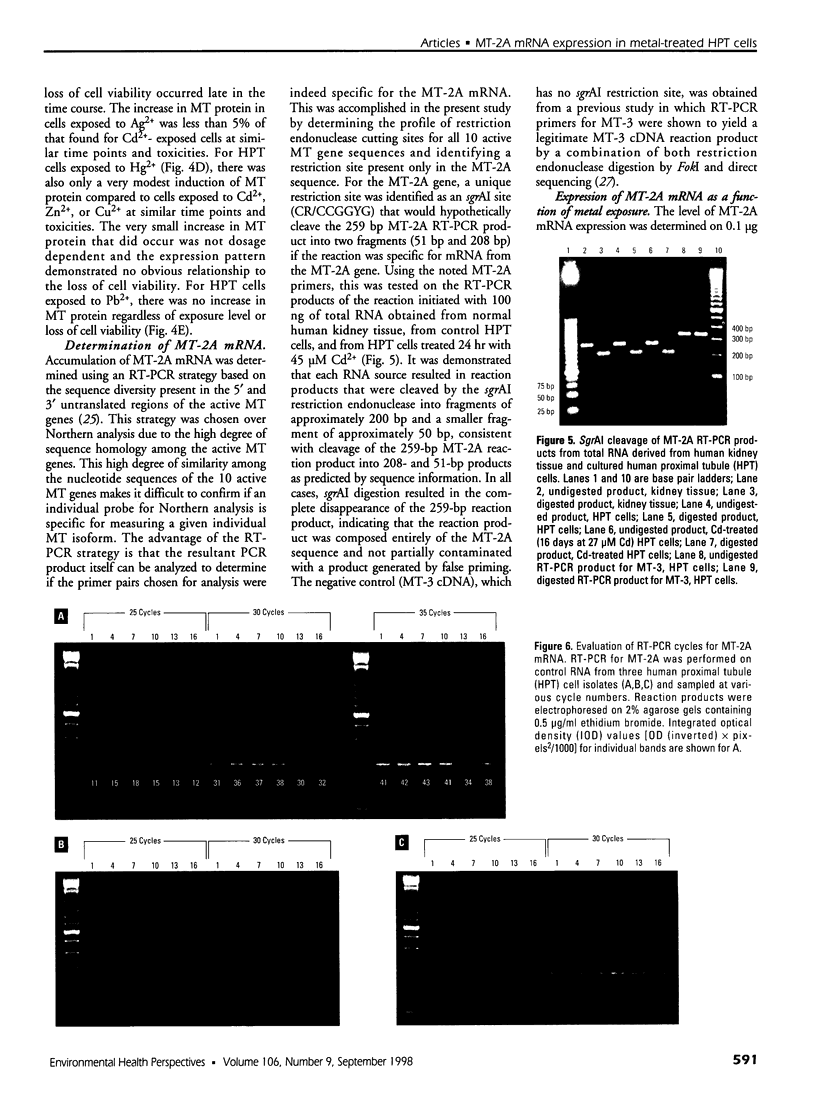
The organization of the human metallothionein (MT) gene family is more complex than the commonly used mouse and rat models. The human MTs are encoded by a family of genes consisting of 10 functional and 7 nonfunctional MT isoforms. One objective of this study was to determine if the accumulation of MT protein in cultures of human proximal tubule (HPT) cells exposed to metals is similar to that expected from the knowledge base obtained from rodent models. To accomplish this objective, HPT cells were exposed to both lethal and sublethal concentrations of Cd2+, Zn2+, Cu2+, Ag2+, Hg2+, and Pb2+ and MT protein levels were determined. The results were in general agreement with animal model studies, although there were some exceptions, mainly in areas where the animal model database was limited. In clear agreement with animal models, Cd2+, Zn2+, and Cu2+ were demonstrated to be potent inducers of MT protein accumulation. In contrast to the similarity in MT protein expression, we obtained evidence that the human renal MT-2 gene has a unique pattern of regulation compared to both animal models and human-derived cell cultures. In the present study, we determined that MT-2A mRNA was not induced by exposure of HPT cells to Cd2+ or the other metals, a finding in contrast to studies in both animal models and other human cell culture systems in which a high level of MT-2 mRNA induction occurs upon exposure to Cd2+ or Zn2+. While MT protein expression may be similar between humans and animal models, this finding provides initial evidence that regulation of the genes underlying MT protein expression may be divergent between species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard A., Roels H., Hubermont G., Buchet J. P., Masson P. L., Lauwerys R. R. Characterization of the proteinuria in cadmium-exposed workers. Int Arch Occup Environ Health. 1976 Oct 21;38(1):19–30. doi: 10.1007/BF00378317. [DOI] [PubMed] [Google Scholar]
- Beyersmann D., Hechtenberg S. Cadmium, gene regulation, and cellular signalling in mammalian cells. Toxicol Appl Pharmacol. 1997 Jun;144(2):247–261. doi: 10.1006/taap.1997.8125. [DOI] [PubMed] [Google Scholar]
- Bosco E., Porta N., Diezi J. Renal handling of cadmium: a study by tubular microinjections. Arch Toxicol Suppl. 1984;7:371–373. doi: 10.1007/978-3-642-69132-4_62. [DOI] [PubMed] [Google Scholar]
- Bremner I. Nutritional and physiologic significance of metallothionein. Methods Enzymol. 1991;205:25–35. doi: 10.1016/0076-6879(91)05080-f. [DOI] [PubMed] [Google Scholar]
- Cherian M. G., Howell S. B., Imura N., Klaassen C. D., Koropatnick J., Lazo J. S., Waalkes M. P. Role of metallothionein in carcinogenesis. Toxicol Appl Pharmacol. 1994 May;126(1):1–5. doi: 10.1006/taap.1994.1083. [DOI] [PubMed] [Google Scholar]
