Abstract
Mutations in the dihydrofolate reductase (dhfr) genes of Plasmodium falciparum and P. vivax are associated with resistance to the antifolate antimalarial drugs. P. vivax dhfr sequences were obtained from 55 P. vivax isolates (isolates Belem and Sal 1, which are established lines originating from Latin America, and isolates from patient samples from Thailand [n = 44], India [n = 5], Iran [n = 2], and Madagascar [n = 2]) by direct sequencing of both strands of the purified PCR product and were compared to the P. vivax dhfr sequence from a P. vivax parasite isolated in Pakistan (isolate ARI/Pakistan), considered to represent the wild-type sequence. In total, 144 P. vivax dhfr mutations were found at only 12 positions, of which 4 have not been described previously. An F→L mutation at residue 57 had been observed previously, but a novel codon (TTA) resulted in a mutation in seven of the nine mutated variant sequences. A new mutation at residue 117 resulted in S→T (S→N has been described previously). These two variants are the same as those observed in the P. falciparum dhfr gene at residue 108, where they are associated with different levels of antifolate resistance. Two novel mutations, I→L at residue 13 and T→M at residue 61, appear to be unique to P. vivax. The clinical, epidemiological, and sequence data suggest a sequential pathway for the acquisition of the P. vivax dhfr mutations. Mutations at residues 117 and 58 arise first when drug pressure is applied. Highly mutated genes carry the S→T rather than the S→N mutation at residue 117. Mutations at residues 57 and 61 then occur, followed by a fifth mutation at residue 13.
With the global spread of chloroquine resistance in Plasmodium falciparum, the combination of sulfadoxine and pyrimethamine (S-P) is now used as first-line antimalarial treatment in an increasing number of countries. This is usually followed rapidly by the emergence of resistance. For example, in Thailand, high-level resistance to S-P arose within 5 years of its deployment in the late 1970s (14, 21). Similar patterns have been observed in other Asian countries, South America, and southern Africa. The combination of antifolate and sulfonamide drugs targets the folate metabolism of the malaria parasite. Molecular clinical and epidemiological studies have clearly shown that resistance to pyrimethamine and sulfadoxine results from specific point mutations in the parasite genes dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps), respectively. These cause alterations in crucial residues in the active sites of these enzymes, resulting in reduced drug affinity (5, 11, 13, 17-20). Detection of these mutations in isolates in blood samples collected in the field has proved very valuable in the mapping of resistance and the monitoring of malaria control measures.
In areas of malaria endemicity outside Africa, the prevalence of P. vivax equals and often exceeds that of P. falciparum. Chloroquine remains the first-line treatment for P. vivax infection, although in most areas where malaria is endemic, empirical antimalarial treatment is given for fevers. In recent years chloroquine-resistant P. vivax parasites have been reported in several locations, with high-level resistance confirmed in parts of Indonesia and New Guinea (1, 2, 6, 9, 12, 15) and, more recently, in Central America. In those countries where S-P was used extensively, such as Thailand, high-grade antifolate resistance has also emerged in P. vivax populations (14, 21). The P. vivax dhps gene has not been well characterized, but the P. vivax dhfr gene has been (3), and a number of point mutations have been identified in parasites from different origins (4, 8). As continuous in vitro culture of P. vivax is generally unavailable, the relationship between the various P. vivax dhfr point mutations and resistance to pyrimethamine relies on epidemiological and clinical investigations. In an extensive study of P. vivax parasites in Thailand, mutations at three of the P. vivax dhfr codons (codons 57, 58 and 117) were found to be highly prevalent and were implicated in clinical antifolate resistance (8). Confirmation that these residues resulted in altered susceptibility to drug action was provided by studies in which the enzymatic activity and drug affinity of the recombinant P. vivax dihydrofolate reductase (DHFR) enzyme were shown to be altered by the introduction of these specific mutations (10). Mutations in P. vivax dhfr codons 58 and 117 are considered to be equivalent to P. falciparum dhfr mutations at residues 59 and 108, respectively, which are associated with pyrimethamine resistance. In our first study, restriction fragment length polymorphism (RFLP) analysis alone was used to identify P. vivax dhfr mutations in Thai isolates (8). Thus, other mutations possibly contributing to pyrimethamine resistance could also have been present in the gene. The dhfr genes from P. vivax isolates from Thailand (n = 44) and several other different geographical origins (n = 11) were therefore sequenced. Previously undescribed mutations were found at positions 13 and 61, and novel mutations were added to those previously described for residues 57 and 117. We then used single-strand conformation polymorphism (SSCP) analysis to confirm the absence of mutations at residue 173 (which corresponds to P. falciparum dhfr residue 164) in 100 Thai isolates. Additional PCR-RFLP protocols for the detection of the novel mutations were then developed.
