Abstract
We used a focal infectivity assay with HeLa H1-JC.37 cells to directly compare susceptibilities of simian immunodeficiency virus (SIV) and human immunodeficiency virus type 1 (HIV-1) to protease inhibitors. SIVmac239 was inhibited by indinavir, saquinavir, and ritonavir, with 50% effective concentrations (means ± standard deviations) of 39 ± 8, 55 ± 3, and 13 ± 5 nM, respectively. The corresponding values for inhibition of HIV-1 were 66 ± 4, 47 ± 10, and 25 ± 14 nM, respectively.
The development of protease inhibitors as potent antiretroviral drugs enabled the first successful drug combinations used for highly active antiretroviral therapy (HAART) (9, 12, 13), and protease inhibitors remain a major component of AIDS therapy. HAART provides long-term suppression of plasma human immunodeficiency virus type 1 (HIV-1) loads to undetectable levels (12, 13, 30) and increased CD4+-T-cell counts (9, 19, 23) in many patients. However, major problems remain, including the emergence of multidrug-resistant virus, latency or persistence, and residual replication of HIV-1, even in patients on suppressive HAART (5, 6, 8, 17, 34, 44). Memory T cells have been identified as one site that harbors latent HIV-1 (10, 46), but it is likely that there are other sites. The sites of residual replication have not been determined and represent a major impediment to eradication of HIV-1 in patients (5, 18). New therapeutic strategies will be necessary to better control or eradicate HIV-1 infection. A highly relevant and predictive animal model of HAART would greatly facilitate development of innovative therapeutic strategies.
Although protease inhibitors have been successful in HAART, they have not been extensively studied in the available animal models of AIDS: feline immunodeficiency virus (FIV) infection of cats or simian immunodeficiency virus (SIV) infection of rhesus macaques. Both of these models have been used extensively for studies of nucleoside analogs (14, 15, 26, 31, 39, 41). However, FIV is not susceptible to the protease inhibitors used in AIDS therapy (36). SIV is susceptible to protease inhibitors that inhibit HIV-1 (1, 3, 25), but direct comparisons of these two viruses by using the same cell line with a quantitative infectivity assay have not been made. The proteases of HIV-1 and SIV have similar biochemical properties (11, 27), but there are substantial differences in several amino acids in the active sites (47). The SIV protease was inhibited by one preclinical inhibitor, SB203386, but the Ki for inhibition was 10 times higher than the Ki for inhibition of the HIV-1 protease (20). In the work reported here, we directly compared the in vitro susceptibilities of SIVmac239 and HIV-1 to three Food and Drug Administration-approved protease inhibitors: indinavir, saquinavir, and ritonavir.
For these comparisons, we used a focal infectivity assay (FIA) (4, 32) with a cell line, HeLa H1-JC.37, that is permissive to infection by both SIV and HIV-1 (21, 33). These cells naturally express CXCR4 and have been engineered to express human genes for CD4 and CCR5 (33). Conditions for the FIA were recently described (29). The viruses used in these studies were SIVmac239 and HIV-1 NL4-3, provided by Paul Luciw (University of California, Davis); SIVmac251, provided by Koen Van Rompay (University of California, Davis); and RT-SHIV (made with a 5′-half clone obtained from Joseph Sodroski [Dana-Farber Cancer Institute, Harvard Medical School] [40] and the 3′-half clone of SIVmac239 [24, 35]). Virus stocks were prepared and stored as previously described (29, 41). Indinavir, saquinavir, and ritonavir used in these studies were provided by Raymond F. Schinazi (Emory University, Decatur, Ga.) and by Mohamed Nasr (Division of AIDS, National Institute of Allergy and Infectious Diseases). Schinazi also provided 3′-azido3′-deoxythymidine (AZT) and 2′,3′-dideoxy-3′-thiacytidine (3TC).