- Coogan T. P., Shiraishi N., Waalkes M. P. Apparent quiescence of the metallothionein gene in the rat ventral prostate: association with cadmium-induced prostate tumors in rats. Environ Health Perspect. 1994 Sep;102 (Suppl 3):137–139. doi: 10.1289/ehp.94102s3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan T. P., Shiraishi N., Waalkes M. P. Minimal basal activity and lack of metal-induced activation of the metallothionein gene correlates with lobe-specific sensitivity to the carcinogenic effects of cadmium in the rat prostate. Toxicol Appl Pharmacol. 1995 May;132(1):164–173. doi: 10.1006/taap.1995.1097. [DOI] [PubMed] [Google Scholar]
- Dalton T., Fu K., Palmiter R. D., Andrews G. K. Transgenic mice that overexpress metallothionein-I resist dietary zinc deficiency. J Nutr. 1996 Apr;126(4):825–833. doi: 10.1093/jn/126.4.825. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Induction of metallothionein-I mRNA in cultured cells by heavy metals and iodoacetate: evidence for gratuitous inducers. Mol Cell Biol. 1984 Mar;4(3):484–491. doi: 10.1128/mcb.4.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering P. L., Fowler B. A. Metal constitution of metallothionein influences inhibition of delta-aminolaevulinic acid dehydratase (porphobilinogen synthase) by lead. Biochem J. 1987 Jul 15;245(2):339–345. doi: 10.1042/bj2450339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hoey J. G., Garrett S. H., Sens M. A., Todd J. H., Sens D. A. Expression of MT-3 mRNA in human kidney, proximal tubule cell cultures, and renal cell carcinoma. Toxicol Lett. 1997 Jul 21;92(2):149–160. doi: 10.1016/s0378-4274(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Hollinger M. A. Toxicological aspects of topical silver pharmaceuticals. Crit Rev Toxicol. 1996 May;26(3):255–260. doi: 10.3109/10408449609012524. [DOI] [PubMed] [Google Scholar]
- Ikebuchi H., Teshima R., Suzuki K., Sawada J., Terao T., Yamane Y. An immunological study of a Pb-thionein like protein in rat liver. Biochem Biophys Res Commun. 1986 Apr 29;136(2):535–541. doi: 10.1016/0006-291x(86)90473-0. [DOI] [PubMed] [Google Scholar]
- Ikebuchi H., Teshima R., Suzuki K., Terao T., Yamane Y. Simultaneous induction of Pb-metallothionein-like protein and Zn-thionein in the liver of rats given lead acetate. Biochem J. 1986 Jan 15;233(2):541–546. doi: 10.1042/bj2330541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irato P., Sturniolo G. C., Giacon G., Magro A., D'Inca R., Mestriner C., Albergoni V. Effect of zinc supplementation on metallothionein, copper, and zinc concentration in various tissues of copper-loaded rats. Biol Trace Elem Res. 1996 Jan;51(1):87–96. doi: 10.1007/BF02790151. [DOI] [PubMed] [Google Scholar]