MATERIALS AND METHODS
P. vivax isolates.
Blood samples were collected on admission from 48 symptomatic patients who acquired P. vivax infections in three separate geographical regions. Isolates from Madagascar or the Comoros Islands (n = 2) were obtained from the Centre Hospitalier de Dunkerque, Rosendael, France (kindly provided by J. Poirriez); isolates from the Indian subcontinent (n = 5) were obtained from Northwick Park Hospital, Harrow, United Kingdom (kindly provided by G. Pasvol); isolates from Iran (n = 2) were kindly provided by Sayeh Jafari; and isolates from Thailand (n = 44) were collected from adult patients admitted to the Bangkok Hospital for Tropical Diseases. All samples were collected on admission before the start of treatment or upon recrudescence or relapse following treatment (see below) and were stored frozen at −30 or −70°C until DNA extraction. The diagnosis was made by microscopic examination of Field- or Giemsa-stained thin and thick blood smears. The presence of P. vivax was confirmed by PCR analysis of an aliquot from each sample (8). Samples from Thailand were collected between 1992 and 1998. Of the Thai patients, 32 had been treated with S-P and observed for 1 month in the Hospital for Tropical Diseases in Bangkok, where no reinfections could have taken place (8). During this observation period, 11 patients had recurrent vivax parasitemias, but blood samples were obtained from only 5 of these patients.
PCR amplification and analysis of P. vivax dhfr.
Template DNA was purified from 1 ml of infected blood by using the QIAamp DNA kit (Qiagen, Hilden, Germany). The DNA was eluted with TE buffer (10 mM Tris-HCl [pH 8.0], 0.1 mM EDTA [pH 8.0]), such that 1 μl of the solution corresponded to 5 μl of whole blood.
The oligonucleotides used to amplify P. vivax dhfr were designed by using a published sequence of the dhfr-ts gene of P. vivax isolate ARI/Pakistan (GenBank accession no. X98123) (3). Nested or seminested PCR amplification strategies were adopted. In the primary reaction, the entire P. vivax dhfr-ts gene (1,876 bp, excluding the stop codon) was amplified by using the oligonucleotide pair VDT OF (5′-ATGGAGGACCTTTCAGATGTATTTGACATT-3′) and VDT OR (5′-GGCGGCCATCTCCATGGTTATTTTATCGTG-3′). The product of this reaction was then used to initiate a second round of amplification to obtain the whole P. vivax dhfr domain (ca. 711 bp) by using the oligonucleotide pair VDT OF and VDF OR (5′-CTTGCTGTAAACCAAAAAGTCCA-3′). Two other secondary amplifications were carried out separately. By using the oligonucleotide pair VDF N3F (5′-ATCCCCAAGCAGTACAAGCCGCTCCCAAAC-3′) and VDF OR, a short fragment close to the 3′ end of P. vivax dhfr (352 bp) was amplified and used for SSCP analysis. The joining regions between the dhfr and the thymidylate synthase (ts) domains were amplified by using the oligonucleotide pair VDT LiF (5′-CTTCCCCGAGTTTGACGAAAGCCAGTTTCG-3′) and VDT LiR (5′-ACTCCCACACCTGTTCTGTCTCCTTGTTTG-3′). By using the oligonucleotide pair VDNF57 (5′-CATGGAAATGCAACTCCGTCGATATGATGT-3′) and VDF NR (5′-TCACACGGGTAGGCGCCGTTGATCCTCGTG-3′), the regions were amplified, and the products were digested with enzymes specific for each variant of position 57 in the P. vivax dhfr gene. The regions flanking the mutations were amplified by PCR with primers VDF13NF (5′-GACCTTTCAGATGTATTTGACATTTACGGC-3′) and VDF NR58 (5′-GGTACCTCTCCCTCTTCCACTTTAGCTTCT-3′), and the products were digested with enzymes specific for position 13 in the P. vivax dhfr gene.
All amplification reactions were carried out in a final volume of 20 μl which included 1 μl of template, i.e., genomic DNA for the primary reaction or the product of that reaction for the secondary amplification. The oligonucleotide primers were each used at a final concentration of 125 nM in the primary reactions and at a final concentration of 250 nM in the secondary reactions. The reaction mixture contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, each of the four deoxynucleoside triphosphates at a concentration of 125 μM, and 0.4 U of AmpliTaq polymerase (Perkin-Elmer Cetus, Norwalk, Conn.). The reactions were carried out in the presence of 2 mM MgCl2 for all oligonucleotide combinations. The cycling parameters for the amplification reactions, which were performed in a PTC-200 thermocycler (M & J Research, Boston, Mass.), were as follows: an initial denaturation step at 95°C for 5 min was followed by 25 (primary reaction) or 30 (secondary reaction with primers VDNF57 and VDF NR and primers VDF13NF and VDF NR58) cycles of annealing at 64°C (58°C for primers VDF N3F and VDF OR, 55°C for primers VDT LiF and VDT LiR, 50°C for primers VDNF57 and VDF NR, and 62°C for primers VDF13NF and VDFNR58) for 2 min, extension at 72°C for 2 min, and denaturation at 94°C for 1 min. After a final annealing step followed by 5 min of extension, the reaction was stopped. The presence of specific PCR products was established by electrophoresis on a 1.5% agarose gel. In the majority of cases sequencing was performed directly with the purified PCR product, and for a subset of isolates the PCR product was cloned into the pCR2.1 vector (Invitrogen, Groningen, The Netherlands); and sequencing was performed with plasmids purified from positive bacterial colonies. Sequence analysis and alignments were performed by using GeneJockey II software (Biosoft, Cambridge, United Kingdom). Nucleotide (base pair) and amino acid numbering was based on the sequence of isolate ARI/Pakistan, and for the sake of clarity, the 18-bp sequence which is inserted or deleted in some P. vivax dhfr genes was not taken into account. The sequences taken into consideration for the analysis excluded those corresponding to the oligonucleotides used for amplification (the first 30 bp and the last 26 bp of the 711 bp of the P. vivax dhfr gene).
