Schistosomiasis is a chronic and debilitating disease that is caused by parasitic trematode worms (schistosomes). It continues to threaten millions of people, particularly the rural poor in the developing world (11, 24). Of the estimated 200 million infected people, more than half have symptoms and 20 million exhibit severe disease manifestations. There are five species of schistosomes that can infect humans, of which Schistosoma mansoni, S. japonicum, and S. haematobium are the most important ones. While infection with the former two species is associated with chronic hepatic and intestinal fibrosis, infection with S. haematobium can lead to ureteric and bladder fibrosis and calcification of the urinary tract (for recent reviews, see references 57 and 68). Schistosomiasis is characterized by a complex life cycle involving a phase of sexual reproduction by adult worms in humans (definitive host) and an asexual phase in specific aquatic snails (intermediate host). Infection occurs when humans come into contact with freshwater that contains free-swimming larval forms of the parasite (cercariae). Cercariae penetrate the intact human skin and transform into schistosomula. Schistosomula travel through the bloodstream for several days before they differentiate into male and female worms and unite. Adult worm pairs residue in the mesenteric veins (S. mansoni and S. japonicum) or the vesical plexus and veins that drain the ureter (S. haematobium). Depending on the species, oviposition commences 4 to 9 weeks postinfection (14, 31, 61) and continues until the worms die. While some eggs are passed through the bladder or intestinal mucosa and are finally excreted through urine or stool, others are trapped in the tissues surrounding the worms. This gives rise to acute granulomatous responses and, in the long term, is the primary cause of morbidity. Finally, the life cycle is completed when eggs reach a freshwater body, hatch, and release tiny miracidia that infect a specific aquatic snail species.
In areas where schistosomiasis is highly endemic, the present goal to mitigate the burden of the disease is to control morbidity. Chemotherapy with praziquantel is the mainstay for morbidity control; e.g., it prevents chronic liver disease or bladder cancer (78). Praziquantel will certainly remain the drug of choice over the next several years, since the 54th World Health Assembly recently put forth a target to treat at least 75% of school-age children in areas with high burdens of schistosomiasis with praziquantel by 2010 (15). Metrifonate, a drug that exhibits activity against S. haematobium singly, has recently been withdrawn from the market because of medical, operational, and economic criteria (27). Oxamniquine is the only alternative antischistosomal drug, but its use is declining (12). Derivatives of artemisinin (e.g., artemether and artesunate), best known for their antimalarial properties, also display activity against schistosomiasis; hence, strategic discussions are under way on how this evidence base can be translated into sound public health actions (68, 69, 84).
Against this background of virtually relying on a single drug for the treatment and control of schistosomiasis (5, 12) and considerable concern about the development of praziquantel resistance (26, 37, 77), it is timely to critically review potential alternatives. Research and development of novel antischistosomal drugs are warranted (5, 24), but this will become feasible only through the creation of innovative and committed public-private partnerships, including academia and the pharmaceutical industry. In the interim, present morbidity control approaches need to be improved and optimized alongside concerted efforts to prolong the useful life span of praziquantel. In this regard, the combination of praziquantel with oxamniquine or an artemisinin derivative might be an obvious and appealing option.
In this minireview we summarize for the first time results from laboratory studies and field trials of combination chemotherapy for the treatment of schistosomiasis. We commence by reviewing the properties of praziquantel, oxamniquine, and artemisinin derivatives, including species- and stage-specific susceptibilities, possible mechanisms of action, pharmacokinetics and pharmacodynamics, toxicologic parameters, and experiences from clinical use. We then summarize the evidence obtained thus far from in vivo experiments and clinical trials that used praziquantel together with oxamniquine or an artemisinin derivative. We conclude with a discussion on the most urgent needs for new tools and approaches for schistosomiasis chemotherapy that will be critical as part of an integrated and sustainable control approach to alleviate the present intolerable burden of schistosomiasis.
ANTISCHISTOSOMAL PROPERTIES OF PRAZIQUANTEL
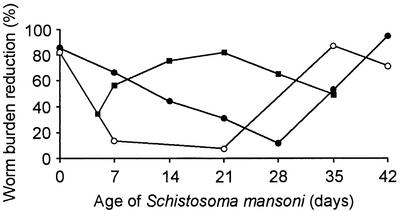
Praziquantel (2-cyclohexylcarbonyl-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one) was synthesized in the 1970s (59). While undergoing initial veterinary screening it showed efficacy against cestodes (33, 62). With regard to schistosomes, efficacy was first demonstrated against S. mansoni in different host animals (32, 52). These findings were confirmed for all other schistosome parasites pathogenic for humans (3, 38, 73). The stage-specific susceptibility of a Puerto Rican strain of S. mansoni harbored in mice treated with three oral doses of praziquantel on alternate days is depicted in Fig. 1 (58). Although only few replicates were used, these studies revealed that the invasive stages (cercariae and very young schistosomula) and the mature worms were affected more than the liver stages of the parasites. These findings were confirmed for S. japonicum (89). However, the underlying causes of the stage-specific susceptibility are far from being fully understood (13).
FIG. 1.
Stage-specific susceptibility of S. mansoni to praziquantel (•), oxamniquine (○), and artemether (▪). Details of the original experiments are provided elsewhere (58, 82). In brief, mice were infected with S. mansoni cercariae and treated orally with an antischistosomal drug (for praziquantel, three doses of 250 mg/kg on alternate days; for oxamniquine, a single dose of 50 mg/kg; for artemether, a single dose of 400 mg/kg) at different time points postinfection (e.g., on days 0, 7, 14, 21, 28, 35, and 42 in the case of praziquantel). Schistosomes were recovered 4 weeks posttreatment. Mean worm burden reductions were calculated by comparison of mean worm burdens in the treatment groups with those from a group of control mice that were also infected with S. mansoni but that remained untreated. It is noteworthy that oviposition is only reached 34 to 35 days after infection (14).
Despite considerable efforts, the mechanism of action of praziquantel has yet to be fully elucidated (2, 13). However, three central features are observed in schistosomes following the administration of praziquantel, and these effects are directly or indirectly associated with Ca2+ redistributions between worm tissues and the surrounding environments. First, worm motor activity is immediately stimulated, followed by strong muscular contraction. Second, praziquantel induces extensive tegumental damage, commencing 5 min posttreatment. Third, treatment is accompanied by metabolic changes altering glycogen content and energy metabolism (13). The drug induces a rapid shift of worms from the mesenteric veins to the liver, known as hepatic shift (48). In the mouse model, praziquantel efficacy depends on host antibody responses, and treatment increases the exposure of schistosome antigens at the worm surface (8, 23, 35). In humans, however, it proved difficult to identify the host-related factors that influence the efficacy of praziquantel (40, 71). Pharmacokinetic studies found that orally administered praziquantel is rapidly and almost completely absorbed (maximum concentrations in serum are reached 1 to 2 h posttreatment) but undergoes extensive first-pass liver clearance and prompt metabolic processing into inactive metabolites. The half-life of the unchanged drug in plasma is about 1 to 2 h (9, 10, 47). Up to 80% of praziquantel is reversibly bound to proteins. Praziquantel elimination is primarily through urine, with about 80% of the drug cleared within 24 h posttreatment (13). Table 1 summarizes the key characteristics of praziquantel.
TABLE 1.