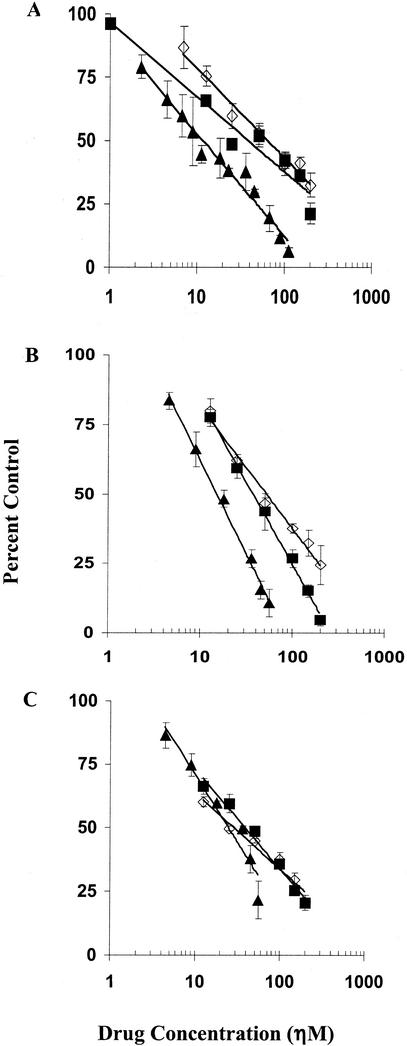
For initial validation of the FIA with SIVmac239, we compared it with two p27 antigen-based assays that utilize either CEMx174 cells or peripheral blood mononuclear cells (PBMC), both of which have previously been used for studies of drug susceptibility of SIV (41-43). The dose-response curves for inhibition of SIVmac239 by indinavir, saquinavir, and ritonavir that were obtained with these three assays are shown in Fig. 1. The concentrations required to inhibit focus formation or p27 production by 50% (EC50) were determined directly from the linear portions of those plots. The results are summarized in Table 1. For each drug, the dose-response curves and EC50 values obtained with the three assays were similar. With any of the drugs, there was no more than a twofold difference in the EC50 values among the three assays.
FIG. 1.
Dose-response curves comparing the susceptibilities of SIVmac239 to indinavir ▪, saquinavir ◊, and ritonavir ▴ in three different assays. (A) Susceptibility of SIV in HeLa cells determined by the FIA. (B) Susceptibility of SIV in CEMx174 cells determined by p27 enzyme-linked immunosorbent assay. (C) Susceptibility of SIV in PBMC determined by p27 enzyme-linked immunosorbent assay. The values are means of at least three experiments (± standard deviations). EC50 values were determined from the best-fit line of the linear portion of the graph. Data were plotted as percentages of control (no drug) versus inhibitor concentration.
TABLE 1.
Inhibition of SIVmac239 by protease inhibitors determined with three drug susceptibility assaysa
| Protease inhibitor | Mean EC50 ± SD (nM)
|
||
|---|---|---|---|
| HeLa H1-JC.37 | CEMx174 | PBMC | |
| Indinavir | 39 ± 8 | 37 ± 7 | 30 ± 4 |
| Saquinavir | 55 ± 3 | 49 ± 6 | 38 ± 8 |
| Ritonavir | 13 ± 5 | 16 ± 2 | 26 ± 10 |
The assays used were the FIA with HeLa H1-JC.37 cells and p27 assays with CEMx174 cells or PBMC. For the three assays, the mean EC50 values were determined from at least three separate experiments.
We used the FIA with HeLa H1-JC.37 cells to directly compare the susceptibilities of SIVmac239 and HIV-1 to these three protease inhibitors. Assay conditions were identical except that foci of HIV-1-infected cells were detected with the HIV-1-specific antibody 22-6 (16), whereas foci of infection by SIV or RT-SHIV were detected with SIV-specific antibodies in serum from SIV-infected rhesus macaques (29). SIV and HIV-1 were very similar in their susceptibilities to each of the three inhibitors (Table 2). All statistical analyses were performed according to the ANOVA analysis of variance. The difference in EC50 values between SIVmac239 and HIV-1 were not significantly different (P > 0.05), except for indinavir (P = 0.005). With all three drugs, the EC50 values obtained with these two viruses were different by no more than twofold. As controls, two nucleoside analogues, AZT and 3TC (Table 2), which are known to inhibit HIV-1 and SIV (2, 7, 28, 38), were evaluated. SIVmac239 and HIV-1 were more similar in susceptibilities to protease inhibitors than to AZT. We also evaluated the susceptibilities of uncloned SIVmac251 and RT-SHIV to these three protease inhibitors (Table 2). Both of these viruses were inhibited by all three protease inhibitors, with EC50 values being similar to those obtained with SIVmac239. There was no more than a twofold difference in EC50 values between SIVmac239 and either of these two viruses.