SSCP analysis.
SSCP analysis was carried out as described previously (22). Briefly, the PCR products were denatured before analysis by adding 50% deionized formamide, 100 mM NaOH, and 0.1% bromophenol blue, followed by incubation at 98°C for 10 min. The samples were placed on ice and loaded onto a nondenaturing 8% polyacrylamide with 5% glycerol and run under constant power (4 W, 8 to 12 mA). A lane containing the PCR product obtained from a reference wild-type strain (strain IP83) was used as a standard for the SSCP patterns. The gels were stained with silver (Silverstain Plus kit; Bio-Rad, Richmond, Calif.) and dried.
RFLP digestions.
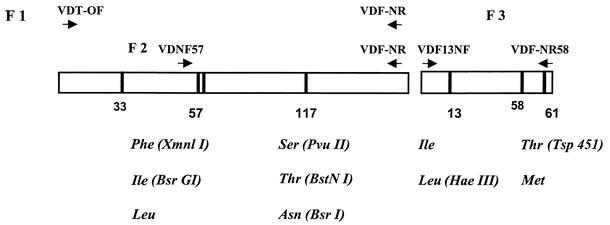
The PCR-RFLP protocols used for the detection of point mutations at residues 57, 58, and 117 have been described previously (8). Additional protocols for the detection of the newly observed mutations (residues 13 and 61) and the identification of the polymorphisms of mutant types at residues 57 and 117 were developed (Fig. 1). The primers used for PCR to create restriction sites were designed so that polymorphisms not described by natural restriction sites could also be detected to distinguish between all of polymorphisms in the P. vivax dhfr gene identified to date.
FIG. 1.
Schematic representation of the PCR system used to detect polymorphisms in the P. vivax dhfr gene. The primers and restriction enzymes used for the detection of variant codons are indicated.
The DNA fragments obtained following PCR amplification or RFLP analysis were subjected to electrophoresis on 3% MetaPhor agarose gels (performed in TBE [Tris-borate-EDTA] buffer). Digestion of 10 μl of the PCR product was performed with 10 U of each restriction enzyme (New England Biolabs Inc., Beverly, Mass.) for 3 h at 37°C in a total volume of 20 μl.
Statistical methods.
Continuous variables which were not normally distributed were compared by the Wilcoxon rank sum test. Categorical variables were compared by the chi-square test or Fisher's exact test, as appropriate.
Nucleotide sequence accession numbers.
The sequences of representative isolates have been submitted to GenBank. See Table 1 for the accession numbers.
TABLE 1.
Mutations present in the dhfr genes amplified from P. vivax isolates
| Isolate | Origin | Residue at codona:
|
PRR48 | GenBank accession no.b | ||||
|---|---|---|---|---|---|---|---|---|
| 13 | 57 | 58 | 61 | 117 | ||||
| Ari/Pakistan | Pakistan | I | F | S | T | S | ||
| Sal 1 | Salvador | I | F | S | T | S | AF525811 | |
| JP95 | Madagascar | I | F | S | T | S | AF525813 | |
| EA1 | Madagascar | I | F | S | T | S | ||
| Iran 1 | Iran | I | F | S | T | S | ||
| Iran 7 | Iran | I | F | S | T | S | ||
| 22/96 | India | I | F | S | T | S | ||
| 10/97 | India | I | F | S | T | S | ||
| 37/96 | India | I | F | S | T | S | ||
| 11/96 | India | I | F | R | T | N | ||
| 22/98 | India | I | F | R | T | N | ||
| Belem | Brazil | I | F | R | T | N | AF525812 | |
| IP30 | Thailand | I | F | S | T | S | ||
| IP83 | Thailand | I | F | S | T | S | AF525814 | |
| IP18 | Thailand | NDc | F | R | T | N | ||
| IP76 | Thailand | ND | F | R | T | N | ||
| IP45/2 | Thailand | I | Ld | Re | T | S | ||
| IP11 | Thailand | I | Ld | Re | T | S | AF525815 | |
| D124 | Thailand | I | F | R | T | N | 2 | |
| IP81 | Thailand | I | F | R | T | N | 3 | |
| IP37 | Thailand | I | F | R | T | N | 5 | |
| IP53 | Thailand | I | F | R | T | N | 5 | |
| IP73 | Thailand | I | F | R | T | N | 10 | AF525816 |
| D123 | Thailand | I | F | R | T | N | 10 | |
| IP41 | Thailand | I | F | R | T | N | 11 | |
| IP21 | Thailand | I | F | R | T | N | 12 | |
| D129 | Thailand | I | F | R | T | N | 12 | |
| D131 | Thailand | I | F | R | T | N | 13 | |
| D127 | Thailand | I | F | R | T | N | 15 | |
| D122 | Thailand | I | F | R | T | N | 16 | AF525817 |
| D128 | Thailand | I | F | R | T | N | 19 | |
| D132 | Thailand | I | F | R | T | N | 23 | |
| D130 | Thailand | I | F | R | T | N | 76 | |
| IP29 | Thailand | I | F | R | T | N | 108 | |
| D121 | Thailand | I | F | R | T | T | 1,330 | |
| IP85 | Thailand | I | F | R | T | N | 1,500 | |
| D126 | Thailand | I | F | R | T | N | 1,500 | |
