Abstract
Because macrolide antibiotics are hypothesized to possess immunomodulatory activity independent of their antimicrobial activity, we evaluated the immunomodulatory effect of clarithromycin in a murine model of lung inflammation induced by either live or UV-killed Mycoplasma pneumoniae. BALB/c mice were intranasally inoculated once with live or UV-killed M. pneumoniae. Clarithromycin (25 mg/kg of body weight) or placebo was subcutaneously administered once daily in both groups of mice. In mice infected with live M. pneumoniae, clarithromycin treatment significantly reduced quantitative M. pneumoniae bronchoalveolar lavage (BAL) culture, pulmonary histopathologic scores (HPS), and airway resistance-obstruction (as measured by plethysmography) compared with placebo. Concentrations of tumor necrosis factor alpha, gamma interferon, interleukin-6 (IL-6), mouse KC (functional IL-8), JE/MCP-1, and MIP-1α in BAL fluid were also significantly decreased in mice infected with live M. pneumoniae given clarithromycin. In contrast, mice inoculated with UV-killed M. pneumoniae had no significant reduction in HPS, airway resistance-obstruction, or BAL cytokine or chemokine concentrations in response to clarithromycin administration. Clarithromycin therapy demonstrated beneficial effects (microbiologic, histologic, respiratory, and immunologic) on pneumonia in the mice infected with live M. pneumoniae; this was not observed in the mice inoculated with UV-killed M. pneumoniae.
While the antimicrobial activity of macrolides is well established and routinely utilized in clinical practice for the treatment of various infectious diseases, macrolides are also hypothesized to have immunomodulatory activity independent of their known antimicrobial properties (9, 14). In particular, macrolides have proven to speculative roles in the management of certain chronic respiratory tract diseases, such as diffuse panbronchiolitis, asthma, chronic obstructive pulmonary disease, and sinusitis, with immunomodulation being the proposed mechanism of action for the decrease in inflammation observed with macrolide therapy (11, 13, 14; C. M. MacLeod, C. Tremblay, and W. Brisco, 3rd Eur. Cong. Chemother., abstr. T243, 2000). An alternative hypothesis is that macrolide antibiotics are treating an unappreciated chronic infection of the respiratory tract with a resultant decrease in inflammation due to this antimicrobial effect (7, 10).
Our laboratory previously developed a murine model of Mycoplasma pneumoniae pneumonia characterized by marked pulmonary inflammation. In addition to infection with live M. pneumoniae, inoculation with UV light-killed M. pneumoniae also induced significant pulmonary inflammation, although to a lesser degree. In both of these models, the inflammatory response is manifested by abnormal pulmonary histopathology and elevated concentrations of cytokines and chemokines in bronchoalveolar lavage (BAL) specimens (8).
The purpose of this study was to characterize the antimicrobial and immunologic activities of clarithromycin using these two models of pulmonary inflammation. Mice were inoculated with live M. pneumoniae to determine the effect of clarithromycin on infection-induced pulmonary inflammation or with UV-killed M. pneumoniae to assess the effect of clarithromycin on pulmonary inflammation independent of its antimicrobial activity.
MATERIALS AND METHODS
Organism and growth conditions.
M. pneumoniae (ATCC 29342) was reconstituted in SP4 broth and subcultured after growth for 24 to 48 h in a flask containing 20 ml of SP4 medium at 37°C. When the broth turned an orange hue (approximately 72 h), the supernatant was decanted, and 2 ml of fresh SP4 broth was added to the flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of the flask. This achieved an M. pneumoniae concentration in the range of 108 to 109 CFU/ml. Aliquots were stored at −80°C. An aliquot of inoculum material was exposed to UV radiation (UV Cross-linker; Fisher Biotech) to obtain dead M. pneumoniae for intranasal inoculation of mice. UV-irradiated M. pneumoniae inoculum was cultured in SP4 broth to confirm its nonviable status. All SP4 media contained nystatin (50 U/ml) and ampicillin (1.0 mg/ml) to inhibit growth of potential contaminants.
Animals and inoculation.