TABLE 2.
Comparison of drug susceptibilities of SIVmac239, HIV-1, SIVmac251, and RT-SHIVa
| Inhibitor | Mean EC50 ± SD (nM)
|
|||
|---|---|---|---|---|
| SIVmac239 | HIV-1 | SIVmac251 | RT-SHIV | |
| Indinavir | 39 ± 8 | 66 ± 4 | 45 ± 3 | 52 ± 5 |
| Saquinavir | 55 ± 3 | 47 ± 10 | 55 ± 9 | 63 ± 10 |
| Ritonavir | 13 ± 5 | 25 ± 14 | 25 ± 4 | 24 ± 2 |
| AZT | 470 ± 40 | 120 ± 40 | NDb | ND |
| 3TC | 550 ± 50 | 360 ± 60 | ND | ND |
These comparisons were made using the FIA. Mean EC50 values were determined from at least three separate experiments.
ND, not determined.
Our data demonstrate that SIVmac239 and HIV-1 are very similar in their susceptibilities to three protease inhibitors that are approved for use in AIDS therapy. These comparisons were made from infections of a single cell line under identical conditions. This precludes differences in cellular uptake or metabolism of drugs, enabling direct comparisons of drug susceptibilities of the two viruses. The SIV-rhesus macaque model has been widely used to study nucleoside inhibitors (26, 41, 45), and our in vitro data suggest that this model may be more broadly useful for studies of HAART combinations that include protease inhibitors. There have been attempts to study HAART combinations that include protease inhibitors in SIV-macaque models (22, 37); however, the efficacy of monotherapy with a protease inhibitor was not demonstrated at the dose used in those studies. Our data suggest that drug combinations that include Food and Drug Administration-approved protease inhibitors can be studied in the SIV-rhesus macaque model. This may enable the study of complications in HAART that are difficult or impossible to investigate in humans, such as more detailed analysis of reservoirs and sites of residual replication in tissues.
As expected, RT-SHIV was similar to SIV in susceptibility to these three protease inhibitors. This is important because RT-SHIV is susceptible to nonnucleoside reverse transcriptase inhibitors whereas SIV is not. The RT-SHIV-rhesus macaque model offers an opportunity to study HAART with combinations of drugs that include all of the classes currently approved for use in therapy of HIV-1.
We plan to characterize mutations in the SIV protease that confer resistance to each of these protease inhibitors. This will provide important structure-function comparisons of the SIV and HIV-1 proteases. If drug-resistant SIV mutants are similar to clinically important HIV-1 mutants, then the model will be useful for evaluation of the virulence and pathogenicity of protease inhibitor-resistant mutants.
Acknowledgments
We thank Timothy B. Matthews for excellent technical assistance. We also thank Michael J. Hofman, Jeffrey P. Murry, and Koen K. Van Rompay for assistance and valuable discussion.
This research was supported by NIH grants AI47070 and RR13697 to T.W.N.
