Abstract
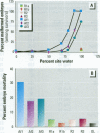
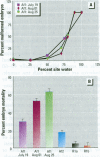
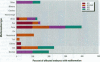
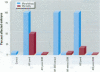
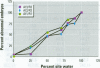
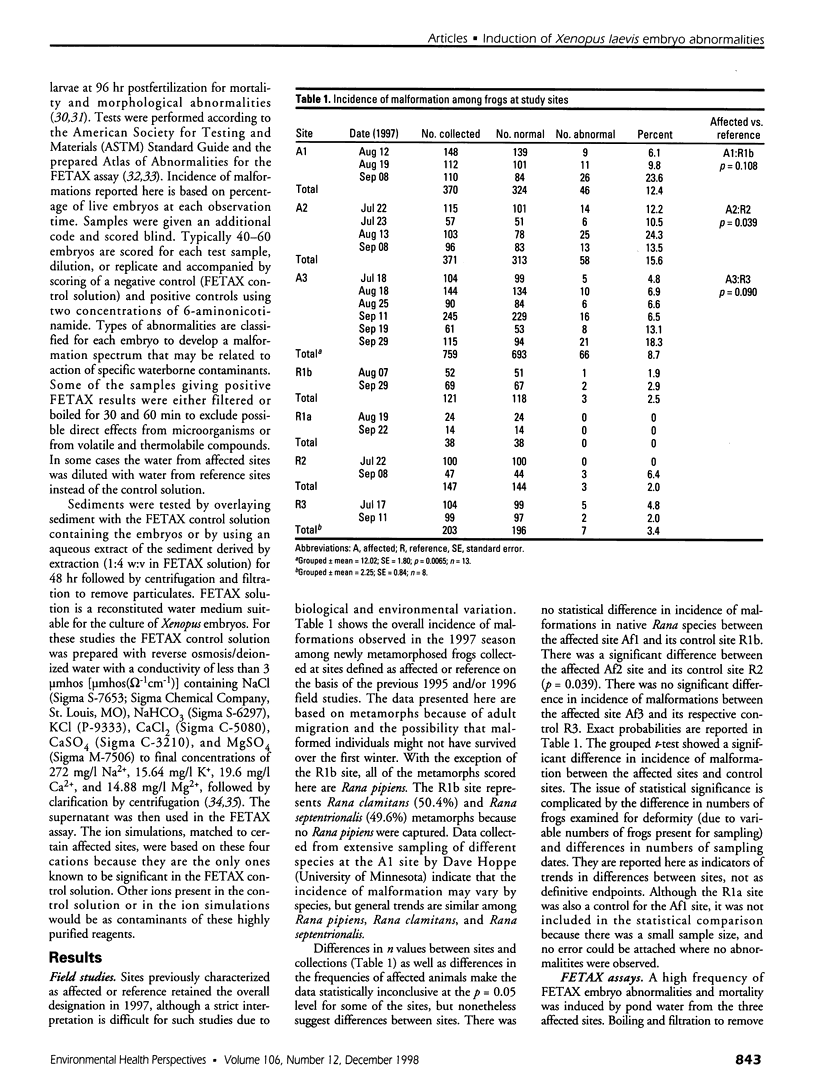
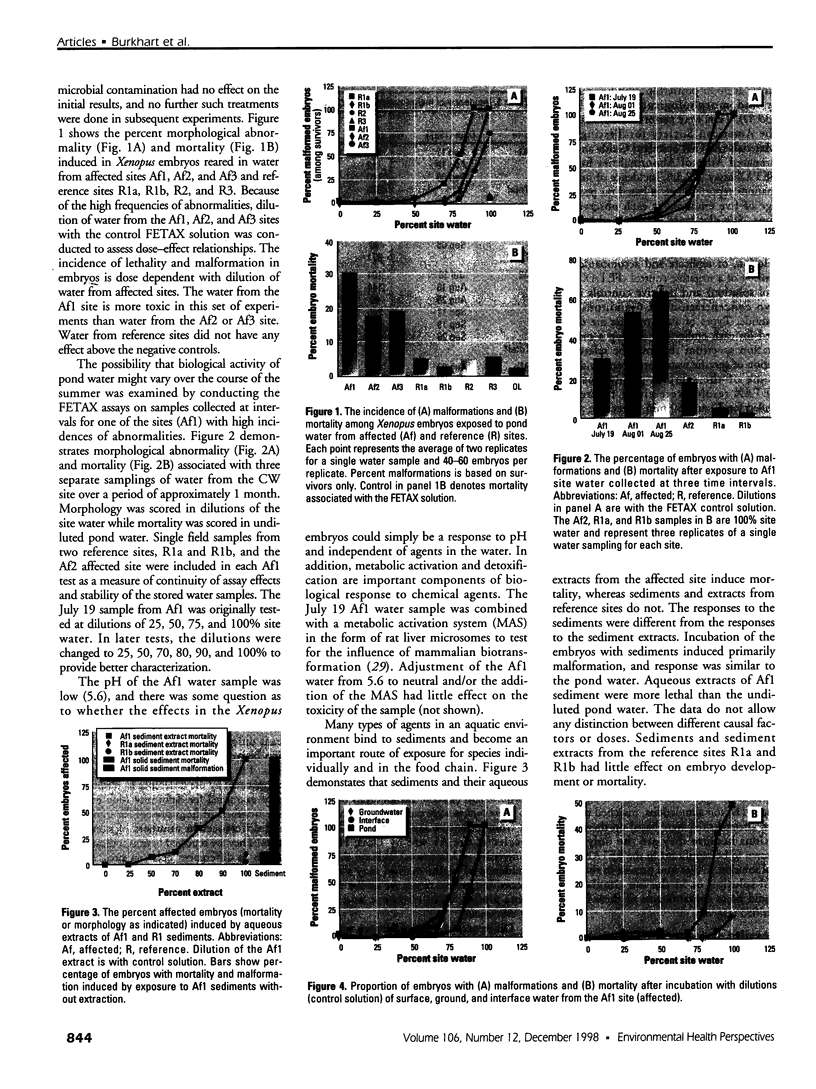
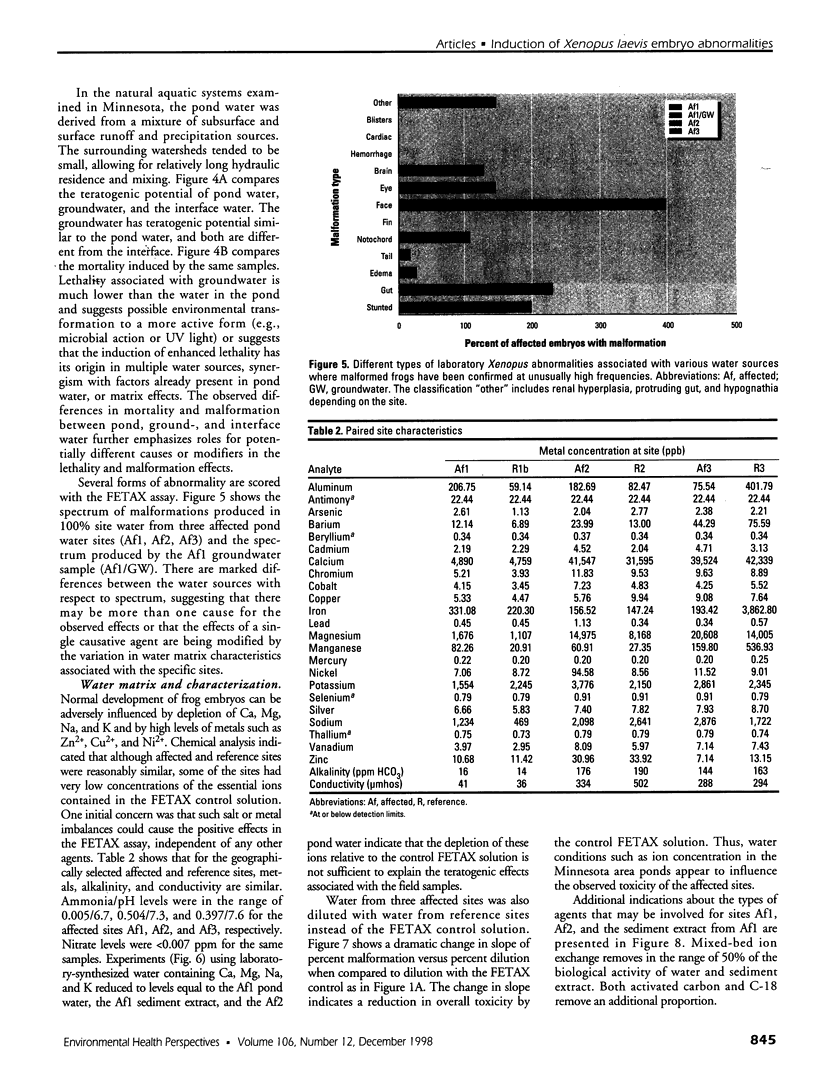
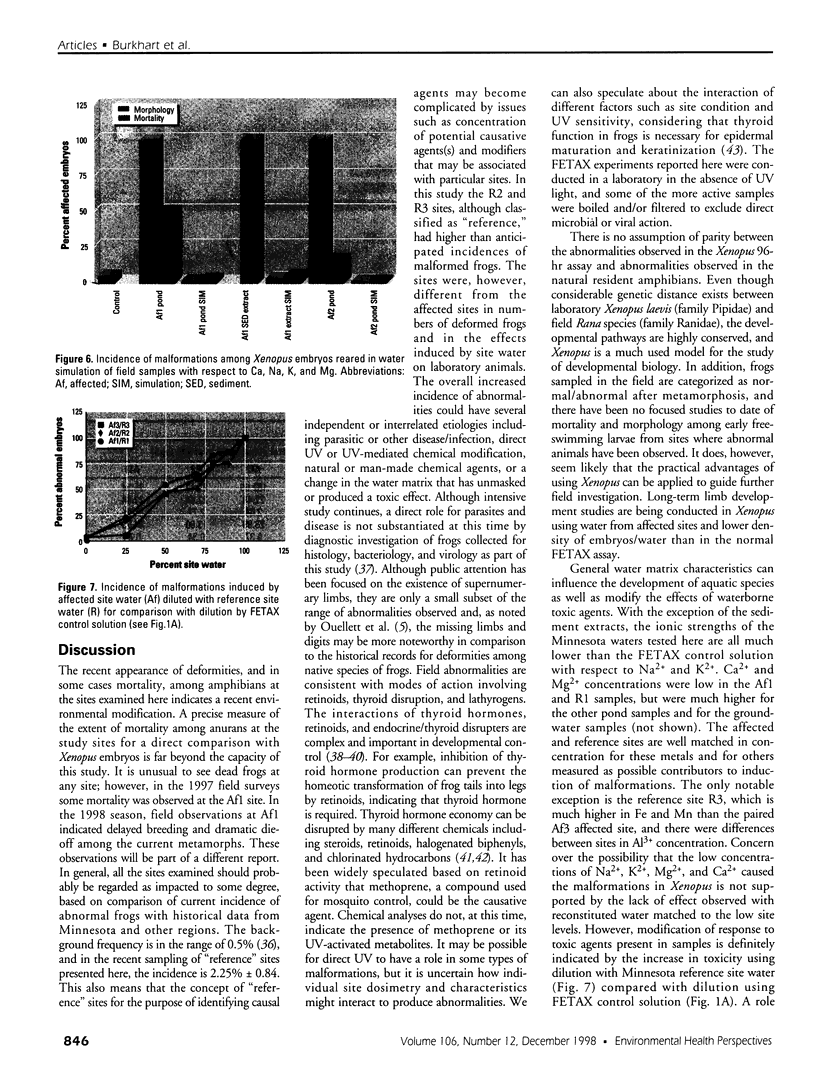
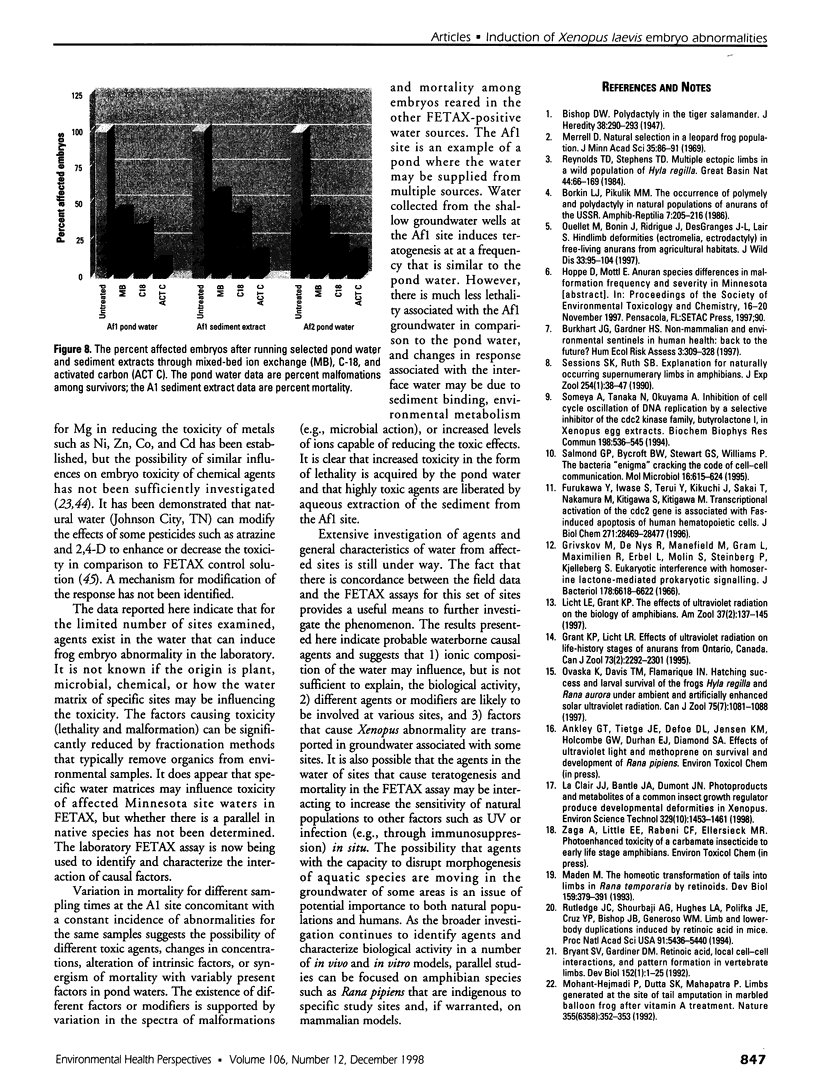
Water samples from several ponds in Minnesota were evaluated for their capacity to induce malformations in embryos of Xenopus laevis. The FETAX assay was used to assess the occurrence of malformations following a 96-hr period of exposure to water samples. These studies were conducted following reports of high incidences of malformation in natural populations of frogs in Minnesota wetlands. The purpose of these studies was to determine if a biologically active agent(s) was present in the waters and could be detected using the FETAX assay. Water samples from ponds with high incidences of frog malformations (affected sites), along with water samples from ponds with unaffected frog populations (reference sites), were studied. Initial experiments clearly showed that water from affected sites induced mortality and malformation in Xenopus embryos, while water from reference sites had little or no effect. Induction of malformation was dose dependent and highly reproducible, both with stored samples and with samples taken at different times throughout the summer. The biological