Abstract
Of 240 pesticides screened for carcinogenicity by the U.S. Environmental Protection Agency Office of Pesticide Programs, at least 24 (10%) produce thyroid follicular cell tumors in rodents. Thirteen of the thyroid carcinogens also induce liver tumors, mainly in mice, and 9 chemicals produce tumors at other sites. Some mutagenic data are available on all 24 pesticides producing thyroid tumors. Mutagenicity does not seem to be a major determinant in thyroid carcinogenicity, except for possibly acetochlor; evidence is less convincing for ethylene thiourea and etridiazole. Studies on thyroid-pituitary functioning, including indications of thyroid cell growth and/or changes in thyroxine, triiodothyronine, or thyroid-stimulating hormone levels, are available on 19 pesticides. No such antithyroid information is available for etridiazole, N-octyl bicycloheptene dicarboximide, terbutryn, triadimefon, and trifluralin. Of the studied chemicals, only bromacil lacks antithyroid activity under study conditions. Intrathyroidal and extrathyroidal sites of action are found: amitrole, ethylene thiourea, and mancozeb are thyroid peroxidase inhibitors; and acetochlor, clofentezine, fenbuconazole, fipronil, pendimethalin, pentachloronitrobenzene, prodiamine, pyrimethanil, and thiazopyr seem to enhance the hepatic metabolism and excretion of thyroid hormone. Thus, with 12 pesticides that mode of action judgments can be made, 11 disrupt thyroid-pituitary homeostasis only; no chemical is mutagenic only; and acetochlor may have both antithyroid and some mutagenic activity. More information is needed to identify other potential antithyroid modes of thyroid carcinogenic action.
Full text
PDF
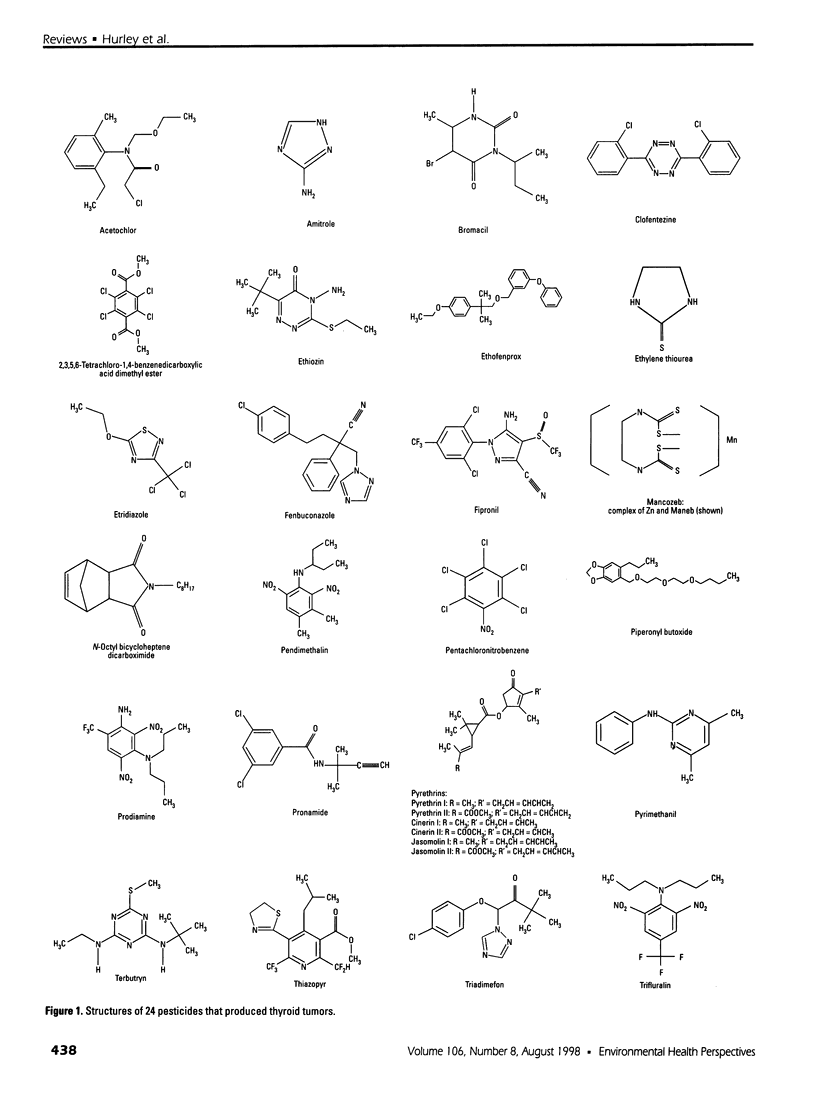


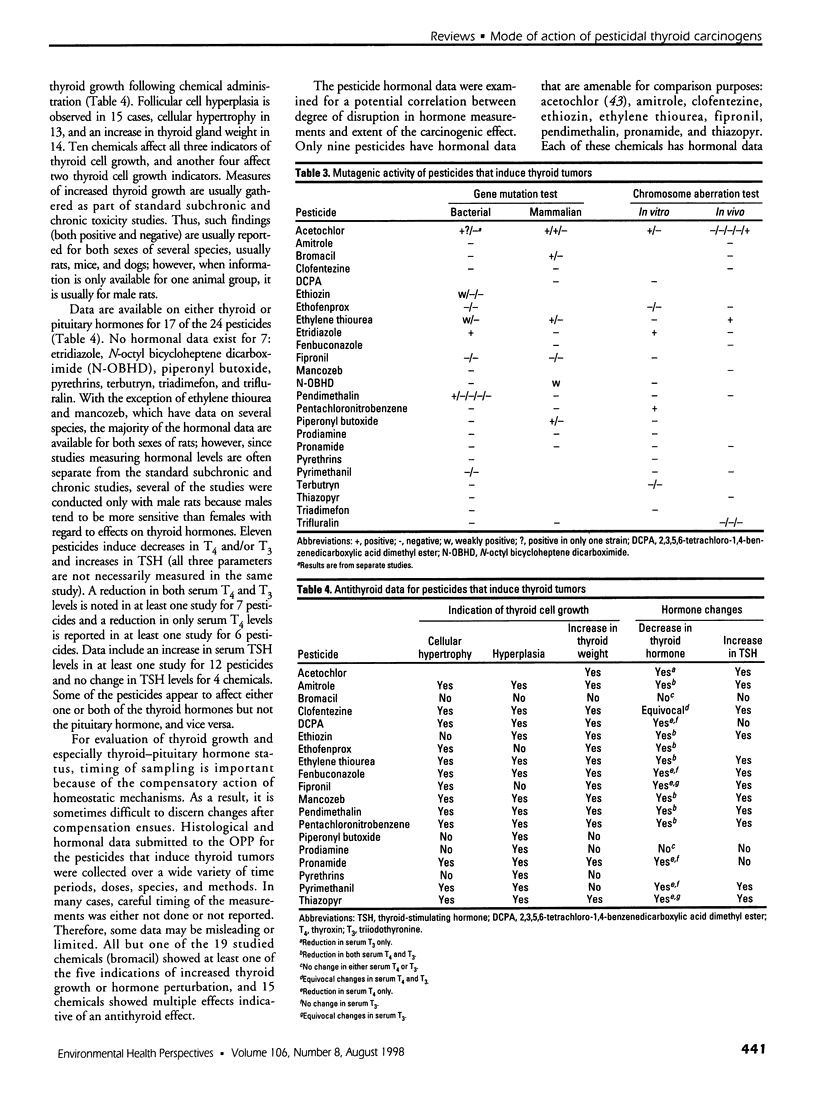

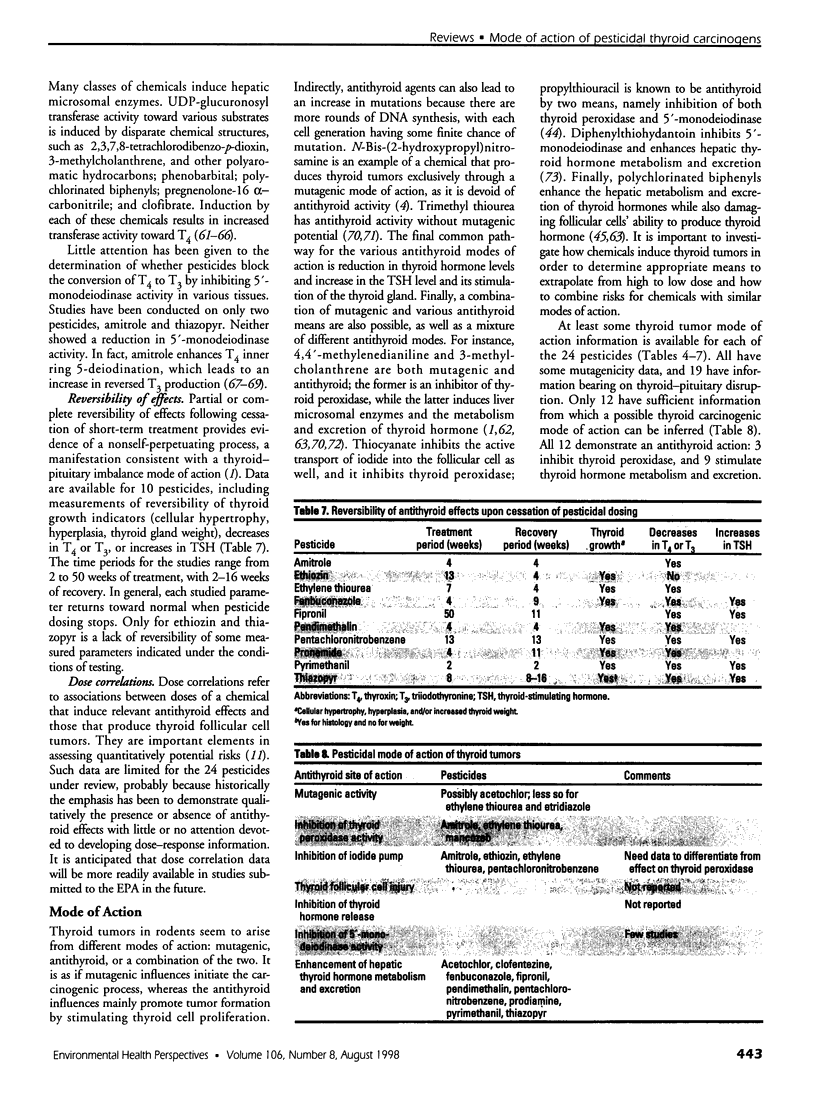


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER N. M. Antithyroid action of 3-amino-1,2,4-triazole. J Biol Chem. 1959 Jan;234(1):148–150. [PubMed] [Google Scholar]
- Arnold D. L., Krewski D. R., Junkins D. B., McGuire P. F., Moodie C. A., Munro I. C. Reversibility of ethylenethiourea-induced thyroid lesions. Toxicol Appl Pharmacol. 1983 Feb;67(2):264–273. doi: 10.1016/0041-008x(83)90233-8. [DOI] [PubMed] [Google Scholar]