REFERENCES
- 1.Ashorn, P., T. J. McQuade, S. Thaisrivongs, A. G. Tomasselli, W. G. Tarpley, and B. Moss. 1990. An inhibitor of the protease blocks maturation of human and simian immunodeficiency viruses and spread of infection. Proc. Natl. Acad. Sci. USA 87:7472-7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini, J., M. Weeger, M. J. Camarasa, E. De Clercq, and K. Uberla. 1995. Sensitivity/resistance profile of a simian immunodeficiency virus containing the reverse transcriptase gene of human immunodeficiency virus type 1 (HIV-1) toward the HIV-1-specific non-nucleoside reverse transcriptase inhibitors. Biochem. Biophys. Res. Commun. 211:850-856. [DOI] [PubMed] [Google Scholar]
- 3.Black, P. L., M. B. Downs, M. G. Lewis, M. A. Ussery, G. B. Dreyer, S. R. Petteway, Jr., and D. M. Lambert. 1993. Antiretroviral activities of protease inhibitors against murine leukemia virus and simian immunodeficiency virus in tissue culture. Antimicrob. Agents Chemother. 37:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesebro, B., and K. Wehrly. 1988. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J. Virol. 62:3779-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun, T. W., and A. S. Fauci. 1999. Latent reservoirs of HIV: obstacles to the eradication of virus. Proc. Natl. Acad. Sci. USA 96:10958-10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates, J. A., N. Cammack, H. J. Jenkinson, A. J. Jowett, M. I. Jowett, B. A. Pearson, C. R. Penn, P. L. Rouse, K. C. Viner, and J. M. Cameron. 1992. (−)-2′-deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob. Agents Chemother. 36:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, O. J., and A. S. Fauci. 1998. Transmission of multidrug-resistant human immunodeficiency virus—the wake-up call. N. Engl. J. Med. 339:341-343. [DOI] [PubMed] [Google Scholar]
- 9.Collier, A. C., R. W. Coombs, D. A. Schoenfeld, R. L. Bassett, J. Timpone, A. Baruch, M. Jones, K. Facey, C. Whitacre, V. J. McAuliffe, H. M. Friedman, T. C. Merigan, R. C. Reichman, C. Hooper, L. Corey, et al. 1996. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N. Engl. J. Med. 334:1011-1017. [DOI] [PubMed] [Google Scholar]
- 10.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 11.Grant, S. K., I. C. Deckman, M. D. Minnich, J. Culp, S. Franklin, G. B. Dreyer, T. A. Tomaszek, Jr., C. Debouck, and T. D. Meek. 1991. Purification and biochemical characterization of recombinant simian immunodeficiency virus protease and comparison to human immunodeficiency virus type 1 protease. Biochemistry 30:8424-8434. [DOI] [PubMed] [Google Scholar]
- 12.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, D. D. Richman, F. T. Valentine, L. Jonas, A. Meibohm, E. A. Emini, and J. A. Chodakewitz. 1997. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337:734-739. [DOI] [PubMed] [Google Scholar]
- 13.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, M. A. Fischl, et al. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann, K., A. Donath, B. Beer, H. F. Egberink, M. C. Horzinek, H. Lutz, G. Hoffmann-Fezer, I. Thum, and S. Thefeld. 1992. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet. Immunol. Immunopathol. 35:167-175. [DOI] [PubMed] [Google Scholar]
- 15.Hayes, K. A., L. J. Lafrado, J. G. Erickson, J. M. Marr, and L. E. Mathes. 1993. Prophylactic ZDV therapy prevents early viremia and lymphocyte decline but not primary infection in feline immunodeficiency virus-inoculated cats. J. Acquir. Immune Defic. Syndr. 6:127-134. [PubMed] [Google Scholar]