Summary of antischistosomal properties of praziquantel, oxamniquine, and artemether
| Property | Praziquantel | Oxamniquine | Artemether |
|---|---|---|---|
| Activity against human schistosomes | S. mansoni, S. japonicum, S. haematobium, S. intercalatum, S. mekongi | S. mansoni | S. mansoni, S. japonicum, S. haematobiuma |
| Stage-specific susceptibility | Cercariae, very young schistosomula, adult worms | Cercariae, very young schistosomula, adult worms | Schistosomula |
| Mechanism of action | Not fully elucidated; Ca2+ of importance | Working hypothesis: activation through schistosome enzyme; alkylation | Working hypothesis: Activation through haemin, cleavage of endoperoxide bridge; alkylation |
| Hepatic shift | Rapid (5 min-1 h) | Moderate (8 h-6 days) | Slow (up to 7 days) |
| Pharmacokinetics (oral formulation) | |||
| Time to maximum concn in serum (h) | 1-2 | 1-4 | 1-2 |
| Elimination half-life (h) | 1-2 | 1.5-2 | 1-3 |
| Metabolites | Not active | Not active | Active (dihydroartemisinin) |
| Resistance | Considerable discussion whether or not there is resistance | Resistance found both in vivo and in humans | No resistance found |
Activities against S. intercalatum and S. mekongi have not yet been assessed.
The very encouraging laboratory findings of the broad-spectrum antischistosomal activity of praziquantel were consistently confirmed in clinical trials designed to test the effect of the drug against the major human schistosome parasites and carried out in different epidemiological settings (19, 74). Consistently, a single oral dose of 40 mg of praziquantel per kg of body weight was safe, showed no or only a few but transient side effects, and resulted in high parasitological cure and egg reduction rates (78). In view of these operational and therapeutic properties and gradually decreasing costs, it is not surprising that millions of people have been treated with praziquantel over the past 20 years. Many millions more patients suffering from schistosomiasis will be treated with this drug over the next several years (12, 15, 21, 78). Awareness about the possible development of praziquantel resistance is growing because of laboratory data and field observations. Experiments with mice revealed the possibility of selecting praziquantel-tolerant schistosome strains after repeated administration of subcurative doses of praziquantel (26). Among other possible explanations, it has been speculated that antimicrobial resistance is responsible for the unusually low cure rates in S. mansoni-infected patients from Senegal (for reviews, see references 18 and 34). In Egypt, patients failed to be completely cured of S. mansoni infections even after praziquantel had been administered three times, which is the most compelling evidence of praziquantel resistance to date (37, 77).
ANTISCHISTOSOMAL PROPERTIES OF OXAMNIQUINE
Oxamniquine (6-hydroxymethyl-2-isopropyl-aminomethyl-7-nitro-1,2,3,4-tetrahydroquinoline) was first described in the late 1960s (56). In contrast to praziquantel, which displays activity against all human schistosome species, the activity of oxamniquine is confined to S. mansoni (13, 41). The stage-specific susceptibility of S. mansoni to oxamniquine exhibits a pattern very similar to that for susceptibility to praziquantel: the invasive stages and the adult worms are significantly more affected than the liver stages (29). This is depicted in Fig. 1 for a Puerto Rican strain of S. mansoni harbored in mice treated with a single oral dose of oxamniquine (58). Adult male worms are considerably more affected by oxamniquine than adult females (29), and Central and East African strains of S. mansoni are significantly less susceptible than South American strains.
The mechanism of action of oxamniquine is reasonably well understood, and the body of evidence suggests that it is closely associated with an irreversible inhibition of the nucleic acid metabolism of the parasites. The following working hypothesis has been put forth: the drug is activated by a single step, in which a schistosome enzyme converts oxamniquine into an ester (probably acetate, phosphate, or sulfate). Subsequently, the ester spontaneously dissociates, while the resulting electrophilic reactant is capable of alkylation of schistosome DNA (13). In contrast to treatment with praziquantel, treatment with oxamniquine results in less specific morphological alterations, and the hepatic shift occurs much more slowly and is completed only 6 days posttreatment. Pharmacokinetic studies with humans after oral administration of oxamniquine revealed many similarities to praziquantel. Oxamniquine is rapidly absorbed (peak concentrations in plasma are reached 1 to 4 h posttreatment), and the half-life of 1.5 to 2 h in plasma is of the same order as that of praziquantel (17, 44). Oxamniquine is extensively metabolized through oxidation processes. Metabolites are inactive and are almost exclusively excreted through urine (13). Table 1 summarizes the key characteristics of oxamniquine.
Oxamniquine at a single oral dose of 15 to 20 mg/kg shows high degrees of efficacy in South America, the Caribbean islands, and West Africa, while higher doses (up to 60 mg/kg given over 2 to 3 days) are required to obtain the desired therapeutic efficacies in Central and East Africa and the Arabian peninsula. Oxamniquine is safe, and side effects are limited to mild but transient dizziness (28). Large-scale application of oxamniquine, sustained over the past two decades, has shown great success in S. mansoni control in Brazil (42). Although S. mansoni-infected patients resistant to oxamniquine have been described repeatedly, these findings are of no public health significance thus far. Despite the success of oxamniquine for schistosomiasis control in Brazil, efforts are under way to replace it with praziquantel, primarily because of the lower price of praziquantel (4, 55).
ANTISCHISTOSOMAL PROPERTIES OF ARTEMISININ DERIVATIVES
Artemisinin, a sesquiterpene lactone with a peroxide group, is the active principle derived from the leaves of Artemisia annua and is best known for its antimalarial properties. Several semisynthetic derivatives with even higher antimalarial activities were developed, namely, arteether, artemether, artesunate, and dihydroartemisinin. The initial work was done in the early 1970s, and over the last decade, derivatives of artemisinin have gained tremendously in importance for the treatment and control of malaria. It is anticipated that their popularity will further increase, particularly also in combination with other antimalarial drugs with unrelated mechanisms of action (51, 75).
The antischistosomal activities of artemisinin, artemether, and artesunate were discovered in the early 1980s, with the initial experiments focusing on S. japonicum (45, 46). More recent studies confirmed that arteether and dihydroartemisinin also display antischistosomal properties (1, 86). Laboratory experiments conducted so far in different animal models found that artemether is active against the three major human schistosome parasites (68, 69, 84). The stage-specific susceptibility of a Liberian strain of S. mansoni harbored in mice treated with a single oral dose of artemether is shown in Fig. 1 (82). Very similar stage-specific susceptibilities have also been reported for S. japonicum (88). In contrast to praziquantel and oxamniquine, artemether exhibits the highest level of activity against 1- to 3-week-old liver stages, while the invasive stages and the adult worms are less susceptible. Adult female worms are somewhat more susceptible to artemether than male worms (69), which is opposite the activity of oxamniquine. There is a need for additional in vivo experiments to assess the stage-specific susceptibilities of S. haematobium to artemether and to comparatively assess the species- and stage-specific susceptibilities of schistosomes to other artemisinin derivatives. A first comparative appraisal revealed that artemether exhibits consistently higher levels of activity against S. mansoni parasites of different ages than artesunate (64).