| IP33 | Thailand | ND | L | R | T | T | AF525818 | |
| IP89 | Thailand | ND | L | R | T | T | ||
| IP69 | Thailand | I | I | R | M | T | 29 | AF525819 |
| IP49 | Thailand | I | I | R | M | T | 13 | |
| IP25 | Thailand | I | I | R | M | T | 7 | |
| IP45/1 | Thailand | I | I | R | M | T | 5 | |
| D125 | Thailand | I | I | R | M | T | 3 | |
| IP77 | Thailand | I | I | R | M | T | 2 | |
| IP93 | Thailand | I | I | R | M | T | 1 | |
| IP13 | Thailand | I | I | R | M | T | 0.5 | |
| IP57 | Thailand | I | L | R | M | T | 2.5 | AF525820 |
| IP17 | Thailand | L | L | R | M | T | 1.5 | |
| IP5 | Thailand | L | L | R | M | T | 1.5 | AF525821 |
| IP28 | Thailand | L | L | R | M | T | ||
| IP40 | Thailand | L | L | R | M | T | ||
Residues which differ from the wild-type are indicated in boldface.
GenBank submissions were made for representative sequences.
ND, not determined.
The codon for these F→L mutations is TTG. The other F→L mutations are encoded by TTA.
The codon for these S→R mutations is CGC. The other S→R mutations are encoded by AGG.
RESULTS
Sequences of amplified P. vivax dhfr fragments.
A total of 55 P. vivax dhfr sequences were obtained and compared to the sequence of the gene from a parasite isolated in Pakistan (ARI/Pakistan) which was considered to represent the wild-type sequence (3, 4). Two P. vivax isolates, isolates Belem and Sal 1, are established lines originating from Latin America. All other isolates were obtained from patients: 44 patients from Thailand, 5 patients from India, 2 patients from Iran, and 2 patients from Madagascar. Among the Thai isolates, 39 were collected on admission from different patients and the remaining 5 represented reccurrence isolates from five of these patients who had been treated with S-P. The P. vivax dhfr sequences obtained differed between the paired admission and reccurrence isolates for only one patient. Thus, sequence analysis was confined to 51 P. vivax dhfr genes, of which 40 (39 admission isolates plus 1 genetically distinct recurrent isolate) were obtained from Thai parasites. All the sequences were obtained by direct sequencing of both strands of the purified PCR product. Thus, in samples in which multiple parasite lines were present, the P. vivax dhfr sequence is likely to represent that of the numerically dominant line. In order to assess whether mutations were introduced as artifacts as a consequence of incorporation errors by the Taq enzyme, duplicate sequences were obtained from five different fragments by direct sequencing and from another seven different fragments following cloning from an independent amplification reaction. All duplicate sequences were identical, indicating that the data obtained reflected the actual P. vivax dhfr sequences. The sequences of the oligonucleotides used to amplify the P. vivax dhfr gene, which corresponded to the first 8 amino acids (aa) and the last 9 aa (aa 228 to 237) of the enzyme, were not included in the analysis.
Size polymorphisms of the P. vivax dhfr sequence resulting from a deletion or a repetition of a unit of 6 aa (THGGDN) have been observed previously (4). These polymorphisms were observed for the 51 P. vivax dhfr sequences analyzed. Amplified genes with two repeated units were denoted variant A, the wild-type gene with a single repeat unit was denoted variant B, and those with the deleted repeat unit are denoted variant C. Among the isolates whose fragments were sequenced, the A variant was observed only once (in an Indian isolate); all the remaining clones were variant B (27 of 51) or variant C (23 of 51). When a nested PCR specific for a fragment spanning this region was performed (8), mixed genotype infections with respect to this size polymorphism were observed in 8 of 40 Thai isolates (variants A and B, variants A and C, and variants A, B, and C in 3, 2, and 3 isolates respectively), whereas variants B and C were found alone in the remaining 14 and 18 isolates, respectively. The P. vivax dhfr genes of non-Thai origin harbored predominantly (10 of 11 isolates) the B variant alone. The other isolate was the A variant (an Indian isolate).
