Abstract
Longitudinal surveillance of Enterobacteriaceae for antimicrobial susceptibility is important because species of this family are among the most significant and prevalent human pathogens. To estimate rates of in vitro antimicrobial susceptibility among hospitalized patients in the United States, data from The Surveillance Network were studied for 14 agents tested against 10 species of Enterobacteriaceae (n = 384,279) isolated from intensive-care-unit (ICU) patients and non-ICU inpatients from 1998 to 2001. Cumulative susceptibility (percent) data for all species of Enterobacteriaceae isolated from ICU patients and non-ICU inpatients, respectively, were ranked as follows: ampicillin-sulbactam (45.5 and 57.2) ≪ ticarcillin-clavulanate (74.8 and 83.5) < trimethoprim-sulfamethoxazole (87.0 and 84.5) ≅ cefotaxime (82.9 and 92.6) = ceftazidime (82.3 and 91.0) = ceftriaxone (86.5 and 93.9) = piperacillin-tazobactam (83.5 and 90.5) < levofloxacin (89.3 and 90.6) = ciprofloxacin (91.0 and 91.7) < gentamicin (91.8 and 94.3) < cefepime (95.0 and 97.9) < amikacin (98.5 and 99.2) < imipenem (100 and 100) = meropenem (100 and 100). Of those agents studied only susceptibilities to ciprofloxacin (94 to 89%) and levofloxacin (93 to 89%) decreased in a stepwise manner from 1998 to 2001. Decreased fluoroquinolone susceptibility was most pronounced for Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. For all species of Enterobacteriaceae, trimethoprim-sulfamethoxazole resistance was more commonly observed in isolates with a single-drug resistance phenotype while gentamicin and fluoroquinolone resistances were more common in isolates resistant to at least one additional class of antimicrobial agent. Ongoing surveillance of Enterobacteriaceae will be particularly important to monitor changes in fluoroquinolone susceptibility, as well as changes in the prevalence of isolates resistant to multiple classes of antimicrobial agents.
Members of the family Enterobacteriaceae are among the most important bacterial human pathogens. They comprise approximately 80% of gram-negative bacteria and 50% of all isolates identified in hospital laboratories in the United States (6). Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterobacter spp., and Serratia marcescens account for the majority of Enterobacteriaceae isolated from clinical specimens. Risk factors for nosocomial gram-negative bacterial infections are widely known and include prior antimicrobial agent use, prolonged hospitalization, advanced age, severity of comorbid illnesses, immunosuppression associated with chemotherapy for malignancy and organ transplantation, invasive monitoring techniques (e.g., indwelling lines, catheters, and endotracheal tubes), and mechanical ventilation. Monitoring for antimicrobial resistance in species of Enterobacteriaceae in hospitalized patients is important because resistance has been reported elsewhere to be associated with increased patient morbidity and mortality, prolonged hospitalization, and increased hospital expenditures particularly for gram-negative bacteremia and ventilator-associated pneumonia (5, 19). Determining susceptibility patterns by location within the hospital (i.e., intensive-care units [ICUs] and non-ICU locations) may identify substantial differences that would be obscured if hospital-wide data were aggregated (7).
Antimicrobial resistance is increasing in many species of Enterobacteriaceae as well as in other gram-negative, gram-positive, and anaerobic bacteria. Current antimicrobial resistance issues for Enterobacteriaceae include the emergence and proliferation of extended-spectrum β-lactamases, β-lactamase-inhibitor-resistant TEM enzymes, stably derepressed and plasmid-encoded AmpC cephalosporinases, fluoroquinolone resistance, and the dissemination of multidrug-resistant (MDR) strains (3). MDR strains have arisen in a multitude of bacterial species including most species of Enterobacteriaceae and are of particular concern because of their potential for widespread dissemination, acquisition of additional resistance elements, and complications in therapeutic management of infected patients, particularly in seriously ill patients. Access to current antimicrobial susceptibility data is of importance to all healthcare providers but is of particular significance to physicians treating hospitalized patients. The present study was undertaken to determine the in vitro activities of 14 commonly tested antimicrobial agents against 10 of the most common, clinically relevant species of Enterobacteriaceae isolated from ICU patients and non-ICU inpatients in U.S. hospitals from 1998 to 2001.
MATERIALS AND METHODS
Antimicrobial susceptibility testing results.
The Surveillance Network (TSN) Database-USA (Focus Technologies, Herndon, Va.) was used as the source of antimicrobial susceptibility testing results for this study. TSN electronically assimilates antimicrobial susceptibility testing and patient demographic data from a network of hospitals in the United States (23). The number of U.S. laboratories participating in TSN increased from 186 in 1998 to 232 in 1999, 258 in 2000, and 270 in 2001. Laboratories are included in TSN based on factors such as hospital bed size, patient population, geographic location, and antimicrobial susceptibility testing methods used (23). Susceptibility testing of patient isolates is conducted onsite by each participating laboratory as a part of their routine diagnostic testing. Only data generated by Food and Drug Administration-approved testing methods with MIC results interpreted according to NCCLS recommendations (17) are included in TSN. In addition, a series of quality-control filters (i.e., critical rule sets) are used to screen susceptibility test results for patterns indicative of testing error; suspect results are removed from the analyzable data set for laboratory confirmation.
The antimicrobial susceptibility testing results included in the present analysis were restricted to 126 U.S. laboratories that participated in TSN from 1998 to 2001 and that reported results for >100 isolates of Enterobacteriaceae per year from hospital inpatients. Isolates were restricted to the first isolate per patient, per bacterial species, per year. Data from ICU patients were analyzed separately and together with data from non-ICU hospital inpatients; data from patients in nursing facilities and hospital outpatients were excluded from the analysis. In TSN, all isolates are not tested with all antimicrobial agents, and variation can be observed for antimicrobial agents of the same class such as extended-spectrum cephalosporins (cefotaxime and ceftriaxone) and fluoroquinolones (ciprofloxacin and levofloxacin) for which similar in vitro activities have previously been demonstrated.
RESULTS
In vitro susceptibilities to 14 antimicrobial agents for clinical isolates of 10 common species of Enterobacteriaceae are depicted in Table 1. Cumulative 1998 to 2001 data are presented separately for isolates from patients in ICUs and for those from non-ICU inpatients. For all Enterobacteriaceae, susceptibility to all agents except trimethoprim-sulfamethoxazole (SXT) was greater for isolates from non-ICU inpatients than for isolates from ICU patients: differences exceeded 5% for ampicillin-sulbactam (11.7%), cefotaxime (9.7%), ticarcillin-clavulanate (8.7%), ceftazidime (8.7%), ceftriaxone (7.4%), and piperacillin-tazobactam (7.0%). Susceptibilities to ampicillin-sulbactam were lower than susceptibilities to any other agent for 9 of the 10 species of Enterobacteriaceae studied: P. mirabilis was the one exception for which susceptibilities were lower to ciprofloxacin, levofloxacin, and SXT than to ampicillin-sulbactam. For all isolates of Enterobacteriaceae studied, ampicillin-sulbactam susceptibilities among both ICU patients (45.5%) and non-ICU inpatients (57.2%) were >25% lower than for ticarcillin-clavulanate, the agent with the next lowest rate of susceptibility.