The exact mechanism of action of artemether against schistosomes remains elusive, but progress has been made in recent years (69). A typical biochemical feature is that, following artemether treatment, adult worms showed significant reductions in their glycogen contents (83). As with praziquantel, artemether also induces severe and extensive tegumental damage; however, the onset of tegumental alterations is considerably slower (69). Another important finding is that in vitro exposure of schistosomes to a medium containing artemether plus hemin results in parasite death, while exposure to artemether or hemin alone showed no effect. Therefore, it has been suggested that artemether might be activated by hemin and sequentially cleaves the endoperoxide bridge and generates free radicals that might form covalent bonds with schistosome-specific proteins (81). The hepatic shift commences within 8 h after artemether administration and is completed within 7 days (80); hence, it is much slower than that after praziquantel treatment but only somewhat slower than that after oxamniquine treatment. Oral formulations of artemisinin derivatives are absorbed rapidly but incompletely (the peak concentrations of most artemisinins in plasma are reached 1 to 2 h posttreatment) and have short half-lives in plasma of 1 to 3 h before undergoing hepatic metabolism (70). Dihydroartemisinin is the principle active metabolite (49). The key features of artemether are summarized in Table 1. In vivo studies in different animal models revealed brain stem neurotoxicity after repeated treatment with high doses of some artemisinin derivatives over at least 7 days (30). However, repeated treatments with high doses of artemether once every 2 weeks, the recommended dose schedule for the prevention of S. japonicum infection, revealed no neurotoxicity (85). Most importantly, there is no clinical evidence of neurological lesions, although several million people have been treated with an artemisinin derivative for malaria (53).
To date, clinical testing of artemether for the prevention of schistosomiasis included 2,670 people who received oral artemether at a dose of 6 mg/kg once every 2 to 4 weeks for periods of up to 6 months. Artemether was safe, showed no or only a few but transient side effects, and was efficacious in reducing the incidence and intensity of infection (50, 67, 79).
COMBINATION CHEMOTHERAPY FOR SCHISTOSOMIASIS
The application of drugs in combination is not a new concept. In fact, the rationale for the use of combination chemotherapy was first developed in the treatment of bacterial infections such as tuberculosis and has subsequently been adapted for chemotherapy for cancer and human immunodeficiency virus infection and AIDS, primarily to delay the emergence of drug resistance. Additional momentum has recently been gained in the field of malaria chemotherapy. Experience from Southeast Asia suggests that the combination of an artemisinin derivative with another antimalarial drug with an unrelated mechanism of action was an effective strategy for prevention of the emergence and spread of drug resistance and might have contributed to the interruption of the transmission of falciparum malaria (51). It has been recommended that this kind of combination be evaluated in sub-Saharan Africa, where it might become the mainstay of malaria chemotherapy (76).
With regard to combination chemotherapy for schistosomiasis, the partner drugs should have different mechanisms of action to reduce the likelihood of resistance development and/or target different developmental stages of the parasite to enhance cure and egg reduction rates. In case the two drugs exhibit synergism, it might be possible to achieve the same or even higher levels of efficacy by using smaller doses of either agent, which might also result in fewer or milder side effects. In view of the present armamentarium against schistosomiasis, praziquantel could be combined with either oxamniquine or an artemisinin derivative. Interestingly, initial laboratory experiments and preliminary clinical trials with praziquantel and oxamniquine combinations were already launched two decades ago. There is new interest in this combination, and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases has recently called for proposals to evaluate its tolerability and efficacy against S. mansoni.
PRAZIQUANTEL AND OXAMNIQUINE COMBINATIONS
The first series of laboratory experiments with adult S. mansoni worms harbored in mice treated simultaneously with praziquantel and oxamniquine reported encouraging results, since treatment outcomes were superior to those expected by simply adding the effects of each drug used singly (60). Additional laboratory testing with combined low doses of praziquantel and oxamniquine (one-third of the curative dose) against different developmental stages of S. mansoni showed only slightly higher worm burden reductions than those achieved with praziquantel administered alone at a curative dose. The combination regimen showed the highest efficacy when it was administered 4 h postinfection. Administration of the two drugs 5 weeks postinfection resulted in a worm burden reduction of only 42%, which was marginally higher than that achieved with praziquantel or oxamniquine monotherapy at either a curative dose or one-third of the curative dose (7).
Two field trials with praziquantel and oxamniquine combinations were carried out shortly after the encouraging laboratory results were published by Shaw and Brammer (60). Both trials were randomized and nonblinded and were designed as dose-ranging studies, with the doses used being considerably lower than those recommended if either drug is used alone. No direct comparisons were made with praziquantel or oxamniquine monotherapy. Most of the study participants were concurrently infected with S. mansoni and S. haematobium, and the therapeutic efficacies were evaluated 1, 3, and 6 months posttreatment for both parasites separately. Table 2 summarizes the key findings assessed 4 weeks after concurrent administration of praziquantel and oxamniquine at different dosage regimens.
TABLE 2.
Effects of praziquantel and oxamniquine combinations for treatment of S. mansoni and S. haematobium infections among schoolchildren from Malawi and Zimbabwea
| Study location | Parasite
|
Treatment (mg/kg)
|
Therapeutic efficacy
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
S. mansoni
|
S. haematobium
|
Praziquantel | Oxamniquine |
S. mansoni
|
S. haematobium
|
|||||
| No. of patients infected | Infection intensity (no. of eggs/g of stool) | No. of patients infected | Infection intensity (no. of eggs/10 ml of urine) | No. (%) of patients cured | Infection intensity (no. of eggs/g of stool [% reduction]) | No. (%) of patients cured | Infection intensity (no. of eggs/10 ml of urine [% egg reduction]) | |||
| Malawi | 22 | 495 | 8 | 4 | NAb | 282 (43) | ||||
| 24 | 624 | 10 | 7.5 | NA | 137 (78) | |||||
| 26 | 328 | 15 | 7.5 | NA | 3 (99) | |||||
| 30 | 241 | 20 | 10 | NA | 1 (100) | |||||
| 29 | 135 | 15 | 7.5 | NA | 1 (99) | |||||
| 27 | 270 | 20 | 10 | NA | 1 (100) | |||||
| Zimbabwe | 10 | 557 | 8 | 4 | 3 (30) | 35 (94) | ||||
| 30 | 208 | 15 | 7.5 | 23 (77) | 8 (96) | |||||
| 18 | 92 | 20 | 10 | 16 (89) | 11 (88) | |||||
| 10 | 575 | 8 | 4 | 0 (0) | 178 (69) | |||||
| 30 | 641 | 15 | 7.5 | 0 (0) | 42 (93) | |||||
| 18 | 750 | 20 | 10 | 0 (0) | 41 (93) | |||||
The first study was carried out with schoolchildren from Malawi aged 6 to 20 years. Complete data records were obtained for 102 children with S. mansoni infection and 56 children with S. haematobium infection. For those with S. mansoni infections, there was a clear trend toward higher egg reduction rates with increasing doses: egg count reductions of 99% or more were found after concurrent administration of 15 to 20 mg of praziquantel per kg and 7.5 to 10 mg of oxamniquine per kg. The combined treatment also resulted in very high S. haematobium egg count reductions. It was safe, and there were only a few side effects, which were self-limiting. The investigators concluded that they had demonstrated a synergistic effect of praziquantel and oxamniquine (54). Care in the acceptance of this conclusion is needed because (i) the sample sizes in the different treatment groups were small (22 to 30 subjects per group), (ii) about half of the study participants were concurrently infected with S. mansoni and S. haematobium, (iii) egg count reductions were used as the only end point, (iv) infection intensities were high prior to drug administration, and (v) only a single stool or urine specimen was examined 1 month posttreatment. In light of the recent findings obtained in different epidemiological settings, one can argue that some light infections were missed; hence, therapeutic efficacies were overestimated (25, 43, 63, 66).