- Ashby J., Kier L., Wilson A. G., Green T., Lefevre P. A., Tinwell H., Willis G. A., Heydens W. F., Clapp M. J. Evaluation of the potential carcinogenicity and genetic toxicity to humans of the herbicide acetochlor. Hum Exp Toxicol. 1996 Sep;15(9):702–735. doi: 10.1177/096032719601500902. [DOI] [PubMed] [Google Scholar]
- Balsam A., Sexton F., Borges M., Ingbar S. H. Formation of diiodotyrosine from thyroxine. Ether-link cleavage, an alternate pathway of thyroxine metabolism. J Clin Invest. 1983 Oct;72(4):1234–1245. doi: 10.1172/JCI111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter R. A., Klaassen C. D. Reduction of thyroid hormone levels and alteration of thyroid function by four representative UDP-glucuronosyltransferase inducers in rats. Toxicol Appl Pharmacol. 1994 Sep;128(1):9–17. doi: 10.1006/taap.1994.1174. [DOI] [PubMed] [Google Scholar]
- Barter R. A., Klaassen C. D. UDP-glucuronosyltransferase inducers reduce thyroid hormone levels in rats by an extrathyroidal mechanism. Toxicol Appl Pharmacol. 1992 Mar;113(1):36–42. doi: 10.1016/0041-008x(92)90006-e. [DOI] [PubMed] [Google Scholar]
- Bookstaff R. C., Murphy V. A., Skare J. A., Minnema D., Sanzgiri U., Parkinson A. Effects of doxylamine succinate on thyroid hormone balance and enzyme induction in mice. Toxicol Appl Pharmacol. 1996 Dec;141(2):584–594. doi: 10.1006/taap.1996.0325. [DOI] [PubMed] [Google Scholar]
- Byrne J. J., Carbone J. P., Hanson E. A. Hypothyroidism and abnormalities in the kinetics of thyroid hormone metabolism in rats treated chronically with polychlorinated biphenyl and polybrominated biphenyl. Endocrinology. 1987 Aug;121(2):520–527. doi: 10.1210/endo-121-2-520. [DOI] [PubMed] [Google Scholar]
- Capen C. C., Martin S. L. The effects of xenobiotics on the structure and function of thyroid follicular and C-cells. Toxicol Pathol. 1989;17(2):266–293. doi: 10.1177/019262338901700205. [DOI] [PubMed] [Google Scholar]
- Cartier L. J., Williams I. K., Holloszy J., Premachandra B. N. Potentiation of thyroxine 5-deiodination by aminotriazole. Biochim Biophys Acta. 1985 Nov 22;843(1-2):68–72. doi: 10.1016/0304-4165(85)90050-9. [DOI] [PubMed] [Google Scholar]
- Contrera J. F., Jacobs A. C., DeGeorge J. J. Carcinogenicity testing and the evaluation of regulatory requirements for pharmaceuticals. Regul Toxicol Pharmacol. 1997 Apr;25(2):130–145. doi: 10.1006/rtph.1997.1085. [DOI] [PubMed] [Google Scholar]