Mice were obtained from commercial vendors (Charles River and Harlan), who confirmed their mycoplasma- and murine virus-free status. The Animal Resource Center at the University of Texas Southwestern Medical Center performed quarterly health surveillance on sentinel mice housed in the mouse storage room. Sentinel mice were examined for antibodies against mouse hepatitis virus, Sendai virus, pneumonia virus of mice, reo-3 virus, mouse encephalitis virus (GD-7), mouse rotavirus (EDIM), minute virus of mice, and Mycoplasma pulmonis. Sentinel mice were also screened for pinworm and mites. The sentinel mice tested negative for these pathogens. Mice were housed in filter-top cages and allowed to acclimate to their new environment for 1 week. Methoxyflurane, an inhaled anesthetic, was used for inoculum sedation. Two-month-old female BALB/c mice were intranasally inoculated once (day 0) with 0.7 × 107 to 4.4 × 107 CFU of live or UV-killed M. pneumoniae in 50 μl of SP4 broth. Directly comparable treatment groups were given inoculum from the same batch. Control mice were inoculated with sterile SP4 broth. All mice were housed in the same animal room and received identical daily care. Animal guidelines were followed in accordance with the Institutional Animal Care and Research Advisory Committee.
Clarithromycin administration.
Clarithromycin (25 mg/kg of body weight) was administered subcutaneously once daily according to the following protocols.
(i) Protocol 1.
Clarithromycin was administered for 4 days, beginning 12 h after inoculation of either live or UV-killed M. pneumoniae. For this protocol, the intravenous formulation of clarithromycin reconstituted in sterile water with 5% dextrose was used.
(ii) Protocol 2.
Clarithromycin was administered for 13 days, beginning 1 day after inoculation with live M. pneumoniae. For this protocol, clarithromycin powder solubilized in ethanol and water with 5% dextrose was used.
(iii) Protocol 3.
Clarithromycin was administered for 7 days, beginning 1 day after inoculation with UV-killed M. pneumoniae. For this protocol, clarithromycin powder solubilized in ethanol and water with 5% dextrose was used.
Ethanol may affect interleukin-6 (IL-6) and IL-8 concentrations, so we used a formulation without ethanol in protocol 1 to avoid this possible confounding effect. We altered the time of clarithromycin therapy initiation in protocol 1 (we started therapy at 12 h, instead of 24 h) to begin the clarithromycin at an earlier point in the inflammation cascade prior to the peak inflammation to see if this altered our findings.
For each protocol, placebo groups received the same treatment regimens with identical solutions not containing clarithromycin. Clarithromycin and placebo treatment groups each contained four to eight mice per time point.
Sample collection.
Samples were taken from the mice at various points during clarithromycin treatment. Mice were anesthetized with an intraperitoneal injection of 75 mg of ketamine per kg and 5 mg of acepromazine per kg before cardiac puncture. BAL specimens were obtained by infusing 0.5 ml of SP4 broth through a 25-gauge needle into the lungs, via the trachea, followed by aspiration of this fluid into a syringe. Whole-lung specimens (including the trachea and both lungs) were collected and fixed with a 10% buffered formalin solution for histologic evaluation.
Culture.
Twenty-five microliters of undiluted sample and serial 10-fold dilutions of BAL fluid in SP4 broth (50 μl of undiluted sample was used for the initial dilution) were immediately cultured on SP4 agar plates at 37°C, while the remaining undiluted BAL specimens were stored at −80°C. Quantification was performed by counting colonies on plated specimens and expressed as log10 CFU per milliliter. If the plated dilutions were negative for growth, but the corresponding 10−1 broth dilution was positive, the specimen was assigned a value of 20 CFU/ml, the lower limit of detection.
Histopathology.
The histopathologic score (HPS) was determined by a single pathologist who was unaware of the treatment status of the animals from which specimens were taken. The HPS was based on grading peribronchiolar or bronchial infiltrate, bronchiolar or bronchial luminal exudate, perivascular infiltrate, and parenchymal pneumonia (neutrophilic alveolar infiltrate). This HPS system assigned values from 0 to 26 (the greater the score, the greater the inflammatory changes in the lung) (1). In our experience, the extent of variation in HPS when the same slide is scored by the same pathologist multiple times has been found to be 0 to 1.
Plethysmography.