The other study was carried out among 58 schoolchildren from Zimbabwe aged 7 to 16 years. All children were concurrently infected with S. mansoni (>100 eggs/g of stool) and S. haematobium (>500 eggs/10 ml of urine). Three different dose combinations were investigated. While a high cure rate of 89% for those with S. mansoni infections was achieved with the highest doses (20 mg of praziquantel per kg and 10 mg of oxamniquine per kg), combination chemotherapy failed to cure S. haematobium infections. High egg count reductions were found for both parasite species. It was concluded that the combination of praziquantel and oxamniquine for the treatment of schistosome infections in Zimbabwean schoolchildren has no curative advantage over praziquantel alone (16). The design of this study has limitations similar to those of the investigation carried out in Malawi, namely, (i) the sample sizes in the different treatment groups were small (10 to 30 subjects per group), (ii) the study participants were concurrently infected with two schistosome species, and (iii) the infection intensities were high prior to treatment. On the other hand, study end points included both cure and egg reduction rates, and the therapeutic efficacy was assessed by screening multiple stool or urine specimens.
COMBINATIONS WITH PRAZIQUANTEL AND ARTEMISININ DERIVATIVES
Since praziquantel and artemether display broad-spectrum antischistosomal activities and the susceptibilities of the different stages to the two drugs are distinctively different, it had been suggested that use of these two compounds in combination might be beneficial for the treatment of infections caused by all human schistosome species. Hence, use of the combination may increase worm burden reductions and, as a consequence, augment cure and egg reduction rates.
Table 3 highlights the key results from two laboratory studies published to date that comparatively assessed worm burden reductions after administration of praziquantel and artemether singly or in combination. The two series of experiments used different host-parasite models and found consistently higher worm burden reductions following treatment with the combination regimen compared to those achieved with the monotherapies. In the first set of experiments, rabbits simultaneously infected with juvenile and adult S. japonicum worms were treated with 50 mg of praziquantel per kg and 15 mg of artemether per kg 1 day apart. The treatment resulted in a worm burden reduction of 82%. This was significantly higher than the reductions achieved after the administration of praziquantel (66%) or artemether (44%) singly at the same doses (87). These results were confirmed with rabbits infected only with adult S. japonicum (65). In the second set of experiments, hamsters with a mixed infection with juvenile and adult S. mansoni worms were simultaneously treated with 75 mg of praziquantel per kg and 150 mg of artemether per kg. The worm burden reduction of 77% was significantly higher than the 2% reduction achieved following praziquantel monotherapy (P < 0.01) but was not significantly different from the 66% reduction obtained with artemether alone (65). Further experiments with a combination of praziquantel and artemether against S. haematobium are warranted. There is also a need for comparative appraisal of schistosome worm burden reductions following treatment with praziquantel in combination with different derivatives of artemisinin.
TABLE 3.
Effects of praziquantel and artemether combinations in animals experimentally infected with juvenile and adult stages of either S. japonicum or S. mansoni
| Group and treatment (reference) | Treatment (mg/kg)
|
No. of animals | Total worm burden (mean ± SD no. of worms) | Total worm burden reduction (%)c | |
|---|---|---|---|---|---|
| Praziquantel | Artemether | ||||
| S. japonicum-infected rabbits (87)a | |||||
| Control | 5 | 301 ± 29 | |||
| Praziquantel | 50 | 7 | 101 ± 45 | 66 | |
| Artemether | 15 | 7 | 168 ± 31 | 44 | |
| Praziquantel + artemether | 50 | 15 | 7 | 54 ± 29 | 82* |
| S. mansoni-infected hamsters (65)b | |||||
| Control | 5 | 36.0 ± 11.5 | |||
| Praziquantel | 75 | 5 | 35.2 ± 14.6 | 2 | |
| Artemether | 150 | 5 | 12.2 ± 5.2 | 66 | |
| Praziquantel + artemether | 75 | 150 | 5 | 8.8 ± 5.2 | 76** |
Rabbits infected with 7- and 14-day-old juvenile and 42-day-old adult S. japonicum worms (infection, 200 cercariae three times subcutaneously).
Hamsters infected with 14- and 21-day-old juvenile and 49-day-old adult S. mansoni worms (infection, 40 cercariae three times subcutaneously).
For the group receiving the combined treatment (praziquantel plus artemether) versus the group receiving praziquantel alone
P was <0.05 or
<0.01 by the t test.
So far, the praziquantel and artemether combinations have not undergone clinical trials. However, a combination of praziquantel and artesunate was used in a nonblinded open-label treatment trial with S. mansoni in Senegal (20) and a randomized controlled clinical trial with S. haematobium in Gabon (6). Table 4 summarizes the results of those two trials. The study in Senegal enrolled a total of 110 S. mansoni-infected patients aged 1 to 60 years. The patients were treated with either praziquantel (single oral dose of 40 mg/kg), artesunate (recommended malaria treatment regimen; total oral dose, 12 mg/kg; an initial dose of 4 mg/kg, followed by four daily doses of 2 mg/kg), or a combination of these two drugs at the doses mentioned above. Cure and egg reduction rates were evaluated 5, 12, and 24 weeks posttreatment by examination of two Kato-Katz thick smears derived from a single stool specimen. It is widely acknowledged that northern Senegal is one of the most intense S. mansoni transmission zones; thus, reinfections must occur rapidly (18). Therefore, only the therapeutic efficacies achieved at 5 weeks posttreatment are considered here. The praziquantel and artesunate combination resulted in cure and egg reduction rates of 69 and 89%, respectively. While this cure rate was significantly higher than the ones observed after praziquantel and artesunate monotherapies, the egg reduction rate was similar to the one achieved following praziquantel treatment (84%) (20).
TABLE 4.
Effects of combination chemotherapy with praziquantel and artesunate against S. mansoni infections in a nonblinded trial in Senegal and against S. haematobium infections in a randomized, double-blind placebo-controlled clinical trial in Gabon compared to either praziquantel or artesunate monotherapy
| Group and treatment (reference) | Baseline
|
Therapeutic efficacy
|
||||
|---|---|---|---|---|---|---|
| No. of patients infected | Infection intensity pretreatmentc
|
No. (%) of patients cured | Infection intensity posttreatmentc
|
|||
| No. of eggs/g of stool | No. of eggs/10 ml of urine | No. of eggs/g of stool | No. of eggs/10 ml urine | |||
| S. mansoni-infected patients from Senegal (20)a | ||||||
| Praziquantel | 36 | 162 | 16 (44) | 26 (84) | ||
| Artesunate | 35 | 205 | 8 (23) | 83 (59) | ||
| Praziquantel + artesunate | 39 | 157 | 27 (69) | 17 (89) | ||
| S. haematobium-infected children from Gabon (6)b | ||||||
| Placebo + placebo | 30 | 22 | 6 (20) | 8.7 (75) | ||
| Praziquantel + placebo | 89 | 37 | 65 (73) | 2.3 (94) | ||
| Artesunate + placebo | 89 | 35 | 24 (27) | 7.0 (80) | ||
| Praziquantel + artesunate | 88 | 32 | 71 (81) | 0.4 (99) | ||
Praziquantel was administered orally at a single dose of 40 mg/kg; artesunate was administered orally at a total dose of 12 mg/kg (4 mg/kg once on day 1, followed by 2 mg/kg once a day on days 2 to 5).
Praziquantel was administered orally at a single dose of 40 mg/kg; artesunate was administered orally at a total dose of 12 mg/kg (4 mg/kg once a day on days 1 to 3).
