Abstract
A Pseudomonas putida strain showing broad-spectrum resistance to β-lactams, including expanded-spectrum cephalosporins and carbapenems, was isolated from a patient with a urinary tract infection at the University Hospital of Varese in northern Italy. The isolate was found to produce metallo-β-lactamase activity and to harbor a 50-kb plasmid, named pVA758, carrying a new blaIMP determinant, named blaIMP-12. Plasmid pVA758 was not self-transferable by conjugation to either Escherichia coli or Pseudomonas aeruginosa but could be introduced by electroporation and maintained in the latter host, where it conferred resistance or decreased susceptibility to various β-lactams. The IMP-12 enzyme is quite divergent from other IMP variants: its closest relatives are IMP-8 and IMP-2 (89 and 88% sequence identity, respectively), and IMP-1 is 85% identical to IMP-12. The blaIMP-12 determinant is carried on an integron-borne gene cassette whose attC recombination site is related to those present in cassettes containing blaIMP-1, blaIMP-6, blaIMP-7, blaIMP-10, and blaIMP-11 and unrelated to that present in cassettes containing blaIMP-2 and blaIMP-8. IMP-12 was overproduced in E. coli by using a T7-based expression system and was purified by cation-exchange chromatography followed by gel filtration. Kinetic analysis revealed that, like other IMP variants, IMP-12 exhibits an overall preference for cephalosporins and carbapenems rather than for penicillins and does not hydrolyze temocillin and aztreonam. However, IMP-12 also exhibits some notable functional differences from other IMP variants, including uniformly poor activity toward penicillins (kcat/Km values, around 104 M−1 · s−1) and a remarkably high Km (around 900 μM) for imipenem.
IMP-type enzymes were the first acquired metallo-β-lactamases to be detected in clinical isolates of Enterobacteriaceae, Pseudomonas aeruginosa, and other nonfastidious gram-negative nonfermenters. IMP-1 was first detected in Japan (12, 26, 30, 35), but subsequently IMP-type enzymes were also detected in other countries of the Far East (2, 9, 10, 36, 38; T. H. Koh, G. S. Babini, N. Woodford, L. H. Sng, L. M. Hall, and D. M. Livermore, Letter, Lancet 353:2162, 1999) as well as in Europe (4, 36; G. Cornaglia, M. L. Riccio, A. Mazzariol, P. Piccoli, L. Lauretti, R. Fontana, and G. M. Rossolini, Letter, Lancet 353:899-900, 1999), Canada (8), and Brazil (6), indicating a wide distribution of these resistance determinants.
Molecular characterization of clinical isolates producing these enzymes revealed the existence of several IMP variants. Currently, 11 variants have been reported: IMP-1 from Japan (1, 26) but also from Singapore (18), Korea (38), and the United Kingdom (34); IMP-3 (14), IMP-6 (37), IMP-10 (13), and IMP-11 (EMBL/GenBank accession no. AB074437) from Japan; IMP-2 from Italy (27); IMP-4 from Hong Kong (2) and China (9); IMP-5 from Portugal (4); IMP-7 from Canada (8) and Malaysia (10); IMP-8 from Taiwan (36); and IMP-9 from China (EMBL/GenBank accession no. AY033653). Some variants are relatively divergent from each other (85 to 96% identity at the amino acid sequence level), namely, IMP-1, IMP-2, IMP-4, IMP-5, IMP-7, IMP-9, and IMP-11 (2, 4, 8, 26, 27; EMBL/GenBank accession no. AY033653 and AB074437), while others appear to be single or double point mutants of one of the above variants (IMP-3, IMP-6, and IMP-10 of IMP-1; IMP-8 of IMP-2) (13, 14, 36, 37).
The very broad substrate specificity of IMP enzymes, including carbapenems, oxyiminocephalosporins, and serine β-lactamase inhibitors (21), accounts for their notable clinical relevance. That is further enhanced by the fact that blaIMP determinants are carried on mobile gene cassettes inserted into chromosome- or plasmid-borne integrons (1, 4, 8, 14, 15, 20, 27, 36, 37; EMBL/GenBank accession no. AY033653 and AB074437), a location which facilitates their horizontal dissemination among different replicons and, eventually, among different strains.
In this paper we report the discovery and characterization of a new plasmid-encoded IMP variant, named IMP-12, produced by a clinical isolate of Pseudomonas putida from a hospital in northern Italy. IMP-12 is quite divergent from any other known IMP variant and exhibits significant functional differences from IMP-1.
MATERIALS AND METHODS
Bacterial strains and genetic vectors.
P. putida VA-758/00 was isolated in December 2000 from an inpatient with a urinary tract infection at the “Circolo” University Hospital of Varese, Italy, and was identified according to standard procedures (17). Escherichia coli MKD-135 (argH rpoB18 rpoB19 recA rpsL) and P. aeruginosa 10145/3 (an rpoB his derivative of reference strain ATCC 10145T) were used as recipients in conjugation experiments. E. coli DH5α (Gibco Life Technologies, Gaithersburg, Md.) and P. aeruginosa PAO1 (11) were used as hosts in electroporation experiments. DH5α was also used as a host for recombinant plasmids. E. coli BL21(DE3) (Novagen, Inc., Madison, Wis.) was used as a host for overexpression of the metallo-β-lactamase gene. Bacteria were grown aerobically at 37°C unless otherwise specified. Plasmids pBC-SK (Stratagene, Inc., La Jolla, Calif.) and pET-9a (Novagen) were used as cloning and expression vectors, respectively, for the blaIMP-12 gene.