TABLE 1.
In vitro antimicrobial agent susceptibilities of 10 species of Enterobacteriaceae reported by 126 clinical laboratories in the United States (cumulative 1998 to 2001 results)
| Organism(s) | Antimicrobial agent | No. of isolate results
|
% Susceptible
|
% Intermediate
|
% Resistant
|
||||
|---|---|---|---|---|---|---|---|---|---|
| ICU | Non-ICU | ICU | Non-ICU | ICU | Non-ICU | ICU | Non-ICU | ||
| E. coli | Amikacin | 7,101 | 82,309 | 99.2 | 99.6 | 0.4 | 0.2 | 0.4 | 0.2 |
| Ampicillin-sulbactam | 9,883 | 86,372 | 58.4 | 59.7 | 18.2 | 20.1 | 23.4 | 20.2 | |
| Cefepime | 4,655 | 42,532 | 98.2 | 99.1 | 0.4 | 0.2 | 1.5 | 0.6 | |
| Cefotaxime | 5,499 | 54,404 | 96.8 | 96.8 | 0.9 | 0.6 | 2.3 | 0.6 | |
| Ceftazidime | 9,877 | 89,839 | 95.9 | 97.4 | 1.2 | 0.9 | 3.0 | 1.7 | |
| Ceftriaxone | 11,384 | 117,495 | 98.0 | 98.9 | 0.6 | 0.5 | 1.5 | 0.7 | |
| Ciprofloxacin | 12,520 | 125,671 | 93.5 | 94.4 | 0.2 | 0.1 | 6.3 | 5.5 | |
| Gentamicin | 15,199 | 158,512 | 93.8 | 95.8 | 0.6 | 0.4 | 5.6 | 3.8 | |
| Imipenem | 9,796 | 90,933 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 6,752 | 86,968 | 89.2 | 92.3 | 0.2 | 0.3 | 10.6 | 7.4 | |
| Meropenem | 1,212 | 9,741 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 7,100 | 59,522 | 92.5 | 95.4 | 3.5 | 2.8 | 4.0 | 1.9 | |
| Ticarcillin-clavulanate | 6,209 | 60,328 | 79.8 | 83.7 | 11.5 | 10.0 | 8.8 | 6.2 | |
| SXT | 14,994 | 163,766 | 81.3 | 81.6 | 0.2 | 0.1 | 18.6 | 18.3 | |
| K. pneumoniae | Amikacin | 5,748 | 32,107 | 98.1 | 98.7 | 0.8 | 0.4 | 1.1 | 0.8 |
| Ampicillin-sulbactam | 7,538 | 36,257 | 69.9 | 75.0 | 14.8 | 12.5 | 15.3 | 12.4 | |
| Cefepime | 3,867 | 18,778 | 95.3 | 96.9 | 0.8 | 0.7 | 3.9 | 2.4 | |
| Cefotaxime | 4,412 | 20,970 | 92.8 | 95.8 | 2.6 | 2.0 | 4.6 | 2.2 | |
| Ceftazidime | 7,258 | 35,556 | 88.6 | 92.3 | 1.8 | 1.2 | 9.6 | 6.6 | |
| Ceftriaxone | 7,533 | 41,302 | 92.9 | 95.7 | 3.0 | 2.0 | 4.2 | 2.3 | |
| Ciprofloxacin | 8,489 | 44,267 | 91.3 | 93.0 | 1.4 | 1.1 | 7.3 | 5.9 | |
| Gentamicin | 10,260 | 55,921 | 91.7 | 94.7 | 1.0 | 0.9 | 7.3 | 4.4 | |
| Imipenem | 7,457 | 36,344 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 4,560 | 31,015 | 91.2 | 93.3 | 2.1 | 1.6 | 6.7 | 5.1 | |
| Meropenem | 960 | 4,725 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 5,675 | 24,981 | 86.9 | 90.0 | 5.7 | 5.1 | 7.4 | 4.9 | |
| Ticarcillin-clavulanate | 5,060 | 23,530 | 83.8 | 87.4 | 4.4 | 4.1 | 11.8 | 8.6 | |
| SXT | 10,026 | 56,688 | 89.2 | 89.1 | 0.3 | 0.2 | 10.5 | 10.7 | |
| E. aerogenes | Amikacin | 1,992 | 6,003 | 98.7 | 97.8 | 0.4 | 0.9 | 0.9 | 1.3 |
| Ampicillin-sulbactam | 2,881 | 7,055 | 23.0 | 29.3 | 18.2 | 21.3 | 58.8 | 49.4 | |
| Cefepime | 1,319 | 3,723 | 88.7 | 98.2 | 0.9 | 0.8 | 10.4 | 1.0 | |
| Cefotaxime | 1,731 | 4,322 | 62.3 | 76.5 | 22.0 | 16.6 | 15.7 | 16.9 | |
| Ceftazidime | 2,866 | 7,422 | 58.8 | 71.2 | 6.6 | 6.0 | 34.6 | 22.9 | |
| Ceftriaxone | 2,921 | 8,197 | 68.0 | 77.9 | 20.2 | 15.9 | 11.8 | 6.2 | |
| Ciprofloxacin | 2,955 | 8,120 | 93.3 | 93.3 | 0.8 | 1.1 | 5.9 | 5.6 | |
| Gentamicin | 3,675 | 10,320 | 92.5 | 96.2 | 0.1 | 0.4 | 7.4 | 3.4 | |
| Imipenem | 2,794 | 7,257 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 1,626 | 5,627 | 95.1 | 94.5 | 1.6 | 1.2 | 3.3 | 4.4 | |
| Meropenem | 435 | 941 | 99.5 | 100 | 0 | 0 | 0.5 | 0 | |
| Piperacillin-tazobactam | 1,818 | 4,593 | 67.5 | 73.6 | 23.6 | 17.5 | 8.9 | 8.9 | |
| Ticarcillin-clavulanate | 1,836 | 4,395 | 55.0 | 67.1 | 11.6 | 10.7 | 33.4 | 22.1 | |
| SXT | 3,530 | 10,365 | 96.1 | 94.8 | 0.2 | 0.2 | 3.7 | 5.0 | |
| E. cloacae | Amikacin | 4,361 | 14,082 | 98.5 | 99.0 | 0.6 | 0.5 | 0.9 | 0.5 |
| Ampicillin-sulbactam | 5,584 | 15,537 | 14.5 | 18.0 | 14.1 | 16.3 | 71.4 | 65.6 | |
| Cefepime | 3,078 | 8,929 | 91.1 | 94.6 | 3.0 | 1.6 | 5.9 | 3.8 | |
| Cefotaxime | 3,705 | 11,404 | 57.8 | 66.3 | 8.3 | 8.4 | 33.9 | 25.3 | |
| Ceftazidime | 5,872 | 17,522 | 59.4 | 67.5 | 3.5 | 3.6 | 37.0 | 28.9 | |
| Ceftriaxone | 6,012 | 18,629 | 63.1 | 69.7 | 8.1 | 7.3 | 28.9 | 23.0 | |
| Ciprofloxacin | 6,152 | 18,077 | 89.7 | 89.6 | 1.7 | 1.5 | 8.6 | 8.9 | |
| Gentamicin | 7,428 | 23,383 | 90.3 | 91.2 | 1.0 | 0.9 | 8.7 | 7.8 | |