Infection intensity is expressed as the geometric mean. The values in parentheses are percent reductions.
The study in Gabon was a randomized, double-blind, placebo-controlled trial and enrolled 296 S. haematobium-infected children aged 5 to 13 years. Four different treatments were administered: (i) a single oral dose of 40 mg of praziquantel per kg plus three daily oral doses of 4 mg of artesunate per kg (total dose, 12 mg/kg, corresponding to the recommended treatment for malaria), (ii) praziquantel plus a placebo, (iii) artesunate plus a placebo, and (iv) a double placebo. Therapeutic efficacy was evaluated 8 weeks posttreatment by determination of quantitative egg counts from two consecutive urine specimens. The highest cure rate of 81% (95% confidence interval [CI], 72 to 89%) was observed among those children who received both praziquantel and artesunate. However, this was not significantly different from the cure rate obtained after praziquantel monotherapy (73%; 95% CI, 64 to 82%). Furthermore, the cure rate of 27% (95% CI, 18 to 36%) in the artesunate monotherapy group was not significantly different from that in the placebo group (20%; 95% CI, 5 to 35%). The investigators acknowledged that their finding of a 20% “cure rate” among the placebo recipients is difficult to explain and might simply be attributable to day-to-day variations in S. haematobium egg output. In terms of egg count reductions, the praziquantel and artesunate combination was beneficial, and the 99% reduction was significantly higher than that for all other groups (6). This is an important finding, since morbidity generally correlates with infection intensities.
OTHER COMBINATIONS
A recent laboratory study investigated a possible additive or synergistic effect of the combination of praziquantel and Ro 15-5458 (a relatively new antischistosomal drug developed by Hoffmann-La Roche, Basel, Switzerland) against two different strains of S. mansoni in mice. The treatment outcomes achieved with a single curative dose of praziquantel or Ro 15-5458 were compared with those achieved with the drugs used in combination at doses that were one-third of the curative doses of the drugs used singly. The investigators found that treatment with the combination at these low doses was beneficial with regard to worm burden reductions and hepatic shift (39). These findings warrant further laboratory investigations, including investigations of mutagenicity and carcinogenicity, as a basis for possible clinical trials with humans (12).
THE WAY FORWARD IN COMBINATION CHEMOTHERAPY
Our review and critical appraisal of laboratory studies and clinical trials with the praziquantel and oxamniquine and the praziquantel and artemisinin combinations clearly reveals that additional studies are warranted. These studies must be well designed and must use sound evaluation criteria. We propose a two-pronged approach to move forward. First, when two antischistosomal agents are administered, there is a need to identify the concentrations of the drugs in the combination that are optimal in terms of both tolerability and therapeutic efficacy. It will be important to adhere to a rigorous diagnostic approach, e.g., microscopic examination of multiple stool and urine specimens collected over consecutive days before and after treatment (22, 25, 43, 63, 66). Therapeutic efficacies should be evaluated 4 to 8 weeks posttreatment.
Second, the evidence base gained during this first step should be sequentially used to design multicenter, double-blind, randomized, placebo-controlled trials. Study end points should include both cure and egg reduction rates, which should be determined by a sensitive diagnostic approach. The treatment outcomes between the combination chemotherapy and the monotherapies should be comparatively assessed. In this regard, the recently completed trial with a combination of praziquantel and artesunate for the treatment of S. haematobium-infected schoolchildren in Gabon can serve as a template (6).
CONCLUSIONS AND RESEARCH NEEDS
Schistosomiasis chemotherapy primarily relies on praziquantel, but the present arsenal also includes oxamniquine and artemisinin derivatives. We have reviewed the properties of these antischistosomal drugs when they are used in monotherapy, have assembled the laboratory and clinical data obtained thus far when praziquantel is used in combination with one of the other compounds, and have presented our views on how to move forward. Although the evidence base is scanty, we argue that praziquantel-oxamniquine is not a promising combination, which we justify on four grounds. First, the activity of oxamniquine is confined to S. mansoni; hence, its combination with praziquantel will most likely not be beneficial in large parts of the world where S. haematobium, S. japonicum, or another schistosome parasite is the predominant species. Second, S. mansoni strains from many parts of sub-Saharan Africa, where >80% of the present burden of schistosomiasis is concentrated (24), are considerably less susceptible to oxamniquine than strains found elsewhere. Third, praziquantel and oxamniquine exhibit similar half-lives; hence, with regard to schistosome susceptibility, this combination is unlikely to improve treatment outcomes. Fourth, the two compounds display virtually the same stage-specific susceptibilities (Fig. 1), with very low levels of activity against schistosomula. Therefore, it is anticipated that combination treatment will fail to kill schistosomula. In turn, these juvenile parasites will mature and commence oviposition several days or weeks posttreatment (14, 31, 61).
The evidence suggests that combinations of praziquantel and artemether (or another artemisinin derivative) are more promising. Both drugs display broad-spectrum antischistosomal activities and act against different parasite stages; hence, the combination covers the entire parasite spectrum in its vertebrate host. Laboratory studies have clearly established a beneficial effect of combining praziquantel with artemether, since worm burden reductions were enhanced. The first results from clinical trials are encouraging, but there is a great need to carry out additional randomized controlled trials in different epidemiological settings. In this context, it is noteworthy that there is some concern that large-scale use of an artemisinin derivative against schistosomiasis might select for resistance in malaria parasites. Although this risk appears to be low, mainly because of the very short elimination half-lives of artemisinins (36, 84), at present, use of these combinations should not be recommended in areas where both schistosome and malaria parasites coexist.
We conclude that even after successful completion of multicenter randomized controlled clinical trials with combinations of extant drugs, there is a pressing need for research into novel antischistosomal agents. The ultimate target should be the development of a compound that is efficacious against all human schistosome species, exhibits high levels of activity against adult and juvenile stages of the parasites (which would give it a comparative advantage over praziquantel), has a good safety profile, and is reasonably priced (5, 24). Innovative financing models analogous to those put in place by the Medicine for Malaria Venture (MMV), in which academia, the private and public sectors, and the pharmaceutical industries are brought together, are appealing and might represent an efficient way forward. In fact, those compounds that are screened for antimalarial activities within the framework of MMV might also be sequentially screened for possible activities against other parasitic agents such as schistosomes. With regard to the development of novel antimalarial agents, the concerted actions of MMV might soon come to provide the first fruits, as a new type of synthetic antimalarial drug candidate—a “peroxide” molecule—exhibits remarkable activity in the mouse model (72). These findings are also likely to be of considerable importance for antischistosomal drug development because of some similarities of this peroxide with artemisinins, which are now increasingly acknowledged to possess not only antimalarial properties but also antischistosomal properties.
Acknowledgments
Jürg Utzinger acknowledges the financial support from the Center for Health and Wellbeing at Princeton University and the Swiss Tropical Institute in Basel.