- 16.Higgins, J. R., N. C. Pedersen, and J. R. Carlson. 1986. Detection and differentiation by sandwich enzyme-linked immunosorbent assay of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus- and acquired immunodeficiency syndrome-associated retroviruslike clinical isolates. J. Clin. Microbiol. 24:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch, M. S., B. Conway, R. T. D'Aquila, V. A. Johnson, F. Brun-Vézinet, B. Clotet, L. M. Demeter, S. M. Hammer, D. M. Jacobsen, D. R. Kuritzkes, C. Loveday, J. W. Mellors, S. Vella, and D. D. Richman. 1998. Antiretroviral drug resistance testing in adults with HIV infection implications for clinical management. J. Am. Chem. Soc. 279:1984-1991. [DOI] [PubMed] [Google Scholar]
- 18.Ho, D. D. 1998. Toward HIV eradication or remission: the tasks ahead. Science 280:1866-1867. [DOI] [PubMed] [Google Scholar]
- 19.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 20.Hoog, S. S., E. M. Towler, B. Zhao, M. L. Doyle, C. Debouck, and S. S. Abdel-Meguid. 1996. Human immunodeficiency virus protease ligand specificity conferred by residues outside of the active site cavity. Biochemistry 35:10279-10286. [DOI] [PubMed] [Google Scholar]
- 21.Kuhmann, S. E., E. J. Platt, S. L. Kozak, and D. Kabat. 1997. Polymorphisms in the CCR5 genes of African green monkeys and mice implicate specific amino acids in infections by simian and human immunodeficiency viruses. J. Virol. 71:8642-8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Grand, R., B. Vaslin, J. Larghero, O. Neidez, H. Thiebot, P. Sellier, P. Clayette, N. Dereuddre-Bosquet, and D. Dormont. 2000. Post-exposure prophylaxis with highly active antiretroviral therapy could not protect macaques from infection with SIV/HIV chimera. AIDS 14:1864-1866. [DOI] [PubMed] [Google Scholar]
- 23.Li, T. S., R. Tubiana, C. Katlama, V. Calvez, H. Ait Mohand, and B. Autran. 1998. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 351:1682-1686. [DOI] [PubMed] [Google Scholar]
- 24.Luciw, P. A., E. Pratt-Lowe, K. E. S. Shaw, J. A. Levy, and C. Cheng-Mayer. 1995. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc. Natl. Acad. Sci. USA 92:7490-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, L. N., K. F. Soike, M. Murphey-Corb, R. P. Bohm, E. D. Roberts, T. J. Kakuk, S. Thaisrivongs, T. J. Vidmar, M. J. Ruwart, and S. R. Davio. 1994. Effects of U-75875, a peptidomimetic inhibitor of retroviral proteases, on simian immunodeficiency virus infection in rhesus monkeys. Antimicrob. Agents Chemother. 38:1277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClure, H. M. 1990. Nonhuman primate models for evaluation of AIDS therapy. Ann. N. Y. Acad. Sci. 616:287-298. [DOI] [PubMed] [Google Scholar]
- 27.Miller, M., M. Jaskolski, J. K. Rao, J. Leis, and A. Wlodawer. 1989. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature 337:576-579. [DOI] [PubMed] [Google Scholar]
- 28.Mitsuya, H., K. J. Weinhold, P. A. Furman, M. H. St. Clair, S. N. Lehrman, R. C. Gallo, D. Bolognesi, D. W. Barry, and S. Broder. 1985. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 82:7096-7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murry, J. P., J. Higgins, T. B. Matthews, V. Y. Huang, K. K. Van Rompay, N. C. Pedersen, and T. W. North. 2003. Reversion of the M184V mutation in simian immunodeficiency virus reverse transcriptase is selected by tenofovir, even in the presence of lamivudine. J. Virol. 77:1120-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 31.Philpott, M. S., J. P. Ebner, and E. A. Hoover. 1992. Evaluation of 9-(2-phosphonylmethoxyethyl) adenine therapy for feline immunodeficiency virus using a quantitative polymerase chain reaction. Vet. Immunol. Immunopathol. 35:155-166. [DOI] [PubMed] [Google Scholar]
- 32.Pincus, S. H., K. Wehrly, and B. Chesebro. 1991. Use of a focal infectivity assay for testing susceptibility of HIV to antiviral agents. BioTechniques 10:336-342. [PubMed] [Google Scholar]
- 33.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richman, D. D. 1995. Clinical significance of drug resistance in human immunodeficiency virus. Clin. Infect. Dis. 21(Suppl. 2):S166-S169. [DOI] [PubMed] [Google Scholar]