activity of the samples was reduced or eliminated when samples were passed through activated carbon. Limited evidence from these samples indicates that the causal factor(s) is not an infectious organism nor are ion concentrations or metals responsible for the effects observed. Results do indicate that the water matrix has a significant effect on the severity of toxicity. Based on the FETAX results and the occurrence of frog malformations observed in the field, these studies suggest that water in the affected sites contains one or more unknown agents that induce developmental abnormalities in Xenopus. These same factors may contribute to the increased incidence of malformation in native species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breen J. J., Hickok N. J., Gurr J. A. The rat TSHbeta gene contains distinct response elements for regulation by retinoids and thyroid hormone. Mol Cell Endocrinol. 1997 Aug 8;131(2):137–146. doi: 10.1016/s0303-7207(97)00099-3. [DOI] [PubMed] [Google Scholar]
- Bryant S. V., Gardiner D. M. Retinoic acid, local cell-cell interactions, and pattern formation in vertebrate limbs. Dev Biol. 1992 Jul;152(1):1–25. doi: 10.1016/0012-1606(92)90152-7. [DOI] [PubMed] [Google Scholar]
- Clements C., Ralph S., Petras M. Genotoxicity of select herbicides in Rana catesbeiana tadpoles using the alkaline single-cell gel DNA electrophoresis (comet) assay. Environ Mol Mutagen. 