REFERENCES
- 1.Abdel Aziz, S. S., and N. M. el-Badawy. 2000. Experimental trials of an artemisinin derivative in treatment of Schistosoma mansoni infected mice. J. Egypt. Soc. Parasitol. 30:295-303. [PubMed] [Google Scholar]
- 2.Andrews, P. 1985. Praziquantel: mechanisms of anti-schistosomal activity. Pharmacol. Ther. 29:129-156. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, P. 1981. A summary of the efficacy of praziquantel against schistosomes in animal experiments and notes on its mode of action. Arzneimittelforschung 31:538-541. [PubMed] [Google Scholar]
- 4.Beck, L., T. C. Favre, O. S. Pieri, L. C. Zani, G. G. Domas, and C. S. Barbosa. 2001. Replacing oxamniquine by praziquantel against Schistosoma mansoni infection in a rural community from the sugar-cane zone of northeast Brazil: an epidemiological follow-up. Mem. Inst. Oswaldo Cruz 96(Suppl.):165-167. [DOI] [PubMed] [Google Scholar]
- 5.Bergquist, N. R. 2002. Schistosomiasis: from risk assessment to control. Trends Parasitol. 18:309-314. [DOI] [PubMed] [Google Scholar]
- 6.Borrmann, S., N. Szlezák, J.-F. Faucher, P.-B. Matsiegui, R. Neubauer, R. K. Binder, B. Lell, and P. G. Kremsner. 2001. Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: a double-blind, randomized, placebo-controlled study. J. Infect. Dis. 184:1363-1366. [DOI] [PubMed] [Google Scholar]
- 7.Botros, S., A. Soliman, N. El-Gawhary, M. Selim, and N. Guirguis. 1989. Effect of combined low dose praziquantel and oxamniquine on different stages of schistosome maturity. Trans. R. Soc. Trop. Med. Hyg. 83:86-89. [DOI] [PubMed] [Google Scholar]
- 8.Brindley, P. J., and A. Sher. 1987. The chemotherapeutic effect of praziquantel against Schistosoma mansoni is dependent on host antibody response. J. Immunol. 139:215-220. [PubMed] [Google Scholar]
- 9.Castro, N., H. Jung, R. Medina, D. González-Esquivel, M. Lopez, and J. Sotelo. 2002. Interaction between grapefruit juice and praziquantel in humans. Antimicrob. Agents Chemother. 46:1614-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro, N., R. Medina, J. Sotelo, and H. Jung. 2000. Bioavailability of praziquantel increases with concomitant administration of food. Antimicrob. Agents Chemother. 44:2903-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitsulo, L., D. Engels, A. Montresor, and L. Savioli. 2000. The global status of schistosomiasis and its control. Acta Trop. 77:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cioli, D. 2000. Praziquantel: is there real resistance and are there alternatives? Curr. Opin. Infect. Dis. 13:659-663. [DOI] [PubMed] [Google Scholar]
- 13.Cioli, D., L. Pica-Mattoccia, and S. Archer. 1995. Antischistosomal drugs: past, present . . . and future? Pharmacol. Ther. 68:35-85. [DOI] [PubMed] [Google Scholar]
- 14.Clegg, J. A. 1965. In vitro cultivation of Schistosoma mansoni. Exp. Parasitol. 16:133-147. [DOI] [PubMed] [Google Scholar]
- 15.Colley, D. G., P. T. LoVerde, and L. Savioli. 2001. Medical helminthology in the 21st century. Science 293:1437-1438. [DOI] [PubMed] [Google Scholar]
- 16.Creasey, A. M., P. Taylor, and J. E. P. Thomas. 1986. Dosage trial of a combination of oxamniquine and praziquantel in the treatment of schistosomiasis in Zimbabwean schoolchildren. Cent. Afr. J. Med. 32:165-167. [PubMed] [Google Scholar]
- 17.Daneshmend, T. K., and M. A. Homeida. 1987. Oxamniquine pharmacokinetics in hepatosplenic schistosomiasis in the Sudan. J. Antimicrob. Chemother. 19:87-93. [DOI] [PubMed] [Google Scholar]
- 18.Danso-Appiah, A., and S. J. De Vlas. 2002. Interpreting low praziquantel cure rates of Schistosoma mansoni infections in Senegal. Trends Parasitol. 18:125-129. [DOI] [PubMed] [Google Scholar]
- 19.Davis, A., and D. H. Wegner. 1979. Multicentre trials of praziquantel in human schistosomiasis: design and techniques. Bull. W. H. O. 57:767-771. [PMC free article] [PubMed] [Google Scholar]
- 20.De Clercq, D., J. Vercruysse, P. Verlé, A. Kongs, and M. Diop. 2000. What is the effect of combining artesunate and praziquantel in the treatment of Schistosoma mansoni infections? Trop. Med. Int. Health 5:744-746. [DOI] [PubMed] [Google Scholar]
- 21.Doenhoff, M., G. Kimani, and D. Cioli. 2000. Praziquantel and the control of schistosomiasis. Parasitol. Today 16:364-366. [DOI] [PubMed] [Google Scholar]
- 22.Doenhoff, M. J. 1998. Is schistosomicidal chemotherapy sub-curative? Implications for drug resistance. Parasitol. Today 14:434-435. [DOI] [PubMed] [Google Scholar]
- 23.Doenhoff, M. J., J. Modha, and J. R. Lambertucci. 1988. Anti-schistosome chemotherapy enhanced by antibodies specific for a parasite esterase. Immunology 65:507-510. [PMC free article] [PubMed] [Google Scholar]
- 24.Engels, D., L. Chitsulo, A. Montresor, and L. Savioli. 2002. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 82:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engels, D., E. Sinzinkayo, and B. Gryseels. 1996. Day-to-day egg count fluctuation in Schistosoma mansoni infection and its operational implications. Am. J. Trop. Med. Hyg. 54:319-324. [DOI] [PubMed] [Google Scholar]
- 26.Fallon, P. G., and M. J. Doenhoff. 1994. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am. J. Trop. Med. Hyg. 51:83-88. [DOI] [PubMed] [Google Scholar]
- 27.Feldmeier, H., and L. Chitsulo. 1999. Therapeutic and operational profiles of metrifonate and praziquantel in Schistosoma haematobium infection. Arzneimittelforschung 49:557-565. [DOI] [PubMed] [Google Scholar]
- 28.Foster, R. 1987. A review of clinical experience with oxamniquine. Trans. R. Soc. Trop. Med. Hyg. 81:55-59. [DOI] [PubMed] [Google Scholar]
- 29.Foster, R., E. T. Mesmer, B. L. Cheetham, and D. F. King. 1971. The control of immature Schistosoma mansoni in mice by UK 3883, a novel 2-aminomethyltetrahydroquinoline derivative. Ann. Trop. Med. Parasitol. 65:221-232. [DOI] [PubMed] [Google Scholar]
- 30.Genovese, R. F., D. B. Newman, and T. G. Brewer. 2000. Behavioral and neural toxicity of the artemisinin antimalarial, arteether, but not artesunate and artelinate, in rats. Pharmacol. Biochem. Behav. 67:37-44. [DOI] [PubMed] [Google Scholar]
- 31.Ghandour, A. 1978. The development of Schistosoma haematobium in the hamster. Ann. Trop. Med. Parasitol. 72:219-225. [DOI] [PubMed] [Google Scholar]
- 32.Gönnert, R., and P. Andrews. 1977. Praziquantel, a new broad-spectrum antischistosomal agent. Z. Parasitenkd. 52:129-150. [DOI] [PubMed] [Google Scholar]