| Imipenem | 5,844 | 16,894 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 3,194 | 13,058 | 88.4 | 89.6 | 2.6 | 2.1 | 9.1 | 8.3 | |
| Meropenem | 800 | 2,453 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 4,174 | 10,806 | 64.6 | 70.8 | 13.6 | 10.3 | 21.8 | 18.9 | |
| Ticarcillin-clavulanate | 3,710 | 10,305 | 53.4 | 63.7 | 9.1 | 9.2 | 37.5 | 27.1 | |
| SXT | 7,120 | 23,332 | 88.4 | 87.9 | 0.1 | 0.2 | 11.6 | 12.0 | |
| C. freundii | Amikacin | 933 | 5,379 | 97.7 | 98.0 | 0.5 | 0.4 | 1.7 | 1.6 |
| Ampicillin-sulbactam | 1,118 | 6,015 | 37.5 | 53.8 | 7.4 | 9.7 | 55.1 | 36.5 | |
| Cefepime | 620 | 3,078 | 96.0 | 98.4 | 1.6 | 0.6 | 2.4 | 1.0 | |
| Cefotaxime | 685 | 3,525 | 54.6 | 72.9 | 21.2 | 15.3 | 24.2 | 11.8 | |
| Ceftazidime | 1,184 | 6,321 | 50.6 | 67.9 | 3.6 | 3.8 | 45.8 | 28.2 | |
| Ceftazidime | 1,184 | 6,321 | 50.6 | 67.9 | 3.6 | 3.8 | 45.8 | 28.2 | |
| Ceftriaxone | 1,227 | 7,348 | 55.8 | 72.4 | 16.4 | 11.9 | 27.8 | 15.7 | |
| Ciprofloxacin | 1,322 | 7,303 | 81.4 | 83.9 | 3.2 | 2.1 | 15.4 | 14.0 | |
| Gentamicin | 1,586 | 9,178 | 83.2 | 89.5 | 1.7 | 1.5 | 15.1 | 9.0 | |
| Imipenem | 1,197 | 6,058 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 731 | 5,075 | 80.6 | 84.1 | 5.1 | 2.4 | 14.4 | 13.5 | |
| Meropenem | 170 | 710 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 798 | 4,005 | 65.9 | 74.7 | 19.7 | 14.7 | 14.4 | 10.6 | |
| Ticarcillin-clavulanate | 788 | 3,641 | 41.4 | 65.7 | 3.7 | 4.3 | 54.9 | 30.0 | |
| SXT | 1,552 | 9,246 | 83.8 | 78.7 | 0.3 | 0.1 | 15.9 | 21.1 | |
| M. morganii | Amikacin | 396 | 3.191 | 99.0 | 99.4 | 0.8 | 0.3 | 0.3 | 0.3 |
| Ampicillin-sulbactam | 518 | 3,767 | 30.3 | 31.3 | 19.9 | 20.1 | 49.8 | 48.6 | |
| Cefepime | 256 | 1,909 | 96.5 | 97.9 | 0.4 | 0.7 | 3.1 | 1.4 | |
| Cefotaxime | 294 | 2,117 | 79.3 | 85.4 | 11.9 | 9.6 | 8.8 | 5.0 | |
| Ceftazidime | 519 | 3,750 | 77.5 | 79.6 | 6.0 | 5.3 | 16.6 | 15.1 | |
| Ceftriaxone | 552 | 4,485 | 92.8 | 92.2 | 5.1 | 5.9 | 2.2 | 1.9 | |
| Ciprofloxacin | 600 | 4,292 | 82.8 | 78.2 | 1.2 | 0.9 | 16.0 | 20.9 | |
| Gentamicin | 718 | 5,422 | 85.5 | 85.4 | 1.7 | 1.5 | 12.8 | 13.1 | |
| Imipenem | 512 | 3,529 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 330 | 3,042 | 80.9 | 78.1 | 1.5 | 1.4 | 17.6 | 20.5 | |
| Meropenem | 75 | 535 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 402 | 2,587 | 92.3 | 92.7 | 3.0 | 2.7 | 4.7 | 4.5 | |
| Ticarcillin-clavulanate | 330 | 2,387 | 85.8 | 82.6 | 7.0 | 7.8 | 7.3 | 9.6 | |
| SXT | 697 | 5,442 | 81.1 | 77.3 | 0.1 | 0.1 | 18.8 | 22.7 | |
| P. mirabilis | Amikacin | 1,693 | 16,648 | 99.2 | 99.4 | 0.4 | 0.3 | 0.4 | 0.3 |
| Ampicillin-sulbactam | 1,991 | 16,446 | 91.0 | 91.9 | 4.8 | 4.2 | 4.2 | 3.9 | |
| Cefepime | 1,131 | 8,345 | 97.3 | 97.4 | 1.1 | 1.1 | 1.6 | 1.5 | |
| Cefotaxime | 1,164 | 10,785 | 99.4 | 99.3 | 0.3 | 0.3 | 0.3 | 0.3 | |
| Ceftazidime | 1,957 | 16,722 | 98.6 | 98.4 | 0.7 | 0.9 | 0.7 | 0.7 | |
| Ceftriaxone | 2,294 | 22,279 | 99.7 | 99.4 | 0.1 | 0.3 | 0.2 | 0.3 | |
| Ciprofloxacin | 2,407 | 22,381 | 90.0 | 86.4 | 1.0 | 0.9 | 9.1 | 12.6 | |
| Gentamicin | 2,957 | 28,885 | 92.5 | 92.0 | 0.7 | 1.5 | 6.7 | 6.6 | |
| Imipenem | 2,037 | 17,180 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 1,387 | 16,413 | 87.7 | 84.0 | 0.8 | 1.6 | 11.5 | 14.4 | |
| Meropenem | 237 | 1,804 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 1,694 | 12,003 | 97.3 | 97.2 | 1.8 | 1.6 | 0.9 | 1.1 | |
| Ticarcillin-clavulanate | 1,305 | 12,036 | 99.6 | 99.6 | 0.3 | 0.3 | 0.1 | 0.1 | |
| SXT | 2,912 | 29,461 | 86.4 | 84.7 | 0.1 | 0.1 | 13.5 | 15.2 | |
| P. vulgaris | Amikacin | 83 | 753 | 98.8 | 99.2 | 1.2 | 0 | 0 | 0.8 |
| Ampicillin-sulbactam | 119 | 878 | 63.0 | 74.0 | 32.8 | 21.0 | 4.2 | 5.0 | |
| Cefepime | 45 | 442 | 97.8 | 98.0 | 0 | 0.7 | 2.2 | 1.4 | |
| Cefotaxime | 64 | 474 | 76.6 | 86.5 | 4.7 | 6.1 | 18.8 | 7.4 | |
| Ceftazidime | 122 | 892 | 97.5 | 97.8 | 1.6 | 1.2 | 0.8 | 1.0 | |
| Ceftriaxone | 133 | 1,071 | 75.9 | 73.6 | 12.8 | 16.6 | 11.3 | 9.8 | |
| Ciprofloxacin | 133 | 1,025 | 100 | 99.1 | 0 | 0.4 | 0 | 0.5 | |
| Gentamicin | 160 | 1,269 | 99.4 | 98.3 | 0.6 | 0.5 | 0 | 1.2 | |
| Imipenem | 116 | 811 | 100 | 99.4 | 0 | 0.6 | 0 | 0 | |
| Levofloxacin | 64 | 620 | 100 | 98.7 | 0 | 0.2 | 0 | 1.1 | |