In vitro susceptibility testing.
Antibiotics were from commercial sources as described previously (21). MICs of β-lactams were determined by a macrodilution broth method (24) by using Mueller-Hinton (MH) broth (Difco Laboratories, Detroit, Mich.) and a bacterial inoculum of 105 CFU per tube. Results were recorded after incubation for 18 h at 37°C and were interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (25).
β-Lactamase assays.
Carbapenemase activity in crude cell extracts was assayed spectrophotometrically as described previously (22) by using 150 μM imipenem as the substrate. Inhibition by EDTA was assayed as described previously (22) by using a final EDTA concentration of 5 mM. Protein concentrations in solution were assayed by the method of Bradford by use of a commercial kit (Bio-Rad [Richmond, Calif.] protein assay) with bovine serum albumin as a standard. Analytical isoelectric focusing (IEF) for detection of β-lactamases was performed as described previously (22) by using crude extracts prepared from cultures grown in antibiotic-free MH broth.
DNA analysis methodology.
Basic recombinant DNA analysis was carried out as described by Sambrook and Russell (28). Genomic DNA was extracted from P. putida as described previously (16). Multiplex PCR for detection of the blaIMP and blaVIM genes was carried out with primers IMP-DIA (IMP-DIA/f, 5′-ggAATAgAgTggCTTAATTCTC; IMP-DIA/r, 5′-gTgATgCgTCYCCAAYTTCACT) and VIM-DIA (VIM-DIA/f, 5′-CAgATTgCCgATggTgTTTgg; VIM-DIA/r, 5′-AggTgggCCATTCAgCCAgA), designed to amplify internal regions of the blaIMP (361 bp) and blaVIM (523 bp) genes, respectively. PCR was carried out in a 50-μl volume by using 50 pmol of each primer, 200 μM deoxynucleoside triphosphates, 10 ng of genomic DNA of the test strain, and 3.5 U of the Expand High Fidelity PCR system (Roche Biochemicals, Mannheim, Germany) in the reaction buffer provided by the manufacturer, with the following cycling parameters: initial denaturation at 94°C for 240 s; 25 cycles of denaturation at 94°C for 60 s, annealing at 52°C for 60 s, and extension at 72°C for 90 s; and a final extension at 72°C for 600 s. Plasmid DNA was extracted by the alkaline lysis method (28). Southern blot analysis was carried out on dried agarose gels, as previously described (33), using a probe labeled with 32P by the random priming technique (28). The probe was made of a mix (molar ratio, 1:1) of blaIMP-1 and blaIMP-2 amplicons obtained with IMP-DIA primers (see above). Mating experiments were performed on MH agar plates. The initial donor/recipient ratio was 0.1. Mating plates were incubated at 30°C for 14 h. E. coli transconjugants were selected on MH agar containing 25 μg of ceftazidime/ml plus 300 μg of rifampin/ml. P. aeruginosa transconjugants were selected on MH agar containing 50 μg of ceftazidime/ml plus 300 μg of rifampin/ml. The detection sensitivity of the assay was ≥1 × 10−8 transconjugants/recipient with either recipient. Electroporation of E. coli and P. aeruginosa was performed by using a Gene Pulser apparatus (Bio-Rad) according to the manufacturer's instructions (for E. coli) or as described previously (5) (for P. aeruginosa). Transformants were selected on MH agar containing ceftazidime at the same concentrations used for selection of transconjugants. PCR amplification of the variable region of type 1 integrons was carried out using primers INT/5CS and INT/3CS, designed on the basis of the 5′-conserved segment (5′-CS) and 3′-CS of type 1 integrons, and the Expand High Fidelity PCR system (Roche Biochemicals), as described previously (27). DNA sequences were determined on PCR amplicons or on plasmid templates as described previously (27). Both strands were sequenced. Similarity searches against sequence databases were performed by using an updated version of the BLAST program at the National Center for Biotechnology Information server (http://www.ncbi.nlm.nih.gov/). The BBL numbering scheme (7) is used throughout this paper.
Overexpression of the blaIMP-12 gene and purification of the IMP-12 enzyme.
