Abstract
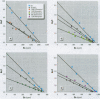
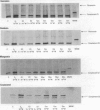
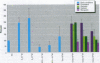
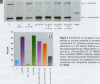
Severe developmental and reproductive disorders in wild animals have been linked to high exposure to persistent environmental chemicals with hormonal activity. These adverse effects of environmental estrogens have raised considerable concern and have received increasing attention. Although numerous chemicals with the capacity to interfere with the estrogen receptor (ER) have been identified, information on their molecular mechanism of action and their relative potency is rather limited. For the endometrium, the lack of information is due to the lack of a suitable experimental model. We investigated the functions of phytoestrogens in an endometrial-derived model, RUCA-I rat endometrial adenocarcinoma cells. The cells were cultured on a reconstituted basement membrane to preserve their functional differentiation and estrogen responsiveness. We assessed the relative binding affinity to the estrogen receptor of the selected phytoestrogens coumestrol, genistein, daidzein, and the putative phytoestrogen mangostin compared to estradiol by a competitive Scatchard analysis. The following affinity ranking was measured: 17beta-estradiol >>> coumestrol > genistein > daidzein >>> mangostin. In addition, we investigated the capacity of these compounds to promote the increased production of complement C3, a well-known estradiol-regulated protein of the rat endometrium. All substances tested increased the production of complement C3, although different concentrations were necessary to achieve equivalent levels of induction compared to estradiol. Mechanistically we were able to demonstrate that the increase of complement C3 production was mediated by primarily increasing its steady-state mRNA level. These findings indicate that RUCA-I cells represent a sensitive model system to elucidate relative potencies and functions of environmental estrogens in an endometrium-derived model.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest Suppl. 1990;201:3–23. [PubMed] [Google Scholar]
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Auger J., Kunstmann J. M., Czyglik F., Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995 Feb 2;332(5):281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- Boyd J., Takahashi H., Waggoner S. E., Jones L. A., Hajek R. A., Wharton J. T., Liu F. S., Fujino T., Barrett J. C., McLachlan J. A. Molecular genetic analysis of clear cell adenocarcinomas of the vagina and cervix associated and unassociated with diethylstilbestrol exposure in utero. Cancer. 1996 Feb 1;77(3):507–513. doi: 10.1002/(SICI)1097-0142(19960201)77:3<507::AID-CNCR12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. L., Bradlow H. L., Wolff M., Woodruff T., Hoel D. G., Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect. 1993 Oct;101(5):372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch H., Goluboff E. T., Olson J. H., Feldshuh J., Broder S. J., Barad D. H. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996 May;65(5):1009–1014. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- Gottardis M. M., Ricchio M. E., Satyaswaroop P. G., Jordan V. C. Effect of steroidal and nonsteroidal antiestrogens on the growth of a tamoxifen-stimulated human endometrial carcinoma (EnCa101) in athymic mice. Cancer Res. 1990 Jun 1;50(11):3189–3192. [PubMed] [Google Scholar]
- Hyder S. M., Shipley G. L., Stancel G. M. Estrogen action in target cells: selective requirements for activation of different hormone response elements. Mol Cell Endocrinol. 1995 Jul;112(1):35–43. doi: 10.1016/0303-7207(95)03581-q. [DOI] [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Pimentel M., Teng C. T., Korach K. S., McLachlan J. A. Cross talk between peptide growth factor and estrogen receptor signaling systems. Environ Health Perspect. 1995 Oct;103 (Suppl 7):35–38. doi: 10.1289/ehp.95103s735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan V. C., Gottardis M. M., Satyaswaroop P. G. Tamoxifen-stimulated growth of human endometrial carcinoma. Ann N Y Acad Sci. 1991;622:439–446. doi: 10.1111/j.1749-6632.1991.tb37886.x. [DOI] [PubMed] [Google Scholar]
- Kuivanen P. C., Capulong R. B., Harkins R. N., DeSombre E. R. The estrogen-responsive 110K and 74K rat uterine secretory proteins are structurally related to complement component C3. Biochem Biophys Res Commun. 1989 Feb 15;158(3):898–905. doi: 10.1016/0006-291x(89)92807-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loukovaara M., Carson M., Adlercreutz H. Regulation of sex-hormone-binding globulin production by endogenous estrogens in vitro. Biochem Biophys Res Commun. 1995 Jan 26;206(3):895–901. doi: 10.1006/bbrc.1995.1127. [DOI] [PubMed] [Google Scholar]