Detection of mutations in the P. vivax dhfr gene.
Sequences totaling ca. 37,000 bp were obtained for the 55 different P. vivax dhfr fragments amplified. Comparison with the published wild-type sequence revealed a total of 144 mutations distributed among only 12 positions. Since mutations might be generated as a result of PCR amplification, the three point mutations which were observed only once (at positions 26, 34, and 671) were excluded from the subsequent analysis. For the remaining nine positions, the only synonymous mutation was observed at position 207 and this was observed in three isolates: isolate Belem from Brazil, an isolate from India, and an isolate from Thailand. The mutation at position 98, which causes a P→L change in codon 33, was observed only in the two isolates from Madagascar, confirming previous studies which indicated that this mutation is specific to parasites from this region and is unlikely to be implicated in pyrimethamine resistance (4). The remaining seven point mutations (at positions 37, 169, 171, 172, 174, 182, and 350) resulted in amino acid substitutions in five residues: residues 13, 57, 58, 61, and 117 (Table 1). The I→L mutation at codon 13 (ATC to CTC at position 37) was observed 4 times (7%), and the T→M mutation at codon 61 (ACG to ATC at position 182) was observed 13 times (23%). Neither of these mutations has been observed previously, and they were found only in Thai samples. Because of its proximity to the end of the amplified fragment, the sequence of codon 13 could not be unambiguously derived for 4 (10%) of the 40 Thai isolates. Mutations at positions 169 and 171 affected codon 57. The F→I mutation at codon 57 resulted from a TTC to a ATA double mutation around position 170 in eight sequences, and the F→L change at codon 57 resulted from one of two observed mutations at position 171 (TTC to TTA in two sequences or TTC to TTG in seven sequences). The S→R change at position 58 also resulted from one of two mutations: at position 172 (AGC to CGC) in 39 sequences (70%) or at position 174 (ACG to AGG) in 2 sequences (3.6%). Finally two different mutations at position 350 resulted in mutations of S→N (AGC to AAC in 22 sequences) or S→T (AGC to ACC in 17 sequences) at residue 117.
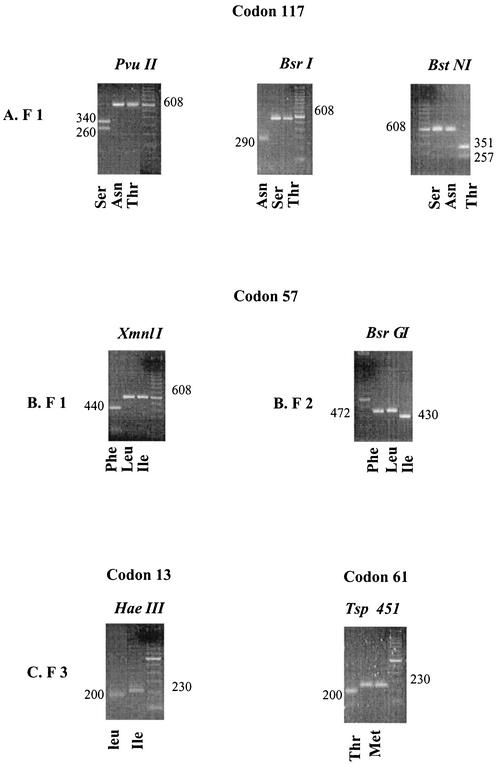
PCR-RFLP protocols for the detection of point mutations at residues 57, 58, and 117 have been described previously (8). Additional protocols for the detection of the newly observed mutations are shown in Fig. 1 and 2.
FIG. 2.
Agarose gels of the restriction digests of the PCR products in tests for the polymorphisms of the dhfr gene. (A) Products (F 1) obtained with primers VDT OF and VDF NR; (B) products (F 2) obtained with primers VDFNF57 and VDF NR; (C) products (F 3) obtained with primers VDFNF13 and VDF NR58. Sizes are indicated in base pairs.
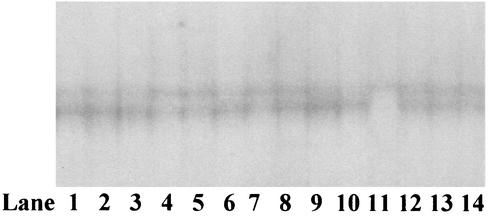
Mutations were not observed at the 3′ ends of the genes from any of the isolates analyzed. In order to confirm the complete sequence conservation of the 3′ end of the P. vivax dhfr gene, this region was amplified from 100 Thai isolates (which included the isolates sequenced) and analyzed by SSCP analysis. The same pattern was observed for all isolates except isolate IP11 (Fig. 3). Three independent sequences were obtained for the P. vivax dhfr 3′-end region of this isolate, but no mutations could be detected, indicating that the original pattern resulted from a point mutation introduced by the amplification reaction. This result also indicates that the SSCP analysis used is capable of resolving two isolates with little sequence difference.
FIG. 3.