Whole-body, unrestrained plethysmography (Buxco, Troy, N.Y.) was utilized to monitor the respiratory dynamics of mice in a quantitative manner before and after methacholine exposure (baseline plethysmography shows airway obstruction; methacholine plethysmography shows airway hyperreactivity). Prior to methacholine exposure, mice were allowed to acclimate to the chamber, and then plethysmograph readings were recorded to establish baseline values. Next, the mice were exposed to aerosolized methacholine (50 mg/ml); after exposure, plethysmograph readings were recorded. Methacholine plethysmography was performed only in the 4-day treatment groups (protocol 1). Enhanced pause (Penh) is a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration. Penh correlates with pulmonary airflow resistance or obstruction. Penh as measured by plethysmography has been previously validated in animal models of airway hyperresponsiveness (5, 6, 15, 18).
BAL cytokines and chemokines.
The concentrations of cytokines and chemokines in BAL specimens were assessed in an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minn.). The cytokines and chemokines examined and their limits of detection were as follows: tumor necrosis factor alpha (TNF-α), 5.1 pg/ml; gamma interferon (IFN-γ), 9.4 pg/ml; IL-6, 3.1 pg/ml; mouse KC (functional IL-8), 2.0 pg/ml; IL-10, 15.6 pg/ml; JE/MCP-1, 1 to 2.0 pg/ml; and MIP-1α, 1.5 pg/ml.
Statistics.
The t test was used to compare indices of groups of animals treated with clarithromycin versus placebo at the same time point, if the data were normally distributed. In the instances where the data were not normally distributed, the Mann-Whitney rank sum test was used for comparisons. A comparison was considered statistically significant if the P value was ≤0.05.
RESULTS
Visual inspection.
No visual differences could be detected between the mice treated with clarithromycin versus placebo in the animals inoculated with live or UV-killed M. pneumoniae.
BAL culture.
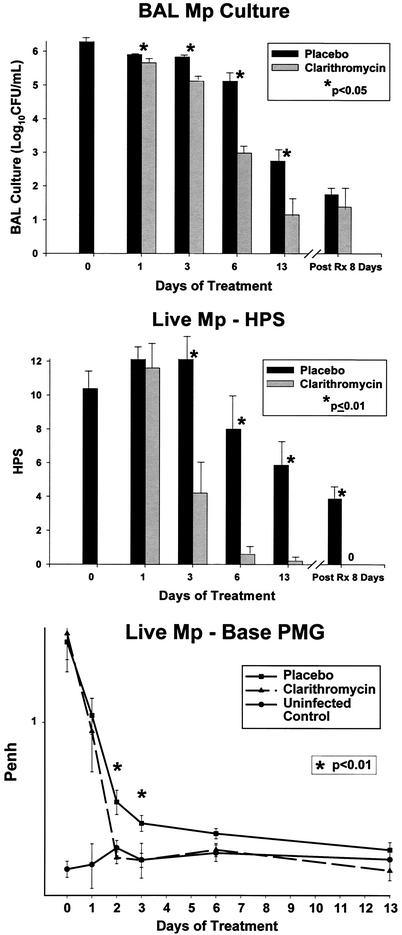
Quantitative M. pneumoniae BAL cultures in mice given live M. pneumoniae were significantly reduced by both the 4-day and 13-day clarithromycin treatment regimens compared with placebo treatment (Fig. 1 and 2). Despite these significant reductions, M. pneumoniae was not eradicated from the animals' airway. There was no significant difference in the number of M. pneumoniae in BAL cultures grown from samples taken 8 days after mice had received clarithromycin for 13 days compared with cultures from the placebo group (Fig. 2).
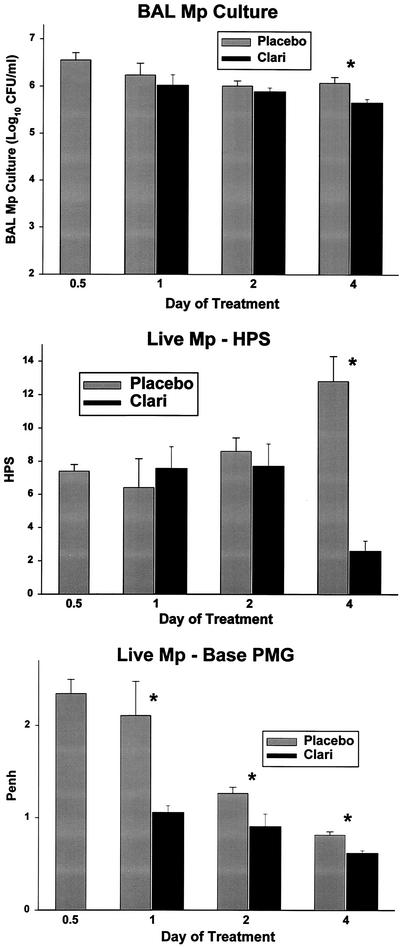
FIG. 1.