| Meropenem | 14 | 105 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 86 | 597 | 98.8 | 97.3 | 1.2 | 2.0 | 0 | 0.7 | |
| Ticarcillin-clavulanate | 80 | 536 | 98.8 | 99.3 | 1.3 | 0.6 | 0 | 0.2 | |
| SXT | 158 | 1,279 | 97.5 | 95.2 | 0 | 0.1 | 2.5 | 4.8 | |
| Providencia spp. | Amikacin | 229 | 2,161 | 97.4 | 99.1 | 1.7 | 0.6 | 0.9 | 0.3 |
| Ampicillin-sulbactam | 267 | 2,172 | 17.6 | 22.6 | 34.8 | 44.2 | 47.6 | 33.2 | |
| Cefepime | 160 | 1,061 | 92.5 | 95.8 | 0.6 | 1.6 | 6.9 | 2.6 | |
| Cefotaxime | 167 | 1,398 | 92.8 | 94.3 | 6.0 | 4.4 | 1.2 | 1.2 | |
| Ceftazidime | 257 | 2,253 | 82.5 | 87.7 | 5.1 | 3.0 | 12.5 | 9.3 | |
| Ceftriaxone | 283 | 2,792 | 95.8 | 98.1 | 2.8 | 1.2 | 1.4 | 0.7 | |
| Ciprofloxacin | 303 | 2,564 | 38.9 | 47.1 | 2.0 | 3.0 | 59.1 | 49.9 | |
| Gentamicin | 356 | 3,382 | 74.7 | 72.4 | 6.7 | 7.7 | 18.5 | 19.8 | |
| Imipenem | 256 | 2,057 | 99.6 | 99.9 | 0.4 | 0.1 | 0 | 0 | |
| Levofloxacin | 176 | 1,963 | 34.1 | 45.6 | 4.5 | 6.1 | 61.4 | 48.3 | |
| Meropenem | 26 | 239 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 217 | 1,454 | 78.8 | 89.5 | 14.7 | 8.3 | 6.5 | 2.2 | |
| Ticarcillin-clavulanate | 170 | 1,458 | 98.8 | 97.3 | 0.6 | 1.6 | 0.6 | 1.1 | |
| SXT | 348 | 3,412 | 69.0 | 71.5 | 0.3 | 0.4 | 30.7 | 28.1 | |
| S. marcescens | Amikacin | 2,703 | 8,112 | 96.8 | 98.4 | 0.6 | 0.7 | 2.6 | 0.9 |
| Ampicillin-sulbactam | 3,751 | 9,463 | 7.3 | 8.6 | 10.8 | 12.7 | 81.9 | 78.7 | |
| Cefepime | 1,917 | 5,191 | 95.4 | 97.2 | 0.8 | 0.9 | 3.9 | 2.0 | |
| Cefotaxime | 2,101 | 5,873 | 87.3 | 88.1 | 6.8 | 7.0 | 5.9 | 4.9 | |
| Ceftazidime | 3,579 | 9,922 | 90.6 | 90.2 | 1.8 | 2.1 | 7.6 | 7.7 | |
| Ceftriaxone | 3,734 | 10,595 | 91.1 | 91.8 | 4.8 | 4.8 | 4.0 | 3.5 | |
| Ciprofloxacin | 3,847 | 10,236 | 91.9 | 89.8 | 1.9 | 2.9 | 6.2 | 7.4 | |
| Gentamicin | 4,634 | 12,911 | 91.5 | 95.3 | 0.5 | 0.9 | 8.0 | 3.8 | |
| Imipenem | 3,684 | 9,435 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 2,224 | 7,386 | 92.4 | 93.2 | 3.1 | 1.9 | 4.6 | 4.9 | |
| Meropenem | 505 | 1,483 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 2,684 | 6,534 | 87.9 | 88.9 | 6.3 | 5.9 | 5.7 | 5.2 | |
| Ticarcillin-clavulanate | 2,127 | 5,824 | 85.6 | 86.3 | 6.6 | 8.8 | 7.9 | 4.9 | |
| SXT | 4,496 | 12,932 | 95.7 | 95.0 | 0.1 | 0.3 | 4.2 | 4.6 | |
| Enterobacteriaceae | Amikacin | 25,239 | 170,745 | 98.5 | 99.2 | 0.6 | 0.3 | 1.0 | 0.5 |
| Ampicillin-sulbactam | 33,645 | 183,962 | 45.5 | 57.2 | 15.0 | 16.5 | 39.6 | 26.4 | |
| Cefepime | 17,048 | 93,988 | 95.0 | 97.9 | 1.1 | 0.6 | 3.9 | 1.5 | |
| Cefotaxime | 19,822 | 115,272 | 82.9 | 92.6 | 6.0 | 3.2 | 11.1 | 4.2 | |
| Ceftazidime | 33,491 | 190,199 | 82.3 | 91.0 | 2.4 | 1.7 | 15.2 | 7.4 | |
| Ceftriaxone | 36,073 | 234,193 | 86.5 | 93.9 | 5.0 | 2.6 | 8.6 | 3.5 | |
| Ciprofloxacin | 38,728 | 243,936 | 91.0 | 91.7 | 1.1 | 0.7 | 7.9 | 7.5 | |
| Gentamicin | 46,973 | 309,183 | 91.8 | 94.3 | 0.8 | 0.8 | 7.4 | 4.9 | |
| Imipenem | 33,693 | 190,498 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 21,044 | 171,167 | 89.3 | 90.6 | 1.6 | 1.0 | 9.0 | 8.4 | |
| Meropenem | 4,434 | 22,736 | 100 | 100 | 0 | 0 | 0 | 0 | |
| Piperacillin-tazobactam | 24,648 | 127,082 | 83.5 | 90.5 | 8.0 | 4.9 | 8.5 | 4.6 | |
| Ticarcillin-clavulanate | 21,615 | 124,440 | 74.8 | 83.5 | 7.8 | 7.5 | 17.5 | 9.0 | |
| SXT | 45,833 | 315,923 | 87.0 | 84.5 | 0.2 | 0.1 | 12.8 | 15.4 | |
Essentially all isolates of Enterobacteriaceae studied were susceptible to the carbapenems, imipenem and meropenem. Three carbapenem-resistant isolates were identified, a resistance rate of 0.001% (3 of 235,042) (data not shown) for all Enterobacteriaceae tested with a carbapenem. Two of the three carbapenem-resistant isolates were Enterobacter aerogenes, and the remaining isolate was E. coli. Ceftazidime-nonsusceptible isolates were more common among ICU patients (4.2 and 11.4%) than among non-ICU inpatients (2.6 and 7.8%) for both E. coli and K. pneumoniae, respectively. Cefotaxime, ceftriaxone, and ceftazidime susceptibilities exceeded 90% for isolates of Enterobacteriaceae from non-ICU inpatients but were up to 10% lower (cefotaxime) among isolates from ICU patients.