- Kelly E. J., Quaife C. J., Froelick G. J., Palmiter R. D. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr. 1996 Jul;126(7):1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- Kido T., Honda R., Tsuritani I., Yamaya H., Ishizaki M., Yamada Y., Nogawa K. Progress of renal dysfunction in inhabitants environmentally exposed to cadmium. Arch Environ Health. 1988 May-Jun;43(3):213–217. doi: 10.1080/00039896.1988.9934935. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Kojima Y. Chemistry and biochemistry of metallothionein. Experientia Suppl. 1987;52:25–61. doi: 10.1007/978-3-0348-6784-9_3. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Schäffer A. Biochemistry of metallothionein. Biochemistry. 1988 Nov 15;27(23):8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Lauwerys R. R., Buchet J. P., Roels H. A., Brouwers J., Stanescu D. Epidemiological survey of workers exposed to cadmium. Arch Environ Health. 1974 Mar;28(3):145–148. doi: 10.1080/00039896.1974.10666455. [DOI] [PubMed] [Google Scholar]
- Liu J., Kershaw W. C., Klaassen C. D. The protective effect of metallothionein on the toxicity of various metals in rat primary hepatocyte culture. Toxicol Appl Pharmacol. 1991 Jan;107(1):27–34. doi: 10.1016/0041-008x(91)90327-b. [DOI] [PubMed] [Google Scholar]
- Maitani T., Watahiki A., Suzuki K. T. Induction of metallothionein after lead administration by three injection routes in mice. Toxicol Appl Pharmacol. 1986 Apr;83(2):211–217. doi: 10.1016/0041-008x(86)90298-x. [DOI] [PubMed] [Google Scholar]
- McKenna I. M., Bare R. M., Waalkes M. P. Metallothionein gene expression in testicular interstitial cells and liver of rats treated with cadmium. Toxicology. 1996 Feb 22;107(2):121–130. doi: 10.1016/0300-483x(95)03252-b. [DOI] [PubMed] [Google Scholar]
- Misra R. R., Crance K. A., Bare R. M., Waalkes M. P. Lack of correlation between the inducibility of metallothionein mRNA and metallothionein protein in cadmium-exposed rodents. Toxicology. 1997 Feb 28;117(2-3):99–109. doi: 10.1016/s0300-483x(96)03557-3. [DOI] [PubMed] [Google Scholar]
- Morcillo M. A., Santamaria J. Mercury distribution and renal metallothionein induction after subchronic oral exposure in rats. Biometals. 1996 Jul;9(3):213–220. doi: 10.1007/BF00817918. [DOI] [PubMed] [Google Scholar]
- Oberdörster G., Cherian M. G., Baggs R. B. Correlation between cadmium-induced pulmonary carcinogenicity, metallothionein expression, and inflammatory processes: a species comparison. Environ Health Perspect. 1994 Sep;102 (Suppl 3):257–263. doi: 10.1289/ehp.102-1567425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Findley S. D., Whitmore T. E., Durnam D. M. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini O., Davenas E., Morin L., Tsangaris G. T., Benveniste J., Manuel Y., Thomas Y. Modulation of stress proteins by Cd2+ in a human T cell line. Eur J Pharmacol. 1994 Apr 4;270(2-3):221–228. doi: 10.1016/0926-6917(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Piotrowski J. K., Trojanowska B., Wiśniewska-Knypl J. M., Bolanowska W. Mercury binding in the kidney and liver of rats repeatedly exposed to mercuric chloride: induction of metallothionein by mercury and cadmium. Toxicol Appl Pharmacol. 1974 Jan;27(1):11–19. doi: 10.1016/0041-008x(74)90169-0. [DOI] [PubMed] [Google Scholar]