SSCP patterns observed for the P. vivax dhfr 3′-end fragments (codons 120 to 203) amplified from Thai isolates. Only one isolate differed from the others (lane 11).
Among the isolates for which a complete P. vivax dhfr sequence was obtained, 30 were collected from patients who had been treated with S-P and for whom the parasite reduction ratio at 48 h (PRR48), which is the ratio between the parasitemia on admission and the parasitemia at 48 h and which is a measure of antimalarial efficacy, had been measured (8). This group can be divided into those patients infected with P. vivax isolates whose dhfr genes harbored two mutations (n = 19) and those infected with isolates whose dhfr genes harbored four or five mutations (n = 11); no isolates with single or triple mutations were detected in the series. The PRR48 values were significantly lower for patients infected with P. vivax isolates whose dhfr genes contained four or five mutations (median PRR48 = 2.5; PRR48 range = 0.5 to 29) than for those infected with P. vivax isolates whose dhfr genes contained two mutations (median PRR48 = 15; PRR48 range = 2 to 1,500) (P = 0.001) (Table 1). Thus, parasite clearance was significantly slower if there were four or five mutations in P. vivax dhfr.
The P. vivax isolates collected in Thailand were obtained either between 1992 and 1995 (indicated by the prefix D; n = 12) or during 1998 (indicated by the prefix IP; n = 29). Only 1 isolate in the former series was found to harbor four or more mutations, whereas 12 isolates in the latter series carried quadruple or quintuple mutations (relative risk = 5.0; 95% confidence interval = 0.72 to 34; P = 0.064).
Conservation of the joining region of the P. vivax dhfr-ts gene.
The DHFR domain is at the amino terminus of the bifunctional enzyme, which includes a thymidylate synthase (TS) domain. The two domains are connected by a joining region. The enzymatic activity of the TS domain seems to depend on the presence of both the DHFR domain and the joining region (16). In P. falciparum, the joining region is conserved between different isolates. We obtained the sequences of the joining regions of 14 P. vivax isolates from Thailand (n = 6), India (n = 3), Iran (n = 2), and Madagascar (n = 3). The sequences were identical except for a single point mutation at codon 241 (G→E), which was present in all three isolates from Madagascar. The joining region that we obtained was quite different at the amino acid level from that published for wild-type isolate ARI/Pakistan (3). Sequence comparison revealed that the reading frame of the published sequence (GenBank accession no. X98123) was shifted by one insertion at the 5′ end of the joining region and three deletions 80 bp later but was restored by an insertion 170 bp later, close to the start of the ts domain.
DISCUSSION
Resistance to the antifolates pyrimethamine and cycloguanil in P. falciparum has been studied extensively both in vitro and in vivo. The loss of susceptibility to these antifolate drugs results from reduced drug binding to the mutant P. falciparum DHFR enzyme. Although gene amplification has been observed in antifolate-resistant bacteria and was initially implicated in malaria parasite resistance, it is now generally accepted that the major mechanism which gives rise to resistance is the introduction of point mutations in defined residues close to the active site of the enzyme. In natural P. falciparum parasite populations, these mutations are restricted to five residues and are present in different combinations, each of which confers different levels of resistance. Epidemiological studies, underpinned by biochemical analysis, strongly suggest that drug pressure leads to the sequential appearance of point mutations and the progressive selection of P. falciparum parasites with increasing levels of resistance. Mutations at codon 108 (which confers low-level resistance) are observed first. Increased resistance to pyrimethamine is seen when mutations at residues 51 or 59 are added, and resistance to cycloguanil is added when mutations at residue 16 are also present. Parasites with P. falciparum dhfr genes containing mutations at residue 164 are highly resistant to both drugs. This mutation has been observed only in genes carrying at least the double mutation at residues 59 and 108. These observations have led to the design of practical PCR-based protocols whereby these mutations can be detected quickly in field isolates. Although the exact contribution of the different mutations to resistance remains to be determined, these protocols should be helpful for the assessment and monitoring of the emergence of drug-resistant parasites without the need for extensive in vivo and in vitro testing.
Isolation of the P. vivax gene for DHFR (3) opened the way to extend these studies to this important human malaria parasite. P. vivax dhfr residues equivalent to those of the P. falciparum gene for DHFR that are known to be associated with resistance have been identified by sequence alignment (4). Initial studies revealed that parasites with mutations in residues 58 and 117, which correspond to residues 59 and 108 of the P. falciparum gene, are found in different countries (4). A mutation at residue 57, for which no equivalent is known in the P. falciparum gene, was also common (8). The majority of the isolates analyzed harbored double or triple mutations at these residues. A mutation at residue 173, which corresponds to residue 164 of the P. falciparum gene, was detected only once, in a parasite from Surinam, but not in 100 samples from Thailand (8). However, it was not clear from these RFLP studies whether other mutations were associated with P. vivax dhfr. Indeed, sequence data for the P. vivax dhfr gene have been restricted to 30 isolates obtained from different geographical regions where, in many cases, neither the level of drug exposure of the P. vivax populations nor the extent of their resistance to pyrimethamine was clearly established (4). We therefore sequenced the dhfr genes from 41 P. vivax isolates from Thailand, where pyrimethamine had been used in combination with sulfadoxine for nearly 20 years (1975 to 1992) and where the clinical responses of vivax malaria to S-P treatment are known to be very poor (14, 21).