- Loukovaara M., Carson M., Palotie A., Adlercreutz H. Regulation of sex hormone-binding globulin production by isoflavonoids and patterns of isoflavonoid conjugation in HepG2 cell cultures. Steroids. 1995 Sep;60(9):656–661. doi: 10.1016/0039-128x(95)00089-9. [DOI] [PubMed] [Google Scholar]
- Lyn-Cook B. D., Blann E., Payne P. W., Bo J., Sheehan D., Medlock K. Methylation profile and amplification of proto-oncogenes in rat pancreas induced with phytoestrogens. Proc Soc Exp Biol Med. 1995 Jan;208(1):116–119. doi: 10.3181/00379727-208-43842. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Webb B., Densmore C. L., Gregory R. R. Effects of coumestrol on estrogen receptor function and uterine growth in ovariectomized rats. Environ Health Perspect. 1995 Jun;103(6):574–581. doi: 10.1289/ehp.95103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz L., Garey J., Adlercreutz H., Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993 May;45(5):399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- Miksicek R. J. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol. 1994 Jun;49(2-3):153–160. doi: 10.1016/0960-0760(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Mittendorf R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology. 1995 Jun;51(6):435–445. doi: 10.1002/tera.1420510609. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Schrader W. T., Mani S., Smith C., Weigel N. L., Conneely O. M., Clark J. H. An alternative ligand-independent pathway for activation of steroid receptors. Recent Prog Horm Res. 1995;50:333–347. doi: 10.1016/b978-0-12-571150-0.50020-2. [DOI] [PubMed] [Google Scholar]
- Okura A., Arakawa H., Oka H., Yoshinari T., Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):183–189. doi: 10.1016/s0006-291x(88)80030-5. [DOI] [PubMed] [Google Scholar]
- Sarkaria J. N., Gibson D. F., Jordan V. C., Fowler J. F., Lindstrom M. J., Mulcahy R. T. Tamoxifen-induced increase in the potential doubling time of MCF-7 xenografts as determined by bromodeoxyuridine labeling and flow cytometry. Cancer Res. 1993 Sep 15;53(18):4413–4417. [PubMed] [Google Scholar]
- Schütze N., Kraft V., Deerberg F., Winking H., Meitinger D., Ebert K., Knuppen R., Vollmer G. Functions of estrogens and anti-estrogens in the rat endometrial adenocarcinoma cell lines RUCA-I and RUCA-II. Int J Cancer. 1992 Dec 2;52(6):941–949. doi: 10.1002/ijc.2910520619. [DOI] [PubMed] [Google Scholar]
- Schütze N., Vollmer G., Wünsche W., Grote A., Feit B., Knuppen R. Binding of 2-hydroxyestradiol and 4-hydroxyestradiol to the estrogen receptor of MCF-7 cells in cytosolic extracts and in nuclei of intact cells. Exp Clin Endocrinol. 1994;102(5):399–408. doi: 10.1055/s-0029-1211311. [DOI] [PubMed] [Google Scholar]
- Tang B. Y., Adams N. R. Effect of equol on oestrogen receptors and on synthesis of DNA and protein in the immature rat uterus. J Endocrinol. 1980 May;85(2):291–297. doi: 10.1677/joe.0.0850291. [DOI] [PubMed] [Google Scholar]
- Vollmer G., Ellerbrake N., Hopert A. C., Knauthe R., Wünsche W., Knuppen R. Extracellular matrix induces hormone responsiveness and differentiation in RUCA-I rat endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol. 1995 Mar;52(3):259–269. doi: 10.1016/0960-0760(94)00173-j. [DOI] [PubMed] [Google Scholar]
- Vollmer G., Hopert A. C., Ellerbrake N., Wünsche W., Knuppen R. Fibronectin is an estrogen-repressed protein in RUCA-I rat endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol. 1995 Aug;54(3-4):131–139. doi: 10.1016/0960-0760(95)00124-i. [DOI] [PubMed] [Google Scholar]
- Vollmer G., Schneider M. R. The rat endometrial adenocarcinoma cell line RUCA-I: a novel hormone-responsive in vivo/in vitro tumor model. J Steroid Biochem Mol Biol. 1996 Apr;58(1):103–115. doi: 10.1016/0960-0760(96)00012-x. [DOI] [PubMed] [Google Scholar]
- Vollmer G., Wünsche W., Schütze N., Feit B., Knuppen R. Methyl and bromo derivatives of estradiol are agonistic ligands for the estrogen receptor of MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1991 Sep;39(3):359–366. doi: 10.1016/0960-0760(91)90047-9. [DOI] [PubMed] [Google Scholar]
- Whitten P. L., Russell E., Naftolin F. Effects of a normal, human-concentration, phytoestrogen diet on rat uterine growth. Steroids. 1992 Mar;57(3):98–106. doi: 10.1016/0039-128x(92)90066-i. [DOI] [PubMed] [Google Scholar]
- Wünsche W., Tenniswood M. P., Schneider M. R., Vollmer G. Estrogenic regulation of clusterin mRNA in normal and malignant endometrial tissue. Int J Cancer. 1998 May 29;76(5):684–688. doi: 10.1002/(sici)1097-0215(19980529)76:5<684::aid-ijc12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]