- Quaife C. J., Findley S. D., Erickson J. C., Froelick G. J., Kelly E. J., Zambrowicz B. P., Palmiter R. D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994 Jun 14;33(23):7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinogi M., Maeizumi S. Effect of preinduction of metallothionein on tissue distribution of silver and hepatic lipid peroxidation. Biol Pharm Bull. 1993 Apr;16(4):372–374. doi: 10.1248/bpb.16.372. [DOI] [PubMed] [Google Scholar]
- Shiraishi N., Hochadel J. F., Coogan T. P., Koropatnick J., Waalkes M. P. Sensitivity to cadmium-induced genotoxicity in rat testicular cells is associated with minimal expression of the metallothionein gene. Toxicol Appl Pharmacol. 1995 Feb;130(2):229–236. doi: 10.1006/taap.1995.1028. [DOI] [PubMed] [Google Scholar]
- Stennard F. A., Holloway A. F., Hamilton J., West A. K. Characterisation of six additional human metallothionein genes. Biochim Biophys Acta. 1994 Aug 2;1218(3):357–365. doi: 10.1016/0167-4781(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Tarnowski B. I., Sens D. A., Nicholson J. H., Hazen-Martin D. J., Garvin A. J., Sens M. A. Automatic quantitation of cell growth and determination of mitotic index using DAPI nuclear staining. Pediatr Pathol. 1993 Mar-Apr;13(2):249–265. doi: 10.3109/15513819309048211. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Klaassen C. D. Concentration of metallothionein in major organs of rats after administration of various metals. Fundam Appl Toxicol. 1985 Jun;5(3):473–477. doi: 10.1016/0272-0590(85)90094-6. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Perantoni A., Bhave M. R., Rehm S. Strain dependence in mice of resistance and susceptibility to the testicular effects of cadmium: assessment of the role of testicular cadmium-binding proteins. Toxicol Appl Pharmacol. 1988 Mar 30;93(1):47–61. doi: 10.1016/0041-008x(88)90024-5. [DOI] [PubMed] [Google Scholar]
- West A. K., Stallings R., Hildebrand C. E., Chiu R., Karin M., Richards R. I. Human metallothionein genes: structure of the functional locus at 16q13. Genomics. 1990 Nov;8(3):513–518. doi: 10.1016/0888-7543(90)90038-v. [DOI] [PubMed] [Google Scholar]
- Yang Y. Y., Woo E. S., Reese C. E., Bahnson R. R., Saijo N., Lazo J. S. Human metallothionein isoform gene expression in cisplatin-sensitive and resistant cells. Mol Pharmacol. 1994 Mar;45(3):453–460. [PubMed] [Google Scholar]
- Zalups R. K., Cherian M. G. Renal metallothionein metabolism after a reduction of renal mass. I. Effect of unilateral nephrectomy and compensatory renal growth on basal and metal-induced renal metallothionein metabolism. Toxicology. 1992;71(1-2):83–102. doi: 10.1016/0300-483x(92)90056-k. [DOI] [PubMed] [Google Scholar]
- Zalups R. K., Lash L. H. Advances in understanding the renal transport and toxicity of mercury. J Toxicol Environ Health. 1994 May;42(1):1–44. doi: 10.1080/15287399409531861. [DOI] [PubMed] [Google Scholar]