Amikacin had higher susceptibilities against all species of Enterobacteriaceae than did gentamicin; the difference between the two aminoglycosides was greatest (>20%) for Providencia spp. Ciprofloxacin and levofloxacin demonstrated similar activities with 89.3 to 91.7% of Enterobacteriaceae isolates susceptible to fluoroquinolones for both ICU patients and non-ICU inpatients. Differences in susceptibility rates for isolates from ICUs and from non-ICU inpatients were similar (<3% difference) for amikacin (0.7%), ciprofloxacin (0.7%), levofloxacin (1.3%), gentamicin (2.5%), and cefepime (2.9%).
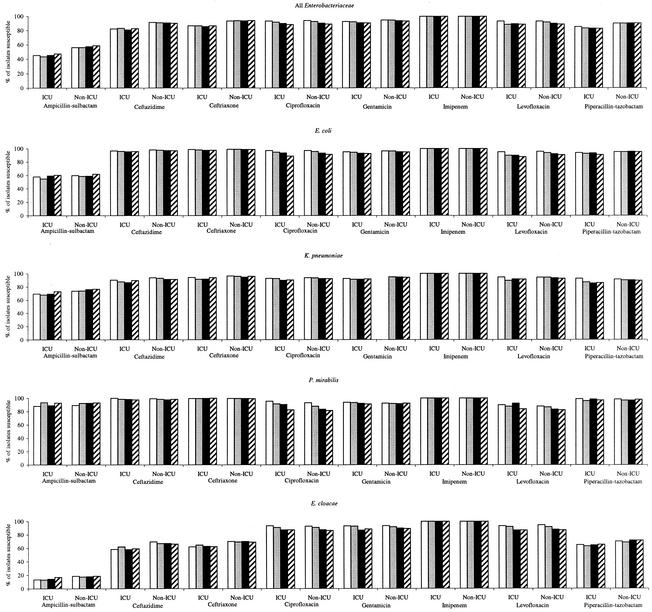
Figure 1 summarizes the susceptibilities to eight antimicrobial agents by year from 1998 to 2001 for all Enterobacteriaceae and, individually, for the four most commonly isolated species of Enterobacteriaceae. Rates of susceptibility for all Enterobacteriaceae from ICU and non-ICU inpatients demonstrated minor variations (<5%) between years for ampicillin-sulbactam, ceftazidime, ceftriaxone, and piperacillin-tazobactam but did not demonstrate a trend toward decreased susceptibility from 1998 to 2001. Imipenem susceptibility was 100% for all Enterobacteriaceae in all four years studied. Gentamicin susceptibility among all Enterobacteriaceae varied by <2% from 1998 to 2001; however, small stepwise decreases over time were observed for Enterobacter cloacae for isolates from both ICU patients (93.4 to 88.6%) and non-ICU inpatients (93.6 to 89.2%). Among all Enterobacteriaceae, susceptibilities declined in a stepwise manner for ciprofloxacin (94 to 89%) and levofloxacin (93 to 89%) from 1998 to 2001. The decline in fluoroquinolone activity was observed for all four species in Fig. 1 but was more apparent for E. coli, P. mirabilis, and E. cloacae than for K. pneumoniae.
FIG. 1.
Annual antimicrobial susceptibilities of Enterobacteriaceae from 1998 to 2001 for ICU patients and non-ICU inpatients. Open bars, 1998 data; lightly shaded bars, 1999 data; black bars, 2000 data; hatched bars, 2001 data.
Table 2 summarizes the prevalence and composition of coresistance phenotypes of 10 species of Enterobacteriaceae for combined ICU and non-ICU inpatient isolates from 2001. Only isolates concurrently tested with ceftazidime, gentamicin, levofloxacin, and SXT (as representatives of different antimicrobial classes) were included in Table 2. Ampicillin-sulbactam was not included in this analysis, and data for ceftazidime, as the β-lactam class representative, cannot be used to estimate ampicillin-sulbactam activity. Pan-susceptible isolates (isolates susceptible to ceftazidime, gentamicin, levofloxacin, and SXT) were most common for Proteus vulgaris (88.2%), S. marcescens (84.2%), and K. pneumoniae (82.7%) and least common for Morganella morganii (57.7%) and Providencia spp. (36.9%). Coresistance phenotypes (isolates resistant to two or more of the drugs ceftazidime, gentamicin, levofloxacin, and SXT) most commonly involved resistance to a fluoroquinolone and SXT. SXT resistance was most common in E. coli (13.0%), and ceftazidime resistance was most common in E. aerogenes (15.6%), E. cloacae (16.4%), and Citrobacter freundii (16.2%). Among antimicrobial-resistant isolates, resistance to a single agent was more common than coresistance for E. coli, E. aerogenes, E. cloacae, C. freundii, P. vulgaris, and S. marcescens but not for K. pneumoniae, M. morganii, P. mirabilis, and Providencia spp. Ceftazidime and SXT resistance was more commonly observed in isolates with a single-drug resistance phenotype than in isolates with other resistances present. Gentamicin and levofloxacin resistance was more commonly associated with coresistance phenotypes than reported as a single resistance phenotype for all species of Enterobacteriaceae except P. vulgaris.
TABLE 2.
Prevalence and composition of coresistance phenotypesa for 10 species of Enterobacteriaceae in 2001
| Organism | No. of isolates | % of isolates pan- susceptibleb | % of isolates resistant to one antimicrobial agent
|
% of isolates resistant to 2 anti- microbial agents | % of isolates resistant to 3 anti- microbial agents | % of isolates pan- resistantc | Most frequent coresistance phenotype(s) (% of total no. of isolates tested) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime | Gentamicin | Levofloxacin | SXT | |||||||
| E. coli | 19,277 | 74.3 | 0.2 | 1.0 | 2.1 | 13.0 | 5.7 | 3.1 | 0.6 | LVX, SXT (3.9); GEN, LVX, SXT (2.7) |
| K. pneumoniae | 7,980 | 82.7 | 1.0 | 0.7 | 1.3 | 4.7 | 4.1 | 3.2 | 2.2 | CTZ, GEN, LVX, SXT (2.2); CTZ, GEN, SXT (1.4) |
| E. aerogenes | 1,727 | 73.4 | 15.6 | 0.4 | 1.3 | 1.0 | 6.0 | 1.8 | 0.6 | CTZ, GEN (4.2); CTZ, GEN, SXT (0.8) |
| E. cloacae | 4,153 | 62.7 | 16.4 | 0.7 | 1.0 | 2.2 | 5.8 | 6.6 | 4.7 | CTZ, GEN, LVX, SXT (4.7); CTZ, GEN, SXT (3.4) |
| C. freundii | 1,238 | 53.1 | 16.2 | 1.5 | 3.0 | 7.6 | 10.7 | 6.0 | 1.9 | LVX, SXT (3.0); CTZ, SXT (2.4) |
| M. morganii | 842 | 57.7 | 6.3 | 0.6 | 1.8 | 5.2 | 13.0 | 9.4 | 6.1 | CTZ, GEN, LVX, SXT (6.1); LVX, SXT (6.0) |
| P. mirabilis | 3,650 | 75.5 | 0.3 | 1.9 | 3.8 | 5.6 | 12.4 | 4.0 | 0.3 | LVX, SXT (6.6); GEN, LVX, SXT (3.5) |
| P. vulgaris | 170 | 88.2 | 1.2 | 1.8 | 0.6 | 8.2 | 1.2 | 0 | 0 | GEN, SXT (1.2) |
| Providencia spp. | 455 | 36.9 | 3.3 | 1.5 | 13.8 | 4.0 | 23.3 | 13.6 | 3.5 | GEN, LVX, SXT (11.2); LVX, SXT (11.2) |
| S. marcescens | 2,345 | 84.2 | 4.4 | 3.2 | 2.0 | 1.6 | 3.5 | 0.9 | 0.2 | CTZ, GEN (1.2); GEN, LVX (0.7) |
Phenotypes were determined by using ceftazidime (CTZ), gentamicin (GEN), levofloxacin (LVX), and SXT susceptibility testing data.