BAL M. pneumoniae (Mp) cultures, HPS, and Penh for mice inoculated with live M. pneumoniae and treated with clarithromycin versus placebo for 1 to 4 days. Treatment began 12 h after inoculation (0.5 day on the x axis). For each pair of bars, placebo treatment is shown on the left and clarithromycin (Clari) treatment is shown on the right. Values shown are the means ± standard errors (error bars) (n = 5 to 7 for clarithromycin data; n = 4 or 5 for placebo data). Values for the clarithromycin and placebo treatments that are significantly different from each other (P ≤ 0.05) are indicated by an asterisk. PMG, plethysmography.
FIG. 2.
BAL M. pneumoniae (Mp) cultures, HPS, and Penh for mice inoculated with live M. pneumoniae and treated with clarithromycin or placebo for 1 to 13 days. Treatment began 1 day after inoculation. Values shown are the means ± standard errors (error bars) (n = 5 for clarithromycin data; n = 7 for placebo data). PMG, plethysmography; Post Rx 8 Days, 8 days after completion of treatment.
Histopathology.
HPSs in mice inoculated with live M. pneumoniae were significantly reduced by both the 4-day and 13-day clarithromycin treatment regimens compared with placebo treatment (Fig. 1 and 2). In contrast to BAL cultures, HPSs assessed 8 days after 13 days of clarithromycin therapy revealed a significant reduction compared with the placebo group (Fig. 2). In mice inoculated with UV-killed M. pneumoniae, clarithromycin did not reduce HPS compared with placebo.
Plethysmography.
Airway obstruction, as measured by baseline Penh, in mice inoculated with live M. pneumoniae was significantly reduced by both clarithromycin treatment regimens compared with placebo treatment (Fig. 1 and 2). In mice inoculated with UV-killed M. pneumoniae, clarithromycin did not reduce baseline Penh compared with placebo.
Penh after methacholine exposure in mice inoculated with live M. pneumoniae was significantly reduced after only one dose of clarithromycin compared with placebo (mean Penh of 6.8 for the placebo group versus 4.6 for the clarithromycin group [P = 0.04]). Penh after methacholine exposure in mice given UV-killed M. pneumoniae was significantly increased after only two doses of clarithromycin compared with placebo (mean Penh of 2.6 for placebo versus 4.3 for clarithromycin [P = 0.05]).
BAL cytokines and chemokines.
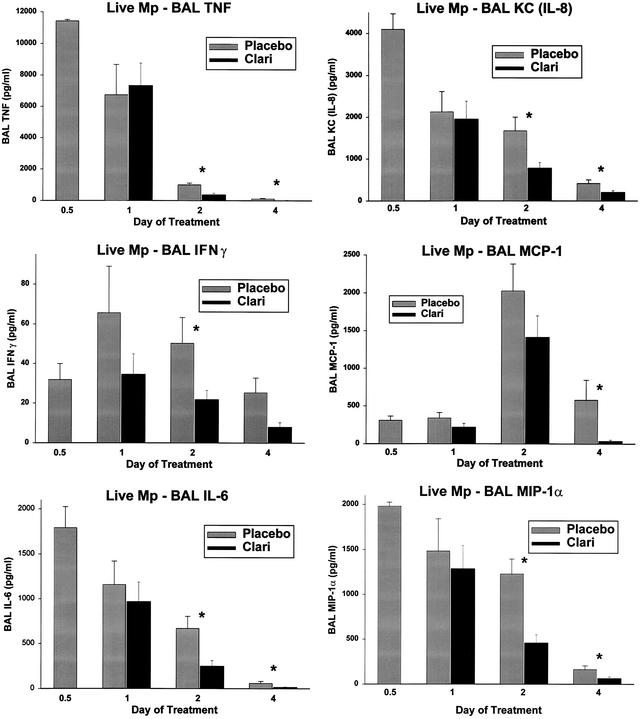
In mice infected with live M. pneumoniae that received the 4-day clarithromycin regimen, concentrations of TNF-α, IFN-γ, IL-6, KC (functional mouse IL-8), JE/MCP-1, and MIP-1α in BAL cultures were significantly decreased from those of the placebo group (Fig. 3); IL-10 was not affected.