- Curran P. G., DeGroot L. J. The effect of hepatic enzyme-inducing drugs on thyroid hormones and the thyroid gland. Endocr Rev. 1991 May;12(2):135–150. doi: 10.1210/edrv-12-2-135. [DOI] [PubMed] [Google Scholar]
- Dalvi R. R., Dalvi P. S. Differences in the effects of piperine and piperonyl butoxide on hepatic drug-metabolizing enzyme system in rats. Drug Chem Toxicol. 1991;14(1-2):219–229. doi: 10.3109/01480549109017878. [DOI] [PubMed] [Google Scholar]
- De Sandro V., Chevrier M., Boddaert A., Melcion C., Cordier A., Richert L. Comparison of the effects of propylthiouracil, amiodarone, diphenylhydantoin, phenobarbital, and 3-methylcholanthrene on hepatic and renal T4 metabolism and thyroid gland function in rats. Toxicol Appl Pharmacol. 1991 Nov;111(2):263–278. doi: 10.1016/0041-008x(91)90030-i. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Niemczura W. P. Suicide inactivation of lactoperoxidase by 3-amino-1,2,4-triazole. Chem Res Toxicol. 1989 Mar-Apr;2(2):100–103. doi: 10.1021/tx00008a005. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Takazawa R. S. Mechanism of thyroid peroxidase inhibition by ethylenethiourea. Chem Res Toxicol. 1990 Mar-Apr;3(2):98–101. doi: 10.1021/tx00014a003. [DOI] [PubMed] [Google Scholar]
- Döhler K. D., Wong C. C., von zur Mühlen A. The rat as model for the study of drug effects on thyroid function: consideration of methodological problems. Pharmacol Ther B. 1979;5(1-3):305–318. doi: 10.1016/0163-7258(79)90099-8. [DOI] [PubMed] [Google Scholar]
- Freudenthal R. I., Kerchner G., Persing R., Baron R. L. Dietary subacute toxicity of ethylene thiourea in the laboratory rat. J Environ Pathol Toxicol. 1978 Sep-Oct;1(1):147–161. [PubMed] [Google Scholar]
- Gerber H., Huber G., Peter H. J., Kämpf J., Lemarchand-Beraud T., Fragu P., Stocker R. Transformation of normal thyroids into colloid goiters in rats and mice by diphenylthiohydantoin. Endocrinology. 1994 Dec;135(6):2688–2699. doi: 10.1210/endo.135.6.7988459. [DOI] [PubMed] [Google Scholar]
- Graham S. L., Hansen W. H. Effects of short-term administration of ethylenethiourea upon thyroid function of the rat. Bull Environ Contam Toxicol. 1972 Jan;7(1):19–25. doi: 10.1007/BF01709170. [DOI] [PubMed] [Google Scholar]