Pan-susceptible isolates were susceptible to ceftazidime, gentamicin, levofloxacin, and SXT.
Pan-resistant isolates were resistant to ceftazidime, gentamicin, levofloxacin, and SXT.
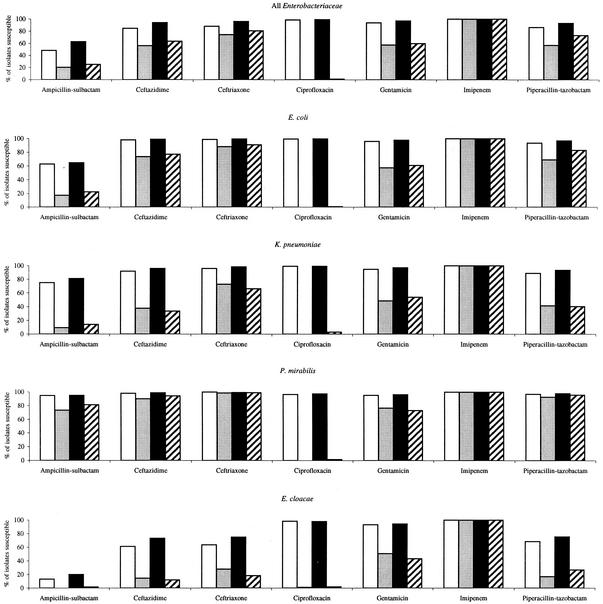
To show the observed relationship between fluoroquinolone susceptibility and susceptibility to other agents in greater detail, Fig. 2 depicts coresistance among fluoroquinolone-susceptible and fluoroquinolone-resistant isolates for ICU patients and non-ICU inpatients for all Enterobacteriaceae and individually for E. coli, K. pneumoniae, P. mirabilis, and E. cloacae. As anticipated, resistance to levofloxacin was most strongly associated with lowered susceptibility to ciprofloxacin but was also associated with lowered susceptibility to ampicillin-sulbactam, ceftazidime, ceftriaxone, gentamicin, and piperacillin-tazobactam; associations were less evident (ampicillin-sulbactam, ceftazidime, and gentamicin) or essentially absent (ceftriaxone and piperacillin-tazobactam) for P. mirabilis compared with other species of Enterobacteriaceae. Differences in isolate susceptibilities between levofloxacin-susceptible and levofloxacin-resistant isolates were greatest for E. cloacae. Levofloxacin-susceptible isolates of Enterobacteriaceae were highly susceptible to antimicrobial agents of other classes.
FIG. 2.
Coresistance among fluoroquinolone-susceptible and fluoroquinolone-resistant isolates of Enterobacteriaceae in 2001 for ICU patients and non-ICU inpatients. Open bars, levofloxacin susceptible, ICU; lightly shaded bars, levofloxacin resistant, ICU; black bars, levofloxacin susceptible, non-ICU; hatched bars, levofloxacin resistant, non-ICU.
The susceptibilities to ampicillin-sulbactam, piperacillin-tazobactam, cefepime, imipenem, gentamicin, and levofloxacin were also studied for ceftazidime-susceptible and ceftazidime-nonsusceptible (intermediate and resistant) isolates of E. coli, K. pneumoniae, and E. cloacae from combined ICU and non-ICU inpatients in 2001. For ceftazidime-susceptible and ceftazidime-nonsusceptible E. coli isolates, susceptibilities (percents) were as follows: ampicillin-sulbactam, 60.3 and 12.5; piperacillin-tazobactam, 95.8 and 65.8; cefepime, 99.8 and 72.4; imipenem, 100 and 100; gentamicin, 95.2 and 45.4; and levofloxacin, 91.4 and 26.8, respectively. For ceftazidime-susceptible and ceftazidime-nonsusceptible K. pneumoniae, susceptibilities (percents) were as follows: ampicillin-sulbactam, 80.1 and 6.5; piperacillin-tazobactam, 93.6 and 37.9; cefepime, 99.9 and 62.8; imipenem, 100 and 100; gentamicin, 97.6 and 36.3; and levofloxacin, 95.8 and 43.1, respectively. For ceftazidime-susceptible and ceftazidime-nonsusceptible E. cloacae, susceptibilities (percents) were as follows: ampicillin-sulbactam, 27.6 and 0.5; piperacillin-tazobactam, 94.7 and 18.1; cefepime, 99.9 and 79.3; imipenem, 100 and 100; gentamicin, 97.6 and 70.2; and levofloxacin, 97.2 and 70.0, respectively.
Table 3 shows the susceptibility of Enterobacteriaceae to selected agents by patient age and specimen source for combined ICU and non-ICU inpatient isolates from 2001. Susceptibilities varied by <5% for patients aged <18 to >65 years for piperacillin-tazobactam, gentamicin, and SXT. Ceftazidime susceptibility was 6.4% lower among patients aged <18 years than among patients aged >65 years. Both ciprofloxacin and levofloxacin demonstrated differences of >10% for isolates from patients aged <18 years versus isolates from patients aged >65 years. Respiratory isolates were less susceptible to ceftazidime and piperacillin-tazobactam than were isolates from other sources. Isolates from all three specimen sources had similar susceptibilities to imipenem (100%), gentamicin (91.2 to 92.7%), ciprofloxacin (88.8 to 89.7%), and levofloxacin (88.9 to 89.7%). Isolates from respiratory sources were more susceptible to SXT than were isolates from blood and urine.
TABLE 3.