1997;29(3):277–288. doi: 10.1002/(sici)1098-2280(1997)29:3<277::aid-em8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Dawson D. A., Bantle J. A. Development of a reconstituted water medium and preliminary validation of the frog embryo teratogenesis assay--Xenopus (FETAX). J Appl Toxicol. 1987 Aug;7(4):237–244. doi: 10.1002/jat.2550070403. [DOI] [PubMed] [Google Scholar]
- Fort D. J., Delphon J., Powers C. R., Helems R., Gonzalez R., Stover E. L. Development of automated methods of identifying toxicants in the environment. Bull Environ Contam Toxicol. 1995 Jan;54(1):104–111. doi: 10.1007/BF00196276. [DOI] [PubMed] [Google Scholar]
- Fort D. J., Stover E. L., Norton D. Ecological hazard assessment of aqueous soil extracts using FETAX. Frog Embryo Teratogenesis Assay--Xenopus. J Appl Toxicol. 1995 May-Jun;15(3):183–191. doi: 10.1002/jat.2550150308. [DOI] [PubMed] [Google Scholar]
- Furlow J. D., Berry D. L., Wang Z., Brown D. D. A set of novel tadpole specific genes expressed only in the epidermis are down-regulated by thyroid hormone during Xenopus laevis metamorphosis. Dev Biol. 1997 Feb 15;182(2):284–298. doi: 10.1006/dbio.1996.8478. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Iwase S., Terui Y., Kikuchi J., Sakai T., Nakamura M., Kitagawa S., Kitagawa M. Transcriptional activation of the cdc2 gene is associated with Fas-induced apoptosis of human hematopoietic cells. J Biol Chem. 1996 Nov 8;271(45):28469–28477. doi: 10.1074/jbc.271.45.28469. [DOI] [PubMed] [Google Scholar]