Primers IMP-12/f (5′-CCgAATTCATATgAAgAAATTATTTgTTTTATgC-3′), containing EcoRI (underlined) and NdeI (boldfaced) restriction sites, and IMP-12/r (5′-CCggATCCTTAgTTACTTggCAgTAATgg-3′), containing a BamHI restriction site (boldfaced), were used to amplify the blaIMP-12 open reading frame by using a genomic DNA preparation of P. putida VA-758/00 as a template. The reaction was performed as described for multiplex PCR (see above) with the following cycling conditions: initial denaturation at 95°C for 240 s; 30 cycles of denaturation at 95°C for 60 s, annealing at 55°C for 90 s, and extension at 72°C for 120 s; and a final extension at 72°C for 600 s. The purified amplicon, digested with EcoRI and BamHI, was cloned into pBC-SK to produce recombinant plasmid pJD758-M, which was subjected to confirmatory sequencing. The 0.74-kb NdeI-BamHI insert of pJD758-M was then subcloned into pET-9a to produce expression vector pET-IMP12. The IMP-12 enzyme was purified from E. coli BL21(DE3)(pET-IMP12) as follows. Bacteria were grown in Buffered Super Broth (20 g of yeast extract/liter, 35 g of tryptone/liter, and 5 g of NaCl/liter, buffered with 50 mM sodium phosphate buffer [pH 7.0]) supplemented with kanamycin (50 μg/ml) at 37°C. When the culture reached an A600 of 0.7, isopropyl-β-d-thiogalactopyranoside (IPTG) was added (final concentration, 1 mM). After 18 h, cells were collected by centrifugation (at 6,000 × g and 4°C for 15 min), resuspended in 50 mM HEPES (pH 7.5) containing 50 μM ZnSO4 (HB buffer) (1/20 of the original culture volume), and disrupted by sonication (5 cycles, for 20 s each, at 45 W). The sample was clarified by centrifugation (at 10,000 × g and 4°C for 1 h) and loaded (flow rate, 2 ml/min) onto an HR 16/5 column packed with 10 ml of Source 15S gel (Amersham Biosciences, Uppsala, Sweden) preequilibrated with HB buffer. Elution was performed using a linear NaCl gradient (0 to 1 M in 100 ml; flow rate, 2 ml/min). Fractions containing β-lactamase activity were pooled, concentrated 20-fold using a Centriplus concentrator (YM10 membrane; Millipore, Bedford, Mass.), and loaded onto a Superdex 75 HR column (Amersham Biosciences) preequilibrated with HB supplemented with 0.15 M NaCl (HBS buffer). Elution was performed with the same buffer at a flow rate of 0.8 ml/min. The purified β-lactamase (final concentration, 0.87 mg/ml) was stored in HBS buffer at −80°C until use.
Protein electrophoretic techniques.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed according to the work of Laemmli (19) by using final acrylamide concentrations of 12 and 5% (wt/vol) for the separating and stacking gels, respectively. After electrophoresis, protein bands were stained with Coomassie brilliant blue R-250.
Determination of kinetic parameters.
The kinetic parameters of the IMP-12 enzyme were determined by using essentially the same methodology previously adopted for characterization of IMP-1 and IMP-2 (21, 27). Hydrolysis of β-lactams was monitored by using a Cary 100 UV-Vis spectrophotometer (Varian Instruments, Walnut Creek, Calif.) equipped with thermostatically controlled cells. The enzyme concentration in the reaction mixture was in the range of 0.17 to 350 nM.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the EMBL/GenBank/DDBJ sequence databases and assigned accession no. AJ420864.
RESULTS
Identification of a P. putida clinical isolate producing a plasmid-encoded IMP-like metallo-β-lactamase.
P. putida VA-758/00 was resistant to several β-lactams (including ampicillin, amoxicillin-clavulanate, ticarcillin, cephalothin, cefuroxime, cefotaxime, ceftazidime, cefepime, imipenem, meropenem, and aztreonam), while it was susceptible to piperacillin, aminoglycosides, and fluoroquinolones (Table 1 and data not shown). The β-lactam resistance pattern was unusual for a member of this species (29). In particular, resistance to carbapenems suggested the production of an acquired carbapenemase. In fact, carbapenemase activity was detected in a crude extract of VA-758/00 (imipenem-hydrolyzing specific activity, 67 ± 4 nmol per min per mg of protein). Carbapenemase activity was inhibited (>90%) by EDTA, suggesting the presence of a metallo-β-lactamase determinant.
TABLE 1.
MICs of various β-lactams for P. putida VA-758/00 and for P. aeruginosa PAO1 harboring plasmid pVA758a
| Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| P. putida VA-758/00 | P. aeruginosa PAO1 (pVA758) | P. aeruginosa PAO1 | |
| Piperacillin | 8 | 8 | 4 |
| Ticarcillin | 512 | 64 | 4 |
| Ceftazidime | 128 | 256 | 2 |
| Cefepime | 128 | 256 | 1 |
| Imipenem | 32 | 8 | 1 |
| Meropenem | 128 | 64 | 0.5 |
| Aztreonam | 32 | 8 | 1 |
| Gentamicin | 4 | 16 | 0.5 |
| Tobramycin | 4 | 32 | 0.5 |
| Amikacin | 1 | 1 | 1 |
The susceptibility of PAO1 is also shown for comparison.
IEF analysis of a crude extract of VA-758/00 revealed the presence of three bands of β-lactamase activity, with pIs of 6.9, 7.8, and 9.1, respectively (data not shown). Multiplex PCR analysis with primers VIM-DIA and IMP-DIA, using genomic DNA extracted from VA-758/00 as a template, yielded a 0.36-kb product, indicating the presence of a blaIMP gene. Restriction of the amplicon with AluI yielded a profile (three fragments of approximately 190, 90, and 80 bp, respectively) compatible with the presence of a blaIMP-2 or blaIMP-8 allele.