Four novel P. vivax dhfr mutations were identified. An F→L mutation at residue 57 has been observed previously, but a novel codon (TTA) was identified in seven of the nine mutant sequences. The other three mutations resulted in previously undescribed residue changes. An S→T mutation was present at residue 117 in 44% of the isolates, and the previously undescribed S→N mutation was present in the remainder. These two variations at this crucial position are the same as those observed at position 108 in the dhfr gene of P. falciparum, where they are associated with different levels of pyrimethamine resistance (17). The last two mutations, I→L at residue 13 and T→M at residue 61, appear to be unique to P. vivax, as they have no known equivalent in the P. falciparum enzyme.
Sequence data for the P. vivax dhfr gene now extend to 81 different isolates. Analysis of the geographical pattern and relationship with drug susceptibility suggests a sequential pathway for the appearance of the mutations. The data have mainly been derived from parasites obtained from Southeast Asia, where substantial antifolate drug pressure has been exerted. In contrast to P. falciparum, P. vivax parasites with a single dhfr mutation were found rarely even among isolates from geographical regions where drug pressure is not thought to have been extensive. The only single mutation, at residue 57, was detected in one isolate from Thailand (4). Double mutants accounted for half of the isolates (41 of 81) analyzed; residues 58 and 117 were altered in 38 of these isolates, and residues 57 and 58 were altered in the remaining 3 isolates. Thus, mutations at residues 117 and 58 appear to arise together when drug pressure is applied. Whether the mutation at position 117 precedes that at position 58, as is the case for the homologous positions in the dhfr gene of P. falciparum, cannot be deduced from these data, although studies with the purified mutated enzyme suggest that the single mutant enzyme at position 117 is functionally impaired (10). The next most frequently encountered group of parasites consisted of those which carry four or five mutations in P. vivax dhfr. Such parasites were found only in Thailand. The additional mutations at residues 61 and 57 resulted in parasites with quadruple mutations, which were observed in 8 of the 41 Thai isolates, and the 4 remaining isolates carried a further mutation at residue 13. It is interesting that all the highly mutated genes carried the S→T mutation rather than the S→N mutation at residue 117. Thus, the mutations at residues 57 and 61, resulting in variants with quadruple mutations, occur after the double mutation, and the fifth mutation at residue 13 is the last to appear.
Two observations provide supportive evidence for the hypothesized sequence of events. First, PRR48 was measured for patients treated with S-P and showed that parasites carrying four or five mutations were cleared significantly more slowly than those carrying the double mutation. PRR48 is a measure of overall antimalarial activity in vivo. It should be noted that interpretation of clinical outcomes is complex, and confounding factors such as the degree of immunity to the infecting strain or the contribution of mutations in another gene (such as the P. vivax dhps gene) probably explain the large degree of overlap observed between the two groups. Second, the parasites with multiple mutations were more prevalent in 1998 than between 1992 and 1995. In Thailand, S-P was replaced as the first-line treatment for falciparum malaria in 1982, but it remained widely available in the market and reappeared in combination with mefloquine (Fansimef; Roche) until the 1990s. It is still freely available in the private sector. Since up to a third of patients treated for P. falciparum infections in Thailand are simultaneously infected with P. vivax (14) and self-medication for fever is still widespread, P. vivax is likely to have remained under pyrimethamine selection pressure.
Ultimately, confirmation of the precise role of these new P. vivax dhfr mutations can be provided only through detailed biochemical and epidemiological investigations. The fact that some of the natural mutations seen in the P. vivax dhfr gene have not been observed at the homologous positions in the P. falciparum dhfr gene could imply that the DHFR enzymes in the two parasites differ in their catalytic activities. Such a difference might be important for the eventual deployment of new potent antifolate drugs, such as WR99210, which are efficient at inhibiting highly pyrimethamine-resistant P. falciparum parasites (7). The effects of the novel mutations on the kinetic parameters of the P. vivax DHFR are being investigated by using recombinant proteins carrying defined combinations of mutated residues.
The simple PCR-RFLP protocols described here can be used for epidemiological mapping to predict the likely efficacies of the antifolates available at present and to monitor the emergence of resistance.
Acknowledgments
We are grateful to the Royal Golden Jubilee Program of the Thailand Research Fund and the Wellcome Trust for supporting this study.