FIG. 3.
Cytokine and chemokine concentrations in BAL specimens from mice inoculated with live M. pneumoniae (Mp) and treated with clarithromycin or placebo for 1 to 4 days. Treatment began 12 h after inoculation (0.5 day on the x axis). For each pair of bars, placebo treatment is shown on the left and clarithromycin (Clari) treatment is shown on the right. Values shown are the means ± standard errors (error bars) (n = 5 to 7 for clarithromycin data; n = 5 for placebo data). Values for the clarithromycin and placebo treatments that are significantly different from each other (P ≤ 0.05) are indicated by an asterisk.
In cultures of BAL specimens from mice infected with live M. pneumoniae that received the 13-day clarithromycin regimen, the concentrations of IFN-γ decreased after only one dose of clarithromycin compared with placebo (median concentration of 34.1 pg/ml for placebo group versus 9.5 pg/ml for clarithromycin treatment group [P = 0.046]), IL-6 decreased after only three doses (mean concentration of 8.8 pg/ml for placebo group versus 5.9 pg/ml for clarithromycin group [P = 0.006]), KC (functional mouse IL-8) decreased after only three doses (mean concentration of 486.9 pg/ml for placebo group versus 21.3 pg/ml for clarithromycin group [P = 0.002]), and JE/MCP-1 decreased after only three doses (median concentration of 165.8 pg/ml for placebo group versus 7.0 pg/ml for clarithromycin group [P = 0.008]) and six doses (median concentration of 12.6 pg/ml for placebo group versus 5.2 pg/ml for clarithromycin group [P = 0.02]); TNF-α, IL-10, and MIP-1α were not measured in this group.
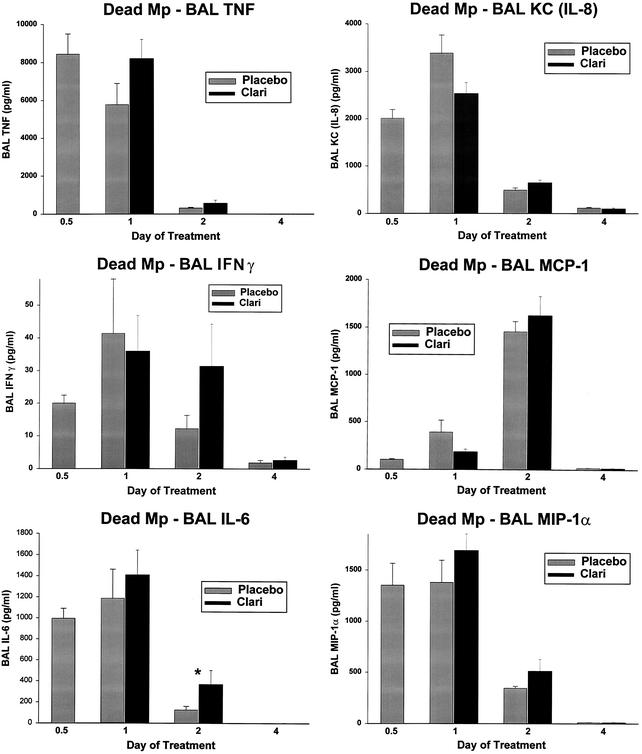
In the mice inoculated with UV-killed M. pneumoniae, neither the 4-day nor 7-day clarithromycin regimen significantly decreased the concentrations of TNF-α, IFN-γ, IL-6, KC (functional mouse IL-8), IL-10, JE/MCP-1, and MIP-1α in BAL cultures compared with the placebo treatment group; BAL IL-6 concentrations were significantly higher for the 4-day clarithromycin group than for the placebo group (Fig. 4).
FIG. 4.
Cytokine and chemokine concentrations in BAL specimens from mice inoculated with dead M. pneumoniae (Mp) and treated with clarithromycin or placebo for 1 to 4 days. Treatment began 12 h after inoculation (0.5 day on the x axis). For each pair of bars, placebo treatment is shown on the left and clarithromycin (Clari) treatment is shown on the right. Values shown are the means ± standard errors (error bars) (n = 7 or 8 for clarithromycin data; n = 5 for placebo data). Values for the clarithromycin and placebo treatments that are significantly different from each other (P ≤ 0.05) are indicated by an asterisk.