- 35.Sawai, E. T., I. H. Khan, P. M. Montbriand, B. M. Peterlin, C. Cheng-Mayer, and P. A. Luciw. 1996. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr. Biol. 6:1519-1527. [DOI] [PubMed] [Google Scholar]
- 36.Slee, D. H., K. L. Laslo, J. H. Elder, I. R. Ollmann, A. Gustchina, K. Kervinen, A. Zdanov, A. Wlodawer, and C.-H. Wong. 1995. Selectivity in the inhibition of HIV and FIV protease: inhibitory and mechanistic studies of pynotide-containing alpha-keto amide and hydroxyethylamine core structures. J. Am. Chem. Soc. 117:11867-11878. [Google Scholar]
- 37.Thiebot, H., F. Louache, B. Vaslin, T. de Revel, O. Neildez, J. Larghero, W. Vainchenker, D. Dormont, and R. Le Grand. 2001. Early and persistent bone marrow hematopoiesis defect in simian/human immunodeficiency virus-infected macaques despite efficient reduction of viremia by highly active antiretroviral therapy during primary infection. J. Virol. 75:11594-11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai, C. C., K. E. Follis, and R. E. Benveniste. 1988. Antiviral effects of 3′-azido-3′-deoxythymidine, 2′,3′-dideoxycytidine, and 2′,3′-dideoxyadenosine against simian acquired immunodeficiency syndrome-associated type D retrovirus in vitro. AIDS Res. Hum. Retrovir. 4:359-368. [DOI] [PubMed] [Google Scholar]
- 39.Tsai, C. C., K. E. Follis, R. F. Grant, R. E. Nolte, C. R. Bartz, R. E. Benveniste, and P. R. Sager. 1993. Effect of dosing frequency on ZDV prophylaxis in macaques infected with simian immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 6:1086-1092. [PubMed] [Google Scholar]
- 40.Uberla, K., C. Stahl-Hennig, D. Böttiger, K. Mätz-Rensing, F. J. Kaup, J. Li, W. A. Haseltine, B. Fleckenstein, G. Hunsmann, and B. Oberg. 1995. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc. Natl. Acad. Sci. USA 92:8210-8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Rompay, K. K., M. L. Marthas, R. A. Ramos, C. P. Mandell, E. K. McGowan, S. M. Joye, and N. C. Pedersen. 1992. Simian immunodeficiency virus (SIV) infection of infant rhesus macaques as a model to test antiretroviral drug prophylaxis and therapy: oral 3′-azido-3′-deoxythymidine prevents SIV infection. Antimicrob. Agents Chemother. 36:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Rompay, K. K., M. G. Otsyula, M. L. Marthas, C. J. Miller, M. B. McChesney, and N. C. Pedersen. 1995. Immediate zidovudine treatment protects simian immunodeficiency virus-infected newborn macaques against rapid onset of AIDS. Antimicrob. Agents Chemother. 39:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Rompay, K. K., M. G. Otsyula, R. P. Tarara, D. R. Canfield, C. J. Berardi, M. B. McChesney, and M. L. Marthas. 1996. Vaccination of pregnant macaques protects newborns against mucosal simian immunodeficiency virus infection. J. Infect. Dis. 173:1327-1335. [DOI] [PubMed] [Google Scholar]
- 44.Wainberg, M. A., and G. Friedland. 1998. Public health implications of antiretroviral therapy and HIV drug resistance. J. Am. Chem. Soc. 279:1977-1983. [DOI] [PubMed] [Google Scholar]
- 45.Watson, A., J. McClure, J. Ranchalis, M. Scheibel, A. Schmidt, B. Kennedy, W. R. Morton, N. L. Haigwood, and S. L. Hu. 1997. Early postinfection antiviral treatment reduces viral load and prevents CD4+ cell decline in HIV type 2-infected macaques. AIDS Res. Hum. Retrovir. 13:1375-1381. [DOI] [PubMed] [Google Scholar]
- 46.Wong, J. K., M. Hezareh, H. F. Günthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 47.Zhao, B., E. Winborne, M. D. Minnich, J. S. Culp, C. Debouck, and S. S. Abdel-Meguid. 1993. Three-dimensional structure of a simian immunodeficiency virus protease/inhibitor complex. Implications for the design of human immunodeficiency virus type 1 and 2 protease inhibitors. Biochemistry 32:13054-13060. [DOI] [PubMed] [Google Scholar]