Abstract
The combination of fluconazole (FLC) and cyclosporine (CY) is fungicidal in FLC-susceptible C. albicans (O. Marchetti, P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard, Antimicrob. Agents Chemother. 44:2373-