- Luo S. Q., Plowman M. C., Hopfer S. M., Sunderman F. W., Jr Mg(2+)-deprivation enhances and Mg(2+)-supplementation diminishes the embryotoxic and teratogenic effects of Ni2+, Co2+, Zn2+, and Cd2+ for frog embryos in the FETAX assay. Ann Clin Lab Sci. 1993 Mar-Apr;23(2):121–129. [PubMed] [Google Scholar]
- Maden M., Corcoran J. Role of thyroid hormone and retinoid receptors in the homeotic transformation of tails into limbs in frogs. Dev Genet. 1996;19(1):85–93. doi: 10.1002/(SICI)1520-6408(1996)19:1<85::AID-DVG9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Maden M. The homeotic transformation of tails into limbs in Rana temporaria by retinoids. Dev Biol. 1993 Oct;159(2):379–391. doi: 10.1006/dbio.1993.1249. [DOI] [PubMed] [Google Scholar]
- Mohanty-Hejmadi P., Dutta S. K., Mahapatra P. Limbs generated at site of tail amputation in marbled balloon frog after vitamin A treatment. Nature. 1992 Jan 23;355(6358):352–353. doi: 10.1038/355352a0. [DOI] [PubMed] [Google Scholar]
- Morgan M. K., Scheuerman P. R., Bishop C. S., Pyles R. A. Teratogenic potential of atrazine and 2,4-D using FETAX. J Toxicol Environ Health. 1996 Jun 7;48(2):151–168. doi: 10.1080/009841096161401. [DOI] [PubMed] [Google Scholar]
- Ouellet M., Bonin J., Rodrigue J., DesGranges J. L., Lair S. Hindlimb deformities (ectromelia, ectrodactyly) in free-living anurans from agricultural habitats. J Wildl Dis. 1997 Jan;33(1):95–104. doi: 10.7589/0090-3558-33.1.95. [DOI] [PubMed] [Google Scholar]