Susceptibility of Enterobacteriaceae to selected antimicrobial agents according to patient age and isolate specimen source in 2001
| Patient age and specimen source | % of isolates susceptible to antimicrobial agent (no. of isolates tested)a
|
||||||
|---|---|---|---|---|---|---|---|
| Ceftazidime | Imipenem | Piperacillin-tazobactam | Gentamicin | SXT | Ciprofloxacin | Levofloxacin | |
| Age | |||||||
| <18 yr | 84.0 (6,812) | 100 (5,705) | 85.9 (4,840) | 92.2 (9,227) | 83.1 (9,194) | 98.3 (7,254) | 98.5 (6,595) |
| 18-65 yr | 89.5 (23,187) | 100 (20,851) | 89.5 (19,997) | 93.6 (36,292) | 83.8 (35,746) | 89.5 (26,135) | 90.3 (25,872) |
| >65 yr | 90.4 (19,703) | 100 (19,592) | 90.7 (18,451) | 93.3 (34,182) | 84.3 (34,034) | 87.0 (24,882) | 86.3 (26,259) |
| Specimen source | |||||||
| Blood | 88.6 (5,002) | 100 (4,921) | 89.3 (4,554) | 91.2 (6,252) | 81.5 (6,068) | 88.8 (4,928) | 89.0 (4,383) |
| Respiratory | 83.5 (10,130) | 100 (9,150) | 84.2 (9,154) | 91.2 (12,481) | 88.8 (12,069) | 89.2 (8,934) | 88.9 (8,510) |
| Urine | 92.8 (27,526) | 100 (25,607) | 92.7 (22,179) | 92.7 (25,607) | 82.8 (51,441) | 89.7 (38,644) | 89.7 (40,171) |
Demographic and specimen source data were not available for all isolates.
DISCUSSION
Antimicrobial resistance impedes effective treatment of patients with infections and is of particular concern for hospitalized patients. Given the prevalence and importance of Enterobacteriaceae as pathogens in hospitalized patients, the propensity for resistant organisms to move from patient to patient, and the mobility of resistance determinants (e.g., plasmids and transposons) between strains of the same and different species, routine surveillance of antimicrobial susceptibilities to all classes of clinically used agents is necessary. Surveillance assists in identifying and understanding trends in resistance; detecting the emergence of new resistance mechanisms; developing, implementing, and monitoring the impact of new empirical antimicrobial prescribing, infection control, and public health guidelines; and identifying outbreaks of resistant organisms. Only current surveillance data are beneficial in determining the best empirical regimens.
The present study provided a number of important observations regarding the susceptibility of Enterobacteriaceae to extended-spectrum cephalosporins, β-lactam-β-lactamase-inhibitor combinations, carbapenems, aminoglycosides, fluoroquinolones, and SXT. Rates of susceptibility to extended-spectrum cephalosporins (cefotaxime, ceftriaxone, and ceftazidime) were steady for Enterobacteriaceae from 1998 to 2001 but were up to 10% lower for isolates from ICU patients than for those from non-ICU inpatients (Table 1 and Fig. 1). Ceftazidime-nonsusceptibility rates in isolates from ICU patients and non-ICU inpatients were 4.2 and 2.6% for E. coli and 11.4 and 7.8% for K. pneumoniae, respectively, and may serve as an estimate of the prevalence of extended-spectrum β-lactamases in these species (9). Differences in susceptibilities to the extended-spectrum cephalosporins of >5% for isolates from ICU patients and non-ICU inpatients were not observed for E. coli, K. pneumoniae, and P. mirabilis but did exist for Enterobacter spp. and other Enterobacteriaceae where susceptibilities were lower among ICU isolates than among isolates from non-ICU inpatients (Fig. 1). Similar observations as those made for extended-spectrum cephalosporins were also evident for β-lactam-β-lactamase-inhibitor combinations (ampicillin-sulbactam, ticarcillin-clavulanate, and piperacillin-tazobactam) (Fig. 1 and Table 1). The in vitro susceptibility of Enterobacteriaceae to ampicillin-sulbactam, relative to the other agents tested, was limited for both ICU patients (45.5%) and non-ICU inpatients (57.2%).
Rates of susceptibility of E. coli to ampicillin-sulbactam observed in the present study (≤60%) were lower than previously reported in the United States (24) and suggest that this agent may not provide physicians and their patients with reliable therapy. The effectiveness of ampicillin-sulbactam in the treatment of E. coli infections is largely dependent on the inhibitory activity of sulbactam, as many isolates harbor the TEM-1 β-lactamase (13). However, sulbactam is a relatively weak inhibitor of TEM-1 (13), and resistance in E. coli can develop by hyperproduction of TEM-1, hyperproduction of chromosomal or plasmid-borne AmpC, and alteration of porin channels and less frequently by β-lactamase-inhibitor-resistant mutants of TEM.
Essentially all isolates in the present study were susceptible to carbapenems, including MDR isolates of Enterobacteriaceae. Carbapenems, such as imipenem, because of their resistance to hydrolysis by most β-lactamases, including those of groups 1, 2b, and 2be (4), are effective agents against a broad range of nosocomial pathogens including Enterobacteriaceae. β-Lactamases that hydrolyze carbapenems are still rare and not a global concern, but vigilant surveillance for these enzymes is important as some of the β-lactamases in this group possess the most extensive substrate hydrolysis profiles of all β-lactamases and plasmid-mediated carbapenem resistance has been reported previously (13, 15).
From 1998 to 2001 fluoroquinolone susceptibility showed the greatest relative decreases of all agents studied, perhaps reflecting the increased use of these agents for common infections such as those of the urinary and respiratory tracts or perhaps as supplements for the agricultural industry. Fluoroquinolone susceptibility was lower in adults than in children, reflecting the patterns of use of this class of agents. The widespread cumulative use of fluoroquinolones (ciprofloxacin and levofloxacin) may be accelerating the development of resistance to these agents and may be the driving force behind stepwise increases in resistance in Enterobacteriaceae and other bacterial species (2, 10, 14, 18, 21). Fluoroquinolone resistance was more commonly found as a component of coresistance phenotypes than as a single-agent resistance phenotype (Table 2 and Fig. 2) as has been reported by others (10, 11, 16, 20, 25). Fluoroquinolone-resistant species of Enterobacteriaceae were frequently also resistant to extended-spectrum cephalosporins, aminoglycosides, and SXT. The potential for commonly encountered gram-negative bacilli to acquire cross-resistance to several antimicrobial agents has been well documented (1, 10, 11, 25). The fact that fluoroquinolone resistance among gram-negative species is found predominantly among MDR isolates suggests that fluoroquinolone resistance will be maintained and perhaps accelerate even if other antimicrobials are used (8, 22). The apparent correlation between fluoroquinolone resistance and resistance to other classes of agents requires careful monitoring, as such resistance profiles seriously limit the therapeutic options available to treat infections caused by these organisms and MDR isolates may pose greater public health problems than isolates that exhibit resistance to a single agent.
A previous report found that increases in fluoroquinolone resistance were significant only in isolates of Enterobacteriaceae from hospitalized patients outside the ICU and from hospital outpatients (7). The authors suggested that factors present outside ICUs such as excessive fluoroquinolone use or inadequate infection control practices may explain their observations. In the present study, fluoroquinolone susceptibilities for Enterobacteriaceae were similar (differing by <5%) for ICU patients and non-ICU inpatients (Table 1) with the exception of Providencia spp., which had lower fluoroquinolone susceptibility rates in isolates from ICU patients. It is notable that rates of resistance to ciprofloxacin and levofloxacin in Table 1 were generally higher (often only marginally higher) in isolates from ICU patients than in isolates from non-ICU inpatients.