- 33.Groll, E. 1984. Praziquantel. Adv. Pharmacol. Chemother. 20:219-238. [DOI] [PubMed] [Google Scholar]
- 34.Gryseels, B., A. Mbaye, S. J. De Vlas, F. F. Stelma, F. Guissé, L. Van Lieshout, D. Faye, M. Diop, A. Ly, L. A. Tchuem-Tchuenté, D. Engels, and K. Polman. 2001. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Trop. Med. Int. Health 6:864-873. [DOI] [PubMed] [Google Scholar]
- 35.Harnett, W., and J. R. Kusel. 1986. Increased exposure of parasite antigens at the surface of adult male Schistosoma mansoni exposed to praziquantel in vitro. Parasitology 93:401-405. [DOI] [PubMed] [Google Scholar]
- 36.Hastings, I. M., W. M. Watkins, and N. J. White. 2002. The evolution of drug-resistant malaria: the role of drug elimination half-life. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 357:505-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismail, M., S. Botros, A. Metwally, S. William, A. Farghally, L. F. Tao, T. A. Day, and J. L. Bennett. 1999. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 60:932-935. [DOI] [PubMed] [Google Scholar]
- 38.James, C., G. Webbe, and G. S. Nelson. 1977. The susceptibility to praziquantel of Schistosoma haematobium in the baboon (Papio anubis) and of S. japonicum in the vervet monkey (Cercopithecus aethiops). Z. Parasitenkd. 52:179-194. [DOI] [PubMed] [Google Scholar]
- 39.Kamel, G., A. Metwally, F. Guirguis, N. G. Nessim, and M. Noseir. 2000. Effect of a combination of the new antischistosomal drug Ro 15-5458 and praziquantel on different strains of Schistosoma mansoni infected mice. Arzneimittelforschung 50:391-394. [DOI] [PubMed] [Google Scholar]
- 40.Karanja, D. M., A. E. Boyer, M. Strand, D. G. Colley, B. L. Nahlen, J. H. Ouma, and W. E. Secor. 1998. Studies on schistosomiasis in western Kenya. II. Efficacy of praziquantel for treatment of schistosomiasis in persons coinfected with human immunodeficiency virus-1. Am. J. Trop. Med. Hyg. 59:307-311. [DOI] [PubMed] [Google Scholar]
- 41.Katz, N. 1980. Current results in the clinical therapy of schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 22(Suppl. 4):8-17. [PubMed] [Google Scholar]
- 42.Katz, N. 1998. Schistosomiasis control in Brazil. Mem. Inst. Oswaldo Cruz 93(Suppl. 1):33-35. [DOI] [PubMed] [Google Scholar]
- 43.Keiser, J., E. K. N′Goran, B. H. Singer, C. Lengeler, M. Tanner, and J. Utzinger. 2002. Association between Schistosoma mansoni and hookworm infections among schoolchildren in Côte d'Ivoire. Acta Trop. 84:31-41. [DOI] [PubMed] [Google Scholar]
- 44.Kokwaro, G. O., and G. Taylor. 1991. Oxamniquine pharmacokinetics in healthy Kenyan African volunteers. East Afr. Med. J. 68:359-364. [PubMed] [Google Scholar]
- 45.Le, W. J., J. Q. You, Y. Q. Yang, J. Y. Mei, H. F. Guo, H. Z. Yang, and C. W. Zhang. 1982. Studies on the efficacy of artemether in experimental schistosomiasis. Acta Pharmaceut. Sin. 17:187-193. (In Chinese.) [PubMed]
- 46.Le, W. J., J. Q. You, and J. Y. Mei. 1983. Chemotherapeutic effect of artesunate in experimental schistosomiasis. Acta Pharmaceut. Sin. 18:619-621. (In Chinese.) [PubMed]
- 47.Mandour, M. E., H. el Turabi, M. M. Homeida, T. el Sadig, H. M. Ali, J. L. Bennett, W. J. Leahey, and D. W. Harron. 1990. Pharmacokinetics of praziquantel in healthy volunteers and patients with schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 84:389-393. [DOI] [PubMed] [Google Scholar]
- 48.Mehlhorn, H., B. Becker, P. Andrews, H. Thomas, and J. K. Frenkel. 1981. In vivo and in vitro experiments on the effects of praziquantel on Schistosoma mansoni: a light and electron microscopic study. Arzneimittelforschung 31:544-554. [PubMed] [Google Scholar]
- 49.Mordi, M. N., S. M. Mansor, V. Navaratnam, and W. H. Wernsdorfer. 1997. Single dose pharmacokinetics of oral artemether in healthy Malaysian volunteers. Br. J. Clin. Pharmacol. 43:363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.N′Goran, E. K., J. Utzinger, H. N. Gnaka, A. Yapi, N. A. N′Guessan, S. D. Kigbafori, C. Lengeler, J. Chollet, S. H. Xiao, and M. Tanner. 2003. Randomized, double-blind, placebo-controlled trial of oral artemether for the prevention of patent Schistosoma haematobium infections. Am. J. Trop. Med. Hyg. 68:24-32. [PubMed] [Google Scholar]
- 51.Nosten, F., and P. Brasseur. 2002. Combination therapy for malaria: the way forward? Drugs 62:1315-1329. [DOI] [PubMed] [Google Scholar]
- 52.Pellegrino, J., F. F. Lima-Costa, M. A. Carlos, and R. T. Mello. 1977. Experimental chemotherapy of schistosomiasis mansoni. XIII. Activity of praziquantel, an isoquinoline-pyrazino derivative, on mice, hamsters and Cebus monkeys. Z. Parasitenkd. 52:151-168. [DOI] [PubMed] [Google Scholar]
- 53.Price, R., M. van Vugt, L. Phaipun, C. Luxemburger, J. Simpson, R. McGready, F. ter Kuile, A. Kham, T. Chongsuphajaisiddhi, N. J. White, and F. Nosten. 1999. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am. J. Trop. Med. Hyg. 60:547-555. [DOI] [PubMed] [Google Scholar]
- 54.Pugh, R. N. H., and C. H. Teesdale. 1983. Synergy of concurrent low dose oxamniquine and praziquantel in schistosomiasis. Br. Med. J. 287:877-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reich, M. R., and A. Fenwick. 2001. Schistosoma haematobium. N. Engl. J. Med. 344:1170.. [PubMed] [Google Scholar]
- 56.Richards, H. C., and R. Foster. 1969. A new series of 2-aminomethyltetrahydroquinoline derivatives displaying schistosomicidal activity in rodents and primates. Nature 222:581-582. [DOI] [PubMed] [Google Scholar]
- 57.Ross, A. G. P., P. B. Bartley, A. C. Sleigh, G. R. Olds, Y. S. Li, G. M. Williams, and D. P. McManus. 2002. Schistosomiasis. N. Engl. J. Med. 346:1212-1220. [DOI] [PubMed] [Google Scholar]
- 58.Sabah, A. A., C. Fletcher, G. Webbe, and M. J. Doenhoff. 1986. Schistosoma mansoni: chemotherapy of infections of different ages. Exp. Parasitol. 61:294-303. [DOI] [PubMed] [Google Scholar]
- 59.Seubert, J., R. Pohlke, and F. Loebich. 1977. Synthesis and properties of praziquantel, a novel broad spectrum anthelmintic with excellent activity against schistosomes and cestodes. Experientia 33:1036-1037. [DOI] [PubMed] [Google Scholar]
- 60.Shaw, J. R., and K. W. Brammer. 1983. The treatment of experimental schistosomiasis with a combination of oxamniquine and praziquantel. Trans. R. Soc. Trop. Med. Hyg. 77:39-40. [DOI] [PubMed] [Google Scholar]