- Ralph S., Petras M. Genotoxicity monitoring of small bodies of water using two species of tadpoles and the alkaline single cell gel (comet) assay. Environ Mol Mutagen. 1997;29(4):418–430. doi: 10.1002/(sici)1098-2280(1997)29:4<418::aid-em11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Rutledge J. C., Shourbaji A. G., Hughes L. A., Polifka J. E., Cruz Y. P., Bishop J. B., Generoso W. M. Limb and lower-body duplications induced by retinoic acid in mice. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5436–5440. doi: 10.1073/pnas.91.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond G. P., Bycroft B. W., Stewart G. S., Williams P. The bacterial 'enigma': cracking the code of cell-cell communication. Mol Microbiol. 1995 May;16(4):615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Sessions S. K., Ruth S. B. Explanation for naturally occurring supernumerary limbs in amphibians. J Exp Zool. 1990 Apr;254(1):38–47. doi: 10.1002/jez.1402540107. [DOI] [PubMed] [Google Scholar]
- Someya A., Tanaka N., Okuyama A. Inhibition of cell cycle oscillation of DNA replication by a selective inhibitor of the cdc2 kinase family, butyrolactone I, in Xenopus egg extracts. Biochem Biophys Res Commun. 1994 Jan 28;198(2):536–545. doi: 10.1006/bbrc.1994.1079. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Lee C. H., Filipcik P., Chang L. Regulation of the mouse cellular retinoic acid-binding protein-I gene by thyroid hormone and retinoids in transgenic mouse embryos and P19 cells. J Endocrinol. 1997 Oct;155(1):35–46. doi: 10.1677/joe.0.1550035. [DOI] [PubMed] [Google Scholar]