Every species of Enterobacteriaceae in the present study demonstrated some percentage of isolates that were coresistant. Based on reports from individual institutions and from different countries, the prevalence and diversity of MDR phenotypes could substantially expand and become problematic (11); therefore, continued monitoring for these phenotypes in the United States is warranted. Multidrug resistance in gram-negative bacteria appears to be primarily the result of the acquisition of resistance genes by horizontal transfer (12); however, clonal spread is also important and is the most likely mechanism by which coresistant strains involving fluoroquinolone resistance spread. Strains of MDR Enterobacteriaceae have been isolated with increasing frequency in hospital settings and are having a significant impact on clinical practice and overall treatment costs (8, 12, 22).
In conclusion, because antimicrobial resistance patterns are continually evolving, surveillance studies will continue to be essential to ensure the provision of safe and effective empirical therapy. Clearly, the current prevalence of coresistant isolates among Enterobacteriaceae isolated in U.S. laboratories suggests that monitoring these phenotypes is important (Table 2). The results of this study confirm those of other investigators suggesting that rates of resistance to extended-spectrum cephalosporins, other β-lactams, and β-lactamase-inhibitor combinations among Enterobacteriaceae such as Enterobacter spp. and C. freundii are quite high and may be increasing. Essentially all Enterobacteriaceae isolated and tested in clinical laboratories from 1998 to 2001 remain susceptible to carbapenems. Ongoing surveillance of Enterobacteriaceae should focus particularly on the continued increases in fluoroquinolone resistance, as well as changes in the prevalence and composition of coresistance phenotypes.
Acknowledgments
We thank the participating institutions in TSN Database-USA, each of whom permits surveillance data collection.
Merck financially supported the study.
REFERENCES
- 1.Acar, J. F., and F. W. Goldstein. 1998. Consequences of increasing resistance to antimicrobial agents. Clin. Infect. Dis. 27(Suppl. 1):S125-S130. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg, H. M., D. Rimland, D. J. Carroll, P. Terry, and I. K. Wachsmuth. 1991. Rapid development of ciprofloxacin resistance in methicillin-susceptible and -resistant Staphylococcus aureus. J. Infect. Dis. 163:1279-1285. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulos, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 6.Eisenstein, B. I., and D. F. Zaleznik. 2000. Enterobacteriaceae, p. 2294-2310. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 7.Fridkin, S. K., H. A. Hill, N. V. Volkova, J. R. Edwards, R. M. Lawton, R. P. Gaynes, J. E. McGowan, Jr., and the Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Project Hospitals. 2002. Temporal changes in prevalence of antimicrobial resistance in 23 U.S. hospitals. Emerg. Infect. Dis. 8:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedrich, L. V., R. L. White, and J. A. Bosso. 1999. Impact of use of multiple antimicrobials on changes in susceptibility of gram-negative aerobes. Clin. Infect. Dis. 28:1017-1024. [DOI] [PubMed] [Google Scholar]
- 9.Hadziyannis, E., M. Touhy, L. Thomas, G. W. Procop, J. A. Washington, and G. S. Hall. 2000. Screening and confirmatory testing for extended spectrum β-lactamases (ESBL) in Escherichia coli, Klebsiella pneumoniae, and Klebsiella oxytoca clinical isolates. Diagn. Microbiol. Infect. Dis. 36:113-117. [DOI] [PubMed] [Google Scholar]
- 10.Hooper, D. C. 2001. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 7:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson, K., K. Rolston, L. Elting, B. LeBlanc, E. Whimby, and D. H. Ho. 1999. Susceptibility surveillance among gram-negative bacilli at a cancer center. Chemotherapy 45:325-334. [DOI] [PubMed] [Google Scholar]
- 12.Leverstein-van Hall, M. A., A. T. A. Box, H. E. M. Blok, A. Paauw, A. C. Fluit, and J. Verhouf. 2002. Evidence of extensive interspecies transfer of integron-mediated antimicrobial resistance genes among multidrug-resistant Enterobacteriaceae in a clinical setting. J. Infect. Dis. 186:49-56. [DOI] [PubMed] [Google Scholar]
- 13.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livermore, D. M., D. James, M. Reacher, C. Graham, T. Nichols, P. Stephens, A. P. Johnson, and R. C. George. 2002. Trends in fluoroquinolone (ciprofloxacin) resistance in Enterobacteriaceae from bacteremias, England and Wales, 1990-1999. Emerg. Infect. Dis. 8:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucet, J. C., S. Chevret, D. Decre, D. Vaniak, A. Macrez, J. P. Bedos, M. Wolff, and B. Regnier. 1996. Outbreak of multiply resistant Enterobacteriaceae in an intensive care unit: epidemiology and risk factors for acquisition. Clin. Infect. Dis. 22:430-436. [DOI] [PubMed] [Google Scholar]
- 16.Meyer, K. S., C. Urban, J. A. Eagan, B. J. Berger, and J. J. Rahal. 1993. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann. Intern. Med. 119:353-358. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing; 11th informational supplement. Vol. 21, no. 1. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Pena, C., J. M. Albareda, R. Pallares, M. Pujol, F. Tubua, and J. Ariza. 1995. Relationship between quinolone use and emergence of ciprofloxacin-resistant Escherichia coli in bloodstream infections. Antimicrob. Agents Chemother. 39:520-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rello, J., M. Gallego, D. Mariscal, R. Sonora, and J. Valles. 1997. The value of routine microbial investigation in ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 156:196-200. [DOI] [PubMed] [Google Scholar]
- 20.Rice, L. B., S. H. Willey, G. A. Papanicolaou, A. A. Medeiros, G. M. Eliopoulos, R. C. Moellering, and G. A. Jacoby. 1990. Outbreak of ceftazidime resistance caused by extended-spectrum β-lactamases at a Massachusetts chronic-care facility. Antimicrob. Agents Chemother. 34:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richard, P., M. H. Delangle, F. Raffi, E. Espaze, and H. Richet. 2001. Impact of fluoroquinolone administration on the emergence of fluoroquinolone-resistant Gram-negative bacilli from gastrointestinal flora. Clin. Infect. Dis. 32:162-166. [DOI] [PubMed] [Google Scholar]
- 22.Sahm, D. F., I. A. Critchley, L. J. Kelly, J. A. Karlowsky, D. C. Mayfield, C. Thornsberry, Y. R. Mauriz, and J. Kahn. 2001. Evaluation of current activities of fluoroquinolones against gram-negative bacilli using centralized in vitro testing and electronic surveillance. Antimicrob. Agents Chemother. 45:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahm, D. F., M. K. Marsilio, and G. Piazza. 1999. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the surveillance network database—USA. Clin. Infect. Dis. 29:259-263. [DOI] [PubMed] [Google Scholar]
- 24.Shungu, D. L., S. Ponticas, and C. J. Gill. 1989. Comparative activity of cefoxitin, ampicillin/sulbactam, and imipenem against clinical isolates of Escherichia coli and Klebsiella pneumoniae. Clin. Ther. 11:315-318. [PubMed] [Google Scholar]
- 25.Weiner, J., J. P. Quinn, P. A. Bradford, R. V. Goering, C. Nathan, K. Bush, and R. A. Weinstein. 1999. Multiple antibiotic-resistant Klebsiella pneumoniae and Escherichia coli in nursing homes. JAMA 281:517-523. [DOI] [PubMed] [Google Scholar]