- Hard G. C. Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environ Health Perspect. 1998 Aug;106(8):427–436. doi: 10.1289/ehp.106-1533202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman J. K., Lockhart A. M. Correlations between chemically related site-specific carcinogenic effects in long-term studies in rats and mice. Environ Health Perspect. 1993 Apr 22;101(1):50–54. doi: 10.1289/ehp.9310150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa Y., Kitahori Y., Kitamura M., Nishioka H., Yane K., Fukumoto M., Ohshima M., Nakaoka S., Nishii S. Relationships between serum thyroid stimulating hormone levels and development of thyroid tumors in rats treated with N-bis-(2-hydroxypropyl)nitrosamine. Carcinogenesis. 1991 May;12(5):873–877. doi: 10.1093/carcin/12.5.873. [DOI] [PubMed] [Google Scholar]
- Hill R. N., Erdreich L. S., Paynter O. E., Roberts P. A., Rosenthal S. L., Wilkinson C. F. Thyroid follicular cell carcinogenesis. Fundam Appl Toxicol. 1989 May;12(4):629–697. [PubMed] [Google Scholar]
- Hotz K. J., Wilson A. G., Thake D. C., Roloff M. V., Capen C. C., Kronenberg J. M., Brewster D. W. Mechanism of thiazopyr-induced effects on thyroid hormone homeostasis in male Sprague-Dawley rats. Toxicol Appl Pharmacol. 1997 Jan;142(1):133–142. doi: 10.1006/taap.1996.8032. [DOI] [PubMed] [Google Scholar]
- Kackar R., Srivastava M. K., Raizada R. B. Studies on rat thyroid after oral administration of mancozeb: morphological and biochemical evaluations. J Appl Toxicol. 1997 Nov-Dec;17(6):369–375. doi: 10.1002/(sici)1099-1263(199711/12)17:6<369::aid-jat449>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kohn M. C., Sewall C. H., Lucier G. W., Portier C. J. A mechanistic model of effects of dioxin on thyroid hormones in the rat. Toxicol Appl Pharmacol. 1996 Jan;136(1):29–48. doi: 10.1006/taap.1996.0004. [DOI] [PubMed] [Google Scholar]
- Krechniak J., Englot B., Wrześniowska K., Hać E. Interaction of lindane and carbaryl on hepatic microsomal enzymes in rats. Bull Environ Contam Toxicol. 1994 Jun;52(6):927–934. doi: 10.1007/BF00200704. [DOI] [PubMed] [Google Scholar]
- Lamb J. C., Huff J. E., Haseman J. K., Murthy A. S., Lilja H. Carcinogenesis studies of 4,4'-methylenedianiline dihydrochloride given in drinking water to F344/N rats and B6C3F1 mice. J Toxicol Environ Health. 1986;18(3):325–337. doi: 10.1080/15287398609530874. [DOI] [PubMed] [Google Scholar]