This study was part of the Wellcome Trust-Mahidol University, Oxford Tropical Medicine Research Programme, funded by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Baird, J. K., H. Basri, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. K., B. Leksana, S. Masbar, D. J. Fryauff, M. A. Sutanihardja, Suradi, F. S. Wignall, and S. L. Hoffman. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 56:621-626. [DOI] [PubMed] [Google Scholar]
- 3.Eldin de Pecoulas, P., L. K. Basco, R. Tahar, T. Ouatas, and A. Mazabraud. 1998. Analysis of the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene sequences. Gene 211:177-185. [DOI] [PubMed] [Google Scholar]
- 4.Eldin de Pécoulas, P., R. Tahar, T. Ouatas, A. Mazabraud, and L. K. Basco. 1998. Sequences variations in the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene and their relationship with pyrimethamine resistance. Mol. Biochem. Parasitol. 92:265-273. [DOI] [PubMed] [Google Scholar]
- 5.Foote, S. J., and A. F. Cowman. 1994. The mode of action and the mechanism of resistance to antimalarial drugs. Acta Trop. 56:157-171. [DOI] [PubMed] [Google Scholar]
- 6.Garg, M., N. Gopinathan, P. Bodhe, and N. A. Khirsagar. 1995. Vivax malaria resistant to chloroquine: case reports from Bombay. Trans. R. Soc. Med. Hyg. 89:656-657. [DOI] [PubMed] [Google Scholar]
- 7.Hekmat-Nejad, M., and P. K. Rathod. 1997. Plasmodium falciparum: kinetic interactions of WR99220 with pyrimethamine-sensitive and pyrimethamine-resistant dihydrofolate reductase. Exp. Parasitol. 87:222-228. [DOI] [PubMed] [Google Scholar]
- 8.Imwong, M., S. Pukrittakayamee, S. Looareesuwan, G. Pasvol, J. Poirriez, N. J. White, and G. Snounou. 2001. Association of genetic mutations in Plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob. Agents Chemother. 45:3122-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyaw, M.-P., M.-P. Kyaw, O. Myint, L. Myint, Z. Thaw, K.-H. Aye, and N.-N. Yin. 1993. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans. R. Soc. Trop. Med. Hyg. 87:687.. [DOI] [PubMed] [Google Scholar]
- 10.Leartsakulpanich, U., M. Imwong, S. Pukrittakayamee, N. J. White, G. Snounou, S. Sirawaraporn, and Y. Yuthavong. 2002. Molecular characterization of dihydrofolate reductase in relation to antifolate resistance in Plasmodium vivax. Mol. Biochem. Parasitol. 119:63-73. [DOI] [PubMed] [Google Scholar]
- 11.Matthews, D. A., R. A. Alden, J. T. Bolin, S. T. Freer, R. Hamlin, N. Xuong, J. Kraut, M. Poe, M. Williams, and K. Hoogsteen. 1977. Dihydrofolate reductase: X-ray structure of the binary complex with methotrexate. Science 197:452-455. [DOI] [PubMed] [Google Scholar]
- 12.Murphy, G. S., H. Basri, Purnomo, E. M. Andersen, M. J. Bangs, D. L. Mount, J. Gorden, A. A. Lal, A. Purwokusumo, S. Harjosuwarno, K. Sorensen, and S. L. Hoffman. 1993. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet 341:96-100. [DOI] [PubMed] [Google Scholar]
- 13.Peterson, D. S., W. K. Milhous, and T. E. Wellems. 1988. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc. Natl. Acad. Sci. USA 85:9114-9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pukrittayakamee, S., A. Chantra, J. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 16.Shallom, S., K. Zhang, L. Jiang, and P. K. Rathod. 1999. Essential protein-protein interactions between Plasmodium falciparum thymidylate synthase and dihydrofolate reductase domains. J. Biol. Chem. 274:37781-37786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirawaraporn, W., P. Prapunwattana, R. Sirawaraporn, Y. Yuthavong, and D. V. Santi. 1993. The dihydrofolate reductase domain of Plasmodium falciparum thymidylate synthase-dihydrofolate reductase: gene synthesis, expression, and anti-folate resistance mutants. J. Biol. Chem. 268:21637-21644. [PubMed] [Google Scholar]
- 18.Snewin, V. A., S. M. England, P. F. G. Sims, and J. E. Hyde. 1989. Characterisation of the dihydrofolate reductase-thymidylate synthase gene from human malaria parasites highly resistant to pyrimethamine. Gene 76:41-52. [DOI] [PubMed] [Google Scholar]
- 19.Thaithong, S., S. W. Chan, S. Songsomboon, P. Wilairat, N. Seesod, T. Sueblinwong, M. Goman, R. Ridley, and G. Beale. 1992. Pyrimethamine resistant mutations in Plasmodium falciparum. Mol. Biochem. Parasitol. 52:149-158. [DOI] [PubMed] [Google Scholar]
- 20.Volz, K. W., D. A. Matthews, R. A. Alden, S. T. Freer, C. Hansch, B. T. Kaufman, and J. Kraut. 1982. Crystal structure of avian dihydrofolate reductase containing phenyltriazine and NADPH. J. Biol. Chem. 257:2528-2536. [PubMed] [Google Scholar]
- 21.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yap, E. P. H., and J. O' D McGee. 1994. Non-isotopic single-strand conformation polymorphism, p. 165-177. In H. G. Griffin and A. M. Griffin (ed.), PCR technology: current innovations. CRC Press, Inc., Boca Raton, Fla.