We have previously shown that SP4 control mice consistently have a lung HPS of zero (no inflammation observed), and the cytokine values for BAL specimens from SP4 control mice are significantly lower than those in mice given live or UV-killed M. pneumoniae (8).
DISCUSSION
We utilized two similar models of pulmonary inflammation to demonstrate the antimycoplasmal activity of clarithromycin and distinguish this from its hypothesized immunologic activity. One model utilized viable M. pneumoniae, and the other model utilized UV-killed M. pneumoniae. Clarithromycin therapy resulted in significant improvement in microbiologic, histologic, respiratory, and immunologic markers of disease severity in the mice inoculated with live M. pneumoniae; this improvement was not noted in the mice inoculated with UV-killed M. pneumoniae. These results suggest that the changes in inflammatory and functional parameters with clarithromycin therapy in the live M. pneumoniae model were likely a consequence of the antimicrobial activity of clarithromycin and not an independent immunologic mechanism. These results raise the question of whether the benefits observed with macrolide administration during chronic respiratory tract conditions, such as diffuse panbronchiolitis, asthma, chronic obstructive pulmonary disease, and sinusitis, are due to the treatment of an unrecognized chronic pathogen, a decrease in the background flora, or an immunologic mechanism.
Clarithromycin therapy had a broad and significant effect on pulmonary cytokine production in the live M. pneumoniae model with reductions in proinflammatory, TH1, TH2, and chemotactic cytokines. However, IL-10 (an anti-inflammatory cytokine) was not affected by clarithromycin in the live M. pneumoniae model. The noted changes in pulmonary cytokines and the associated improvement in pulmonary histopathology provide further insight into the immunopathogenesis of M. pneumoniae respiratory infection and suggest that these cytokines play a significant role in orchestrating the lung inflammatory response.
These results also raise the question of what aspect of M. pneumoniae pathogenesis induces a pulmonary immune response that can be suppressed by an antimicrobial agent. It is noteworthy that there was a significant difference in the HPS in the mice infected with live M. pneumoniae eight days after clarithromycin therapy was stopped, while there was no difference in the microbiologic outcome (quantitative M. pneumoniae BAL cultures) at this time. It is known that M. pneumoniae persists in the human respiratory tract after the resolution of acute symptoms, with or without appropriate antibiotic therapy (2, 4, 16). The significance of the chronic persistence of this pathogen in the airway after the resolution of acute symptoms is not well defined, but it is hypothesized to play a role in chronic respiratory conditions (7), such as asthma.
It is possible that a higher dosage or longer treatment time of clarithromycin therapy may be required to demonstrate an independent immunologic effect of clarithromycin. We did not determine clarithromycin pharmacokinetics in the model. A clarithromycin dose of 25 mg/kg administered subcutaneously in mice has been shown to yield peak serum drug concentrations in mice similar to those attained with doses of 250 to 500 mg of oral clarithromycin in adult humans (3) (http://www.pdr.net). The area under the curve (AUC) of this dose in mice has been shown to be 3.4 μg · h/ml with approximately 55% protein binding compared with an AUC of approximately 38 to 44 μg · h/ml with 70% protein binding in humans given 500 mg of clarithromycin orally twice a day [BID] (3, 12, 17; Carol A. Olson [Abbott Laboratories, Chicago, Ill.], personal communication). Thus, while the peak clarithromycin concentrations in serum are in the same range, the AUC of clarithromycin in mice is lower than that found in humans receiving standard clarithromycin doses. Because the half-life of clarithromycin in serum is much shorter in mice than in humans, some investigators have suggested that a higher dose of clarithromycin should be used in murine models to achieve an AUC similar to that seen in humans given 500 mg orally BID. However, this dose would yield peak serum drug concentrations in mice considerably higher than those seen in adult humans given 500 mg orally BID (17). The pharmacodynamic index of clarithromycin that determines the antimicrobial activity may not be the same as that which determines the proposed immunomodulatory activity of the drug. The relative importance of clarithromycin concentrations in tissues, such as the lung, compared with concentrations in serum will also need to be considered in this regard.
Additionally, although the two models are qualitatively similar in terms of pulmonary inflammation, the live M. pneumoniae model does have an increased level of pulmonary inflammation in terms of HPS, cytokines and chemokines, and plethysmography indices (8). This difference may have led to a blunted immunomodulatory response with clarithromycin therapy in the UV-killed M. pneumoniae model compared with the live M. pneumoniae model. Future studies with respiratory pathogens other than M. pneumoniae will be required to further characterize the multifaceted activities of clarithromycin in infections of the respiratory tract.