- 61.Smith, M., J. A. Clegg, and G. Webbe. 1976. Culture of Schistosoma haematobium in vivo and in vitro. Ann. Trop. Med. Parasitol. 70:101-107. [DOI] [PubMed] [Google Scholar]
- 62.Thomas, H., and R. Gönnert. 1977. The efficacy of praziquantel against cestodes in animals. Z. Parasitenkd. 52:117-127. [DOI] [PubMed] [Google Scholar]
- 63.Utzinger, J., M. Booth, E. K. N′Goran, I. Müller, M. Tanner, and C. Lengeler. 2001. Relative contribution of day-to-day and intra-specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology 122:537-544. [DOI] [PubMed] [Google Scholar]
- 64.Utzinger, J., J. Chollet, Z. W. Tu, S. H. Xiao, and M. Tanner. 2002. Comparative study of the effects of artemether and artesunate on juvenile and adult Schistosoma mansoni in experimentally infected mice. Trans. R. Soc. Trop. Med. Hyg. 96:318-323. [DOI] [PubMed] [Google Scholar]
- 65.Utzinger, J., J. Chollet, J. Q. You, J. Y. Mei, M. Tanner, and S. H. Xiao. 2001. Effect of combined treatment with praziquantel and artemether on Schistosoma japonicum and Schistosoma mansoni in experimentally infected animals. Acta Trop. 80:9-18. [DOI] [PubMed] [Google Scholar]
- 66.Utzinger, J., E. K. N′Goran, A. N′Dri, C. Lengeler, and M. Tanner. 2000. Efficacy of praziquantel against Schistosoma mansoni with particular consideration on intensity of infection. Trop. Med. Int. Health 5:771-778. [DOI] [PubMed] [Google Scholar]
- 67.Utzinger, J., E. K. N′Goran, A. N′Dri, C. Lengeler, S. H. Xiao, and M. Tanner. 2000. Oral artemether for prevention of Schistosoma mansoni infection: randomised controlled trial. Lancet 355:1320-1325. [DOI] [PubMed] [Google Scholar]
- 68.Utzinger, J., S. H. Xiao, J. Keiser, M. G. Chen, J. Zheng, and M. Tanner. 2001. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr. Med. Chem. 8:1841-1860. [DOI] [PubMed] [Google Scholar]
- 69.Utzinger, J., S. H. Xiao, E. K. N′Goran, R. Bergquist, and M. Tanner. 2001. The potential of artemether for the control of schistosomiasis. Int. J. Parasitol. 31:1549-1562. [DOI] [PubMed] [Google Scholar]
- 70.van Agtmael, M. A., T. A. Eggelte, and C. J. van Boxtel. 1999. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol. Sci. 20:199-205. [DOI] [PubMed] [Google Scholar]
- 71.van Lieshout, L., F. F. Stelma, F. Guissé, S. T. Falcao Ferreira, K. Polman, G. J. van Dam, M. Diakhate, S. Sow, A. Deelder, and B. Gryseels. 1999. The contribution of host-related factors to low cure rates of praziquantel for the treatment of Schistosoma mansoni in Senegal. Am. J. Trop. Med. Hyg. 61:760-765. [DOI] [PubMed] [Google Scholar]
- 72.Walgate, R. 2002. New malaria drug candidate could cure in a single dose Bull. W. H. O. 80:685-686. [PMC free article] [PubMed] [Google Scholar]
- 73.Webbe, G., and C. James. 1977. A comparison of the susceptibility to praziquantel of Schistosoma haematobium, S. japonicum, S. mansoni, S. intercalatum and S. mattheei in hamsters. Z. Parasitenkd. 52:169-177. [DOI] [PubMed] [Google Scholar]
- 74.Wegner, D. H. G. 1984. The profile of the trematodicidal compound praziquantel. Arzneimittelforschung 34:1132-1136. [PubMed] [Google Scholar]
- 75.White, N. 1999. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 354:739-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White, N. J., F. Nosten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. Kokwaro, J. Ouma, T. T. Hien, M. E. Molyneux, T. E. Taylor, C. I. Newbold, T. K. Ruebush II, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 77.William, S., S. Botros, M. Ismail, A. Farghally, T. A. Day, and J. L. Bennett. 2001. Praziquantel-induced tegumental damage in vitro is diminished in schistosomes derived from praziquantel-resistant infections. Parasitology 122:63-66. [DOI] [PubMed] [Google Scholar]
- 78.World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: first report of the Joint WHO Expert Committees. WHO Tech. Rep. Ser., in press. [PubMed]
- 79.Xiao, S. H., M. Booth, and M. Tanner. 2000. The prophylactic effects of artemether against Schistosoma japonicum infections. Parasitol. Today 16:122-126. [DOI] [PubMed] [Google Scholar]
- 80.Xiao, S. H., and B. A. Catto. 1989. In vitro and in vivo studies of the effect of artemether on Schistosoma mansoni. Antimicrob. Agents Chemother. 33:1557-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao, S. H., J. Chollet, J. Utzinger, H. Matile, J. Y. Mei, and M. Tanner. 2001. Artemether administered together with haemin damages schistosomes in vitro. Trans. R. Soc. Trop. Med. Hyg. 95:67-71. [DOI] [PubMed] [Google Scholar]
- 82.Xiao, S. H., J. Chollet, N. A. Weiss, R. N. Bergquist, and M. Tanner. 2000. Preventive effect of artemether in experimental animals infected with Schistosoma mansoni. Parasitol. Int. 49:19-24. [DOI] [PubMed] [Google Scholar]
- 83.Xiao, S. H., P. J. Hotez, and M. Tanner. 2000. Artemether, an effective new agent for chemoprophylaxis against shistosomiasis in China: its in vivo effect on the biochemical metabolism of the Asian schistosome. Southeast Asian J. Trop. Med. Public Health 31:724-732. [PubMed] [Google Scholar]
- 84.Xiao, S. H., M. Tanner, E. K. N′Goran, J. Utzinger, J. Chollet, R. Bergquist, M. G. Chen, and J. Zeng. 2002. Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia. Acta Trop. 82:175-181. [DOI] [PubMed] [Google Scholar]
- 85.Xiao, S. H., Y. Q. Yang, Q. Q. You, J. Utzinger, H. F. Guo, P. Y. Jiao, J. Y. Mei, J. Guo, R. Bergquist, and M. Tanner. 2002. Potential long-term toxicity of repeated orally administered doses of artemether in rats. Am. J. Trop. Med. Hyg. 66:30-34. [DOI] [PubMed] [Google Scholar]
- 86.Xiao, S. H., J. W. Yin, J. Y. Mei, J. Q. You, Y. Li, and H. J. Jiang. 1992. Effect of arteether on Schistosoma japonicum. Acta Pharmaceut. Sin. 27:161-165. (In Chinese.) [PubMed]
- 87.Xiao, S. H., J. Q. You, J. Y. Mei, H. F. Guo, P. Y. Jiao, and M. Tanner. 2000. Effect of praziquantel together with artemether on Schistosoma japonicum parasites of different ages in rabbits. Parasitol. Int. 49:25-30. [DOI] [PubMed] [Google Scholar]
- 88.Xiao, S. H., J. Q. You, Y. Q. Yang, and C. Z. Wang. 1995. Experimental studies on early treatment of schistosomal infection with artemether. Southeast Asian J. Trop. Med. Public Health 26:306-318. [PubMed] [Google Scholar]
- 89.Xiao, S. H., W. J. Yue, Y. Q. Yang, and J. Q. You. 1987. Susceptibility of Schistosoma japonicum to different developmental stages to praziquantel. Chin. Med. J. 100:759-768. [PubMed] [Google Scholar]