- Littlefield N. A., Gaylor D. W., Blackwell B. N., Allen R. R. Chronic toxicity/carcinogenicity studies of sulphamethazine in B6C3F1 mice. Food Chem Toxicol. 1989 Jul;27(7):455–463. doi: 10.1016/0278-6915(89)90032-x. [DOI] [PubMed] [Google Scholar]
- Littlefield N. A., Sheldon W. G., Allen R., Gaylor D. W. Chronic toxicity/carcinogenicity studies of sulphamethazine in Fischer 344/N rats: two-generation exposure. Food Chem Toxicol. 1990 Mar;28(3):157–167. doi: 10.1016/0278-6915(90)90004-7. [DOI] [PubMed] [Google Scholar]
- McClain R. M. Mechanistic considerations for the relevance of animal data on thyroid neoplasia to human risk assessment. Mutat Res. 1995 Dec;333(1-2):131–142. doi: 10.1016/0027-5107(95)00139-5. [DOI] [PubMed] [Google Scholar]
- McClain R. M. The significance of hepatic microsomal enzyme induction and altered thyroid function in rats: implications for thyroid gland neoplasia. Toxicol Pathol. 1989;17(2):294–306. doi: 10.1177/019262338901700206. [DOI] [PubMed] [Google Scholar]
- McConnell E. E. Thyroid follicular cell carcinogenesis: results from 343 2-year carcinogenicity studies conducted by the NCI/NTP. Regul Toxicol Pharmacol. 1992 Oct;16(2):177–188. doi: 10.1016/0273-2300(92)90056-f. [DOI] [PubMed] [Google Scholar]
- Owen N. V., Worth H. M., Kiplinger G. F. The effects of long-term ingestion of methimazole on the thyroids of rats. Food Cosmet Toxicol. 1973 Aug;11(4):649–653. doi: 10.1016/s0015-6264(73)80336-0. [DOI] [PubMed] [Google Scholar]
- Parker S. L., Tong T., Bolden S., Wingo P. A. Cancer statistics, 1997. CA Cancer J Clin. 1997 Jan-Feb;47(1):5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- Paynter O. E., Burin G. J., Jaeger R. B., Gregorio C. A. Goitrogens and thyroid follicular cell neoplasia: evidence for a threshold process. Regul Toxicol Pharmacol. 1988 Mar;8(1):102–119. doi: 10.1016/0273-2300(88)90009-8. [DOI] [PubMed] [Google Scholar]
- Saito K., Kaneko H., Sato K., Yoshitake A., Yamada H. Hepatic UDP-glucuronyltransferase(s) activity toward thyroid hormones in rats: induction and effects on serum thyroid hormone levels following treatment with various enzyme inducers. Toxicol Appl Pharmacol. 1991 Oct;111(1):99–106. doi: 10.1016/0041-008x(91)90138-5. [DOI] [PubMed] [Google Scholar]
- Scammell J. G., Fregly M. J. The effect of 3-amino-1,2,4-triazole on hepatic and renal deiodination of L-thyroxine to 3,5,3'-triiodothyronine. Toxicol Appl Pharmacol. 1981 Aug;60(1):45–51. doi: 10.1016/0041-008x(81)90133-2. [DOI] [PubMed] [Google Scholar]
- Semler D. E., Chengelis C. P., Radzialowski F. M. The effects of chronic ingestion of spironolactone on serum thyrotropin and thyroid hormones in the male rat. Toxicol Appl Pharmacol. 1989 Apr;98(2):263–268. doi: 10.1016/0041-008x(89)90231-7. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Grossman S. J., Gerson R. J., Gordon L. R., Deluca J. G., Majka J. A., Wang R. W., Germershausen J. I., MacDonald J. S. Studies on the mechanism of simvastatin-induced thyroid hypertrophy and follicular cell adenoma in the rat. Toxicol Pathol. 1991;19(3):197–205. doi: 10.1177/019262339101900301. [DOI] [PubMed] [Google Scholar]
- Strum J. M., Karnovsky M. J. Aminotriazole goiter. Fine structure and localization of thyroid peroxidase activity. Lab Invest. 1971 Jan;24(1):1–12. [PubMed] [Google Scholar]
- Tsutsui T., Maizumi H., Barrett J. C. Amitrole-induced cell transformation and gene mutations in Syrian hamster embryo cells in culture. Mutat Res. 1984 Aug;140(4):205–207. doi: 10.1016/0165-7992(84)90078-2. [DOI] [PubMed] [Google Scholar]
- Wilson A. G., Thake D. C., Heydens W. E., Brewster D. W., Hotz K. J. Mode of action of thyroid tumor formation in the male Long-Evans rat administered high doses of alachlor. Fundam Appl Toxicol. 1996 Sep;33(1):16–23. doi: 10.1006/faat.1996.0138. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D., Stringer B. M., Williams E. D. Desensitisation of rat thyroid to the growth-stimulating action of TSH during prolonged goitrogen administration. Persistence of refractoriness following withdrawal of stimulation. Acta Endocrinol (Copenh) 1982 Dec;101(4):562–569. doi: 10.1530/acta.0.1010562. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D., Stringer B. M., Williams E. D. Dissociation of growth and function in the rat thyroid during prolonged goitrogen administration. Acta Endocrinol (Copenh) 1982 Oct;101(2):210–216. doi: 10.1530/acta.0.1010210. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D., Stringer B. M., Williams E. D. Goitrogen-induced thyroid growth in the rat: a quantitative morphometric study. J Endocrinol. 1982 Jul;94(1):131–140. doi: 10.1677/joe.0.0940131. [DOI] [PubMed] [Google Scholar]