The models utilized in this investigation present a novel approach to assess both the antimycoplasmal activity and immunologic activity of new therapeutic agents. In addition, unrestrained whole-body plethysmography provides useful physiologic indices to access pulmonary function as an outcome of therapy for models of respiratory infections.
Acknowledgments
This work was funded in part by grants from Abbott Laboratories and the American Lung Association (O.R.).
REFERENCES
- 1.Cimolai, N., G. P. Taylor, D. Mah, and B. J. Morrison. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36:465-478. [DOI] [PubMed] [Google Scholar]
- 2.Denny, F. W., W. A. Clyde, Jr., and W. P. Glezen. 1971. Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control. J. Infect. Dis. 123:74-92. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes, P. B., B. Bailer, R. Swanson, C. W. Hanson, E. McDonald, N. Ramer, D. Hardy, N. Shipkowitz, R. R. Bower, and E. Gade. 1986. In vitro and in vivo evaluation of A-56268, a new macrolide. Antimicrob. Agents Chemother. 30:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foy, H. M., J. T. Grayston, G. E. Kenny, E. R. Alexander, and R. McMahan. 1966. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA 197:859-866. [PubMed] [Google Scholar]
- 5.Gonzalo, J. A., C. M. Lloyd, D. Wen, J. P. Albar, T. N. Wells, A. Proudfoot, A. C. Martinez, M. Dorf, T. Bjerke, A. J. Coyle, and J. C. Gutierrez-Ramos. 1998. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188:157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmograph. Am. J. Respir. Crit. Care Med. 156:766-775. [DOI] [PubMed] [Google Scholar]
- 7.Hardy, R. D., H. S. Jafri, K. Olsen, J. Hatfield, J. Iglehart, B. B. Rogers, P. Patel, G. Cassell, G. H. McCracken, and O. Ramilo. 2002. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect. Immun. 70:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy, R. D., H. S. Jafri, K. Olsen, M. Wordemann, J. Hatfield, B. B. Rogers, P. Patel, L. Duffy, G. Cassell, G. H. McCracken, and O. Ramilo. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 69:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labro, M. T. 1998. Anti-inflammatory activity of macrolides: a new therapeutic potential? J. Antimicrob. Chemother. 41(Suppl. B):37-46. [DOI] [PubMed] [Google Scholar]
- 10.Martin, R. J., M. Kraft, H. W. Chu, E. A. Berns, and G. H. Cassell. 2001. A link between chronic asthma and chronic infection. J. Allergy Clin. Immunol. 107:595-601. [DOI] [PubMed] [Google Scholar]
- 11.Miyatake, H., F. Taki, H. Taniguchi, R. Suzuki, K. Takagi, and T. Satake. 1991. Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma. Chest 99:670-673. [DOI] [PubMed] [Google Scholar]
- 12.Nilsen, O. G. 1995. Pharmacokinetics of macrolides. Comparison of plasma, tissue and free concentrations with special reference to roxithromycin. Infection 23:S5-S9. [DOI] [PubMed] [Google Scholar]
- 13.Sakito, O., J. Kadota, S. Kohno, K. Abe, R. Shirai, and K. Hara. 1996. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration 63:42-48. [DOI] [PubMed] [Google Scholar]
- 14.Scaglione, F., and G. Rossoni. 1998. Comparative anti-inflammatory effects of roxithromycin, azithromycin and clarithromycin. J. Antimicrob. Chemother. 41(Suppl. B):47-50. [DOI] [PubMed] [Google Scholar]
- 15.Schwarze, J., E. Hamelmann, K. L. Bradley, K. Takeda, and E. W. Gelfand. 1997. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Investig. 100:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, C. B., W. T. Friedewald, and R. M. Chanock. 1967. Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy. N. Engl. J. Med. 276:1172-1175. [DOI] [PubMed] [Google Scholar]
- 17.Tessier, P. R., M. Kim, W. Zhou, D. Xuan, C. Li, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Pharmacodynamic assessment of clarithromycin in a murine model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 46:1425-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Schaik, S. M., G. Enhorning, I. Vargas, and R. C. Welliver. 1998. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J. Infect. Dis. 177:269-276. [DOI] [PubMed] [Google Scholar]