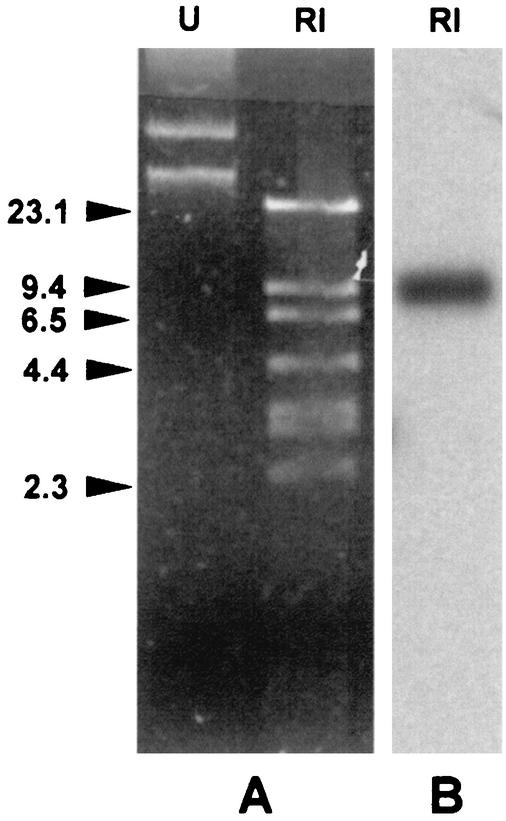
Agarose gel electrophoresis of a plasmid DNA preparation from VA-758/00 revealed the presence of plasmid DNA that was recognized by a blaIMP-1/2 probe mix in a Southern blot hybridization (Fig. 1). The blaIMP-containing plasmid, named pVA758, was purified and estimated to be approximately 50 kb, based on restriction analysis (Fig. 1).
FIG. 1.
(A) Agarose gel electrophoresis of a plasmid DNA preparation from P. putida VA-758/00, either undigested (lane U) or digested with EcoRI (lane RI). Identical plasmid profiles (not shown) were observed with β-lactam-resistant transformants obtained by electroporation of the plasmid preparation into P. aeruginosa PAO1. (B) Results of Southern blot analysis of the digested plasmid preparation by using a blaIMP-1/2 probe mix. DNA size standards (in kilobases) are shown on the left.
The potential for conjugational transfer of pVA758 was examined in biparental mating experiments using either P. aeruginosa 10145/3 or E. coli MKD-135 as the recipient, but in neither case could plasmid transfer be detected. However, pVA758 could be introduced into P. aeruginosa PAO1 by electroporation, yielding transformants that carried a plasmid apparently identical to pVA758 and produced EDTA-inhibitable imipenemase activity (data not shown). PAO1(pVA758) exhibited resistance to ceftazidime, cefepime, meropenem, gentamicin, and tobramycin and decreased susceptibility to imipenem, piperacillin, ticarcillin, and aztreonam (Table 1). IEF analysis of a crude extract of PAO1(pVA758) revealed the presence of two bands of β-lactamase activity with pIs of 7.8 and 9.1, respectively, neither of which was detectable in a crude extract of PAO1 (data not shown). This finding suggested that pVA758 encoded another β-lactamase in addition to the IMP-type enzyme. The additional enzyme could be responsible for the decreased susceptibility to aztreonam observed with PAO1(pVA758) compared to that for PAO1. Attempts at introducing pVA758 into E. coli DH5α by electroporation were unsuccessful, suggesting that the plasmid was unable to replicate in that host.
Structure of the blaIMP determinant and of its genetic environment.
PCR mapping using primers INT/5CS and INT/3CS, designed on the basis of the 5′-CS and 3′-CS of type 1 integrons (24), and pVA758 as a template yielded a 2.3-kb amplicon which was recognized by the blaIMP-1/2 probe mix in Southern blot hybridization (data not shown). Amplicon sequencing revealed the presence of two gene cassettes bounded by a 5′-CS and a 3′-CS typical of the sul1-associated type 1 integrons (31).
The first cassette carries a new blaIMP allele, named blaIMP-12, which, at the nucleotide sequence level, is 10% divergent from blaIMP-2 and blaIMP-8 (the closest homologues) and 15% divergent from blaIMP-1. The attC recombination site of the blaIMP-12-containing cassette is 125 bp long and is very similar to those of the cassettes carrying blaIMP-7 and blaIMP-5 (94 and 91% sequence identity, respectively) and also clearly related to those of the cassettes carrying blaIMP-11 (83% sequence identity) and blaIMP-1 or closely related alleles (80 to 81% sequence identity). On the other hand, it is apparently unrelated to the attC recombination site of the blaIMP-2- and blaIMP-8-containing cassettes (Fig. 2).
FIG. 2.
Sequence alignment of the attC recombination sites of blaIMP-containing gene cassettes. Descriptions and sources of the sequences are as follows: IMP-1, attC site of the blaIMP-1-containing cassette of integron In31 from P. aeruginosa 101/1477 (20); IMP-1/6/10, attC site of the blaIMP-1-containing cassette from Serratia marcescens AK9373 (1), which is identical to that of the blaIMP-6-containing cassette from S. marcescens KU3838 (37) and to those of the blaIMP-10-containing cassettes from P. aeruginosa PAI97 and Alcaligenes xylosoxidans AXI2 (13); IMP-11, attC site of the blaIMP-11-containing cassette from P. aeruginosa PAI112 (EMBL/GenBank accession no. AB074437); IMP-5, attC site of the blaIMP-5-containing cassette from Acinetobacter baumannii 65FFC (4); IMP-7, attC site of the blaIMP-7-containing cassette from P. aeruginosa 98/P/6327 (8); IMP-12, attC site of the blaIMP-12-containing cassette from P. putida VA-758/00 (this report); IMP-2/8, attC site of the blaIMP-2-containing cassette of In42 from A. baumannii AC-54/97 (27), which is identical to that of the blaIMP-8-containing cassette from Klebsiella pneumoniae KPO787 (36). The inverse core site is boxed; the positions of the 2L and 2R core sites (32) are indicated by arrows. Conserved residues in the first group of sequences are shaded; in the IMP-2/8 sequence, residues identical to those conserved within the first group are shaded.
The second cassette carries an aacA4 determinant and is identical to the cassette found in In42 (25). The presence of this determinant was consistent with the modification of the aminoglycoside susceptibility profile observed for PAO1(pVA758) (Table 1). Interestingly, in the P. putida isolate, the presence of this determinant was not associated with an overt phenotype of resistance to gentamicin and tobramycin (Table 1).
Comparison of IMP-12 with other IMP-type enzymes.
The IMP-12 protein is quite divergent from other members of the IMP family. Its closest relatives are IMP-8 and IMP-2 (88.6 and 88.2% amino acid sequence identity, respectively), while the most distant IMP variant is IMP-9 (81.6% sequence identity).
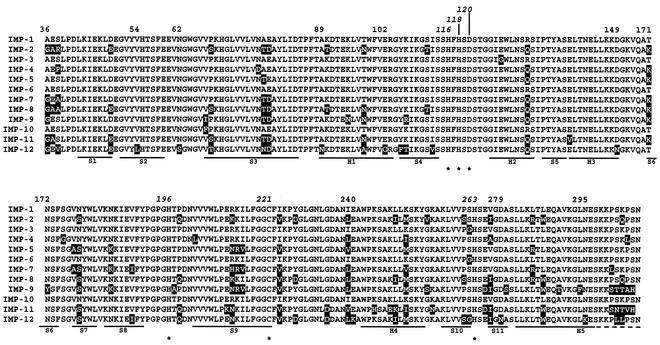
Multiple sequence alignment analysis including the 12 IMP variants revealed that IMP-12 carries unique residues at several positions where invariant residues are found in the other IMP enzymes, namely, at positions 54 (Leu versus Val), 62 (Ser versus Asn), 89 (Asn versus Ala), 102 (Gly versus Glu), 105 (Phe versus Tyr), 113 (Val versus Ile), 149 (Asn versus Asp), 240 (Lys versus Glu), 279 (Asn versus Asp), and 295 (Lys versus Asn) (Fig. 3). Compared to the other IMP enzymes, IMP-12 also carries unique residues at positions where variability was already detected, namely, at positions 38 (Val versus Ser, Arg, Ala, or Pro), 68 (Thr versus Pro or Ser), 78 (Asn versus Ala or Thr), 97 (Ala versus Thr or Asn), 106 (Thr versus Lys or Arg), 301 (Leu versus Ser, Thr, or Asn), and 302 (Leu versus Lys, Gln, or Thr) (Fig. 3).
FIG. 3.
Amino acid alignment of the sequence of the IMP-12 protein with those of other IMP-type enzymes. Stars indicate residues involved in the coordination of zinc ions. Substitutions observed in other variants, compared to IMP-1, are shown on a solid background. Secondary-structure elements (H, helices; S, strands) of IMP-1 (3) are also indicated below the sequences. Numbering is according to the BBL scheme (7). References for the various sequences are as follows: references 26 (IMP-1), 27 (IMP-2), 14 (IMP-3), 2 (IMP-4), 4 (IMP-5), 37 (IMP-6), 8 (IMP-7), 36 (IMP-8), and 13 (IMP-10); EMBL/GenBank accession no. AY033653 (IMP-9) and AB074437 (IMP-11); and this study (IMP-12).
Purification and characterization of the IMP-12 enzyme.
The IMP-12 enzyme was purified from E. coli BL21(DE3)(pET-IMP12) by means of a cation-exchange chromatography step, followed by a gel permeation chromatography step. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the purified protein preparation appeared to contain a single 28-kDa band, and it was estimated to be >95% pure (data not shown). The pI of the purified protein, determined by analytical IEF, was >9 (data not shown). This value is in agreement with the theoretical pI calculated for mature IMP-12 (9.17) assuming the presence of a signal peptide of 17 amino acids (Fig. 3) and is similar to the pI (9 ± 0.2) previously reported for IMP-1 (18).
IMP-12 was capable of hydrolyzing several β-lactam substrates including penicillins, narrow- to expanded-spectrum cephalosporins, and carbapenems. No hydrolysis of aztreonam or temocillin was detected (Table 2). The best substrates were cephalosporins and carbapenems (kcat/Km ratios, >105 M−1 · s−1), while penicillins were uniformly poorer substrates (kcat/Km ratios, around 104 M−1 · s−1) (Table 2). The poor activity on piperacillin is consistent with the relatively low MICs of this agent for both VA-758/00 and PAO1(pVA758).
TABLE 2.
Kinetic parameters of the purified IMP-12 enzymea
| Substrate |
kcat (s−1)
|
Km (μM)
|
kcat/Km (M−1 · s−1)
|
|||
|---|---|---|---|---|---|---|
| IMP-12 | IMP-1 | IMP-12 | IMP-1 | IMP-12 | IMP-1 | |
| Ampicillin | 18 | 950 | 1,500 | 200 | 1.2 × 104 | 4.8 × 106 |
| Piperacillin | NDb | ND | ND | ND | 2.3 × 104 | 7.2 × 105 |
| Carbenicillin | 3.7 | ND | 175 | ND | 2.1 × 104 | 2.0 × 104 |
| Ticarcillin | 6.9 | 1.1 | 470 | 740 | 1.5 × 104 | 1.5 × 103 |
| Temocillin | <0.1c | —d | — | NIe | — | — |
| Nitrocefin | 570 | 63 | 72 | 27 | 7.9 × 106 | 2.3 × 106 |
| Cephalothin | 118 | 48 | 16 | 21 | 7.4 × 106 | 2.3 × 106 |
| Cefotaxime | 56 | 1.3 | 22 | 4 | 2.5 × 106 | 3.3 × 105 |
| Cefuroxime | 61 | 8 | 7 | 37 | 8.7 × 106 | 2.2 × 105 |
| Ceftazidime | 6.7 | 8 | 15 | 44 | 4.5 × 105 | 1.8 × 105 |
| Cefepime | 15 | 7 | 26 | 11 | 5.8 × 105 | 6.4 × 105 |
| Imipenem | 240 | 46 | 920 | 39 | 2.6 × 105 | 1.2 × 106 |
| Meropenem | 9.5 | 5 | 7.2 | 10 | 1.3 × 106 | 5.0 × 105 |
| Aztreonam | <0.1c | <0.01 | — | NI | — | — |
The corresponding values previously measured for IMP-1 (21) are also shown for comparison. Data are means of three measurements. Standard deviations never exceeded 10%.
ND, not determined (first-order kinetic reaction in the range of the tested concentrations; with IMP-12, a piperacillin concentration up to 1 mM was assayed).
No hydrolysis detected by using an enzyme concentration of 350 nM in the reaction mixture.
—, not calculated.
NI, no interaction with the substrate.
The catalytic efficiencies of IMP-12 are similar to those of IMP-1 overall with some β-lactam substrates (carbenicillin, nitrocefin, cephalothin, ceftazidime, cefepime, and meropenem) but notably different with some penicillins (ampicillin, piperacillin, and ticarcillin), cefuroxime, cefotaxime, and imipenem (Table 2). The above differences usually resulted from differences of both individual kinetic parameters. A remarkable feature of IMP-12, compared to IMP-1, was the much higher Km for imipenem (Table 2).
DISCUSSION
The IMP enzymes are broad-spectrum metallo-β-lactamases, encoded by mobile genetic elements, that are successfully spreading among nosocomial gram-negative pathogens worldwide. Several variants of IMP enzymes that may diverge from each other by single or several amino acid substitutions (up to approximately 15% of the amino acid sequence) have been identified. In this work a new IMP variant, IMP-12, which is quite divergent (from 11 to 18%) from all other known IMP variants, was identified in a P. putida clinical isolate from northern Italy. This is the first report of an IMP-type enzyme in P. putida in Europe, and it underscores the diversity of existing IMP-type variants and of bacterial hosts that can acquire them.
Considering the epidemiological distribution of IMP variants, and data from comparative structural analysis of their genes and cognate genetic elements, some conclusions can be drawn concerning the origin and evolution of the blaIMP resistance determinants. (i) There is a blaIMP gene pool which is geographically widespread and includes several allelic variants. The original source of this gene pool remains unknown, but it is likely represented by some environmental species or group of species from which blaIMP genes eventually escape to opportunistic pathogens that colonize the hospital environment, such as pseudomonads, acinetobacters, and some Enterobacteriaceae. (ii) In the latter hosts blaIMP genes have always been found on integron-borne gene cassettes, and they are most likely acquired when already part of these elements. This allows exploitation of the integron recombination system for rapid dissemination in the clinical setting, under the selective pressure generated by antimicrobial agents. The finding of the same gene cassette in different isolates and in different integrons (1, 15, 20) underscores this potential for dissemination. (iii) Gene cassettes carrying blaIMP variants are equipped with attC recombination sites that belong to two different lineages: a “long” type, including members that are 119 to 129 bp long and clearly related to each other, found in the blaIMP-1, blaIMP-5, blaIMP-6, blaIMP-7, blaIMP-10, blaIMP-11, and blaIMP-12 cassettes, and a “short” type, 72 bp long, found in the blaIMP-2 and blaIMP-8 cassettes (Fig. 2). blaIMP variants located within similar cassette frameworks could originate from the same ancestral cassette, while the occurrence of notably divergent blaIMP variants located in different cassette frameworks (e.g., IMP-1 and IMP-2) points to a different phylogeny of the cassettes, either at the time of assembly or due to shuffling of recombination sites. (iv) The appearance of quite divergent IMP variants carried on different cassette frameworks, in epidemiologically unrelated clinical isolates, most likely reflects independent acquisition of the corresponding resistance genes and suggests that recruitment of similar genes by opportunistic gram-negative pathogens could be a relatively common and widespread phenomenon.
Biochemical characterization of IMP-12 revealed both common features with, and notable functional differences from, IMP-1 and other IMP-type enzymes (IMP-2, IMP-3, IMP-4, IMP-6, and IMP-10) for which kinetic data are available (2, 13, 14, 21, 27, 37). Common features of these enzymes include an overall preference for cephalosporins and carbapenems rather than for penicillins and a lack of activity toward temocillin. The functional differences concern individual kinetic parameters with various substrates, which can eventually affect the hydrolytic efficiency for the corresponding substrate. In particular, IMP-12 is less efficient than IMP-1 and other IMP variants at hydrolysis of penicillins (kcat/Km values were around 104 M−1 · s−1 for all compounds tested). Another notable feature of IMP-12 is the very high Km (almost 1 mM) for imipenem. A similar feature was previously reported for IMP-3, which differs from IMP-1 by Glu-to-Gly and Ser-to-Gly substitutions at positions 126 and 262, respectively (11), and for which the critical role of the latter substitution in drastically lowering the affinity for imipenem was confirmed through site-directed mutagenesis experiments (14). Interestingly, the S262G substitution, which is not present in IMP variants with higher imipenem affinities, was also present in IMP-12, where it might, at least in part, contribute to that behavior. However, it should be noted that kinetic data reported for IMP-6 (37), a natural IMP-1 mutant harboring only the S262G substitution, were at variance (Km for imipenem, 110 μM) with those reported by Iyobe et al. (14). Investigation of this aspect is currently under way. The poor affinity for imipenem exhibited by IMP-12 is likely the cause for the relatively low increase in the imipenem MIC (compared to that of meropenem) for P. aeruginosa PAO1 producing the IMP-12 enzyme, and it underscores the notion that enzyme affinity for the substrate can be critical to expression of the resistance phenotype in species of low outer membrane permeability, such as P. aeruginosa, even though the hydrolytic efficiency is relatively high (in this case the kcat/Km ratio was around 2 × 105 M−1 · s−1).
A unique structural feature of IMP-12 is a substitution (Asn-62 to Ser) in the “flap” region (positions 58 to 67 [3]), which is highly conserved in all IMP variants (Fig. 3) and was found to be a sensitive structural component of IMP-1 in codon randomization and selection experiments (23). In that work, only two substitutions (Pro or Ala) were found to be tolerated, at position 62, if enzyme activity toward ampicillin was to be maintained (23). Therefore, it might be speculated that the presence of a different residue (Ser) at that position could contribute to the reduced activity toward ampicillin observed for IMP-12 relative to IMP-1. Further investigation of these aspects is under way.
Acknowledgments
This work was supported by the European research network on metallo-β-lactamases (contract FMRX-CT98-0232) and by grant 2001068755_003 from MIUR. (PRIN 2001).
REFERENCES
- 1.Arakawa, Y., M. Murakami, K. Suzuki, H. Ito, R. Wacharotayankun, S. Ohsuka, N. Kato, and M. Ohta. 1995. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob. Agents Chemother. 39:1612-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu, Y. W., M. Afzal-Shah, E. T. Houang, M. I. Palepou, D. J. Lyon, N. Woodford, and D. M. Livermore. 2001. IMP-4, a novel metallo-β-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 45:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Concha, N. O., C. A. Janson, P. Rowling, S. Pearson, C. A. Cheever, B. P. Clarke, C. Lewis, M. Galleni, J. M. Frere, D. J. Payne, J. H. Bateson, and S. S. Abdel-Meguid. 2000. Crystal structure of the IMP-1 metallo-β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry 39:4288-4298. [DOI] [PubMed] [Google Scholar]
- 4.Da Silva, G. J., M. Correia, C. Vital, G. Ribeiro, J. C. Sousa, R. Leitão, L. Peixe, and A. Duarte. 2002. Molecular characterization of blaIMP-5, a new integron-borne metallo-β-lactamase from an Acinetobacter baumannii nosocomial isolate in Portugal. FEMS Microbiol. Lett. 215:33-39. [DOI] [PubMed] [Google Scholar]
- 5.Farinha, M. A., and A. M. Kropinski. 1990. High efficiency electroporation of Pseudomonas aeruginosa using frozen cell suspensions. FEMS Microbiol. Lett. 70:221-226. [DOI] [PubMed] [Google Scholar]
- 6.Gales, A. C., M. C. B. Tognim, A. O. Reis, R. N. Jones, and H. S. Sader. 2003. Emergence of an IMP-like metallo-enzyme in an Acinetobacter baumannii clinical strain from a Brazilian teaching hospital. Diagn. Microbiol. Infect. Dis. 45:77-79. [DOI] [PubMed] [Google Scholar]
- 7.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, and J. M. Frere. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibb, A. P., C. Tribuddharat, R. A. Moore, T. J. Louie, W. Krulicki, D. M. Livermore, M. F. Palepou, and N. Woodford. 2002. Nosocomial outbreak of carbapenem-resistant Pseudomonas aeruginosa with a new blaIMP allele, blaIMP-7. Antimicrob. Agents Chemother. 46:255-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkey, P. M., J. Xiong, H. Ye, H. Li, and F. H. M'Zali. 2001. Occurrence of a new metallo-β-lactamase IMP-4 carried on a conjugative plasmid in Citrobacter youngae from the People's Republic of China. FEMS Microbiol. Lett. 194:53-57. [DOI] [PubMed] [Google Scholar]
- 10.Ho, S. E., G. Subramaniam, S. Palasubramaniam, and P. Navaratnam. 2002. Carbapenem-resistant Pseudomonas aeruginosa in Malaysia producing IMP-7 β-lactamase. Antimicrob. Agents Chemother. 46:3286-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holloway, B. W. 1955. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13:572-581. [DOI] [PubMed] [Google Scholar]
- 12.Ito, H., Y. Arakawa, S. Ohsuka, R. Wacharotayankun, N. Kato, and M. Ohta. 1995. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob. Agents Chemother. 39:824-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyobe, S., H. Kusadokoro, A. Takahashi, S. Yomoda, T. Okubo, A. Nakamura, and K. O'Hara. 2002. Detection of a variant metallo-β-lactamase, IMP-10, from two unrelated strains of Pseudomonas aeruginosa and an Alcaligenes xylosoxidans strain. Antimicrob. Agents Chemother. 46:2014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyobe, S., H. Kusadokoro, J. Ozaki, N. Matsumura, S. Minami, S. Haruta, T. Sawai, and K. O'Hara. 2000. Amino acid substitutions in a variant of IMP-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:2023-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyobe, S., S. Minami, and H. Yamada. 1996. Insertion of a carbapenemase gene cassette in an integron of a Pseudomonas aeruginosa plasmid. J. Antimicrob. Chemother. 38:1114-1115. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J. L. 1994. Similarity analysis of DNAs, p. 655-682. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 17.Kiska, D. L., and P. H. Gilligan. 1999. Pseudomonas, p. 517-525. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington D.C.
- 18.Koh, T. H., L. H. Sng, G. S. Babini, N. Woodford, D. M. Livermore, and L. M. Hall. 2001. Carbapenem-resistant Klebsiella pnuemoniae in Singapore producing IMP-1 β-lactamase and lacking an outer membrane protein. Antimicrob. Agents Chemother. 45:1939-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Laraki, N., M. Galleni, I. Thamm, M. L. Riccio, G. Amicosante, J. M. Frere, and G. M. Rossolini. 1999. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob. Agents Chemother. 43:890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laraki, N., N. Franceschini, G. M. Rossolini, P. Santucci, C. Meunier, E. de Pauw, G. Amicosante, J. M. Frere, and M. Galleni. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Materon, I. C., and T. Palzkill. 2001. Identification of residues critical for metallo-β-lactamase function by codon randomization and selection. Protein Sci. 10:2556-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing; 12th informational supplement M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Osano, E., Y. Arakawa, R. Wacharotayankun, M. Ohta, T. Horii, H. Ito, F. Yoshimura, and N. Kato. 1994. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riccio, M. L., N. Franceschini, L. Boschi, B. Caravelli, G. Cornaglia, R. Fontana, G. Amicosante, and G. M. Rossolini. 2000. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob. Agents Chemother. 44:1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Sanford, J. P. 1995. Pseudomonas species (including melioidosis and glanders), p. 2003-2009. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 30.Senda, K., Y. Arakawa, K. Nakashima, H. Ito, S. Ichiyama, K. Shimokata, N. Kato, and M. Ohta. 1996. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob. Agents Chemother. 40:349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes, H. W., and R. M. Hall. 1989. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 3:1669-1683. [DOI] [PubMed] [Google Scholar]
- 32.Stokes, H. W., D. B. O'Gorman, G. D. Recchia, M. Parsekhian, and R. M. Hall. 1997. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol. Microbiol. 26:731-745. [DOI] [PubMed] [Google Scholar]
- 33.Tsao, S. G., C. F. Brunk, and R. E. Pearlman. 1983. Hybridization of nucleic acids directly in agarose gels. Anal. Biochem. 131:365-372. [DOI] [PubMed] [Google Scholar]
- 34.Tysall, L., M. W. Stockdale, P. R. Chadwick, M. F. Palepou, K. J. Towner, D. M. Livermore, and N. Woodford. 2002. IMP-1 carbapenemase detected in an Acinetobacter clinical isolate from the UK. J. Antimicrob. Chemother. 49:217-218. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe, M., S. Iyobe, M. Inoue, and S. Mitsuhashi. 1991. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 35:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan, J. J., W. C. Ko, and J. J. Wu. 2001. Identification of a plasmid encoding SHV-12, TEM-1, and a variant of IMP-2 metallo-β-lactamase, IMP-8, from a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:2368-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yano, H., A. Kuga, R. Okamoto, H. Kitasato, T. Kobayashi, and M. Inoue. 2001. Plasmid-encoded metallo-β-lactamase (IMP-6) conferring resistance to carbapenems, especially meropenem. Antimicrob. Agents Chemother. 45:1343-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yum, J. H., K. Yi, H. Lee, D. Yong, K. Lee, J. Kim, G. M. Rossolini, and Y. Chong. 2002. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the blaVIM-2 gene cassettes. J. Antimicrob. Chemother. 49:837-840. [DOI] [PubMed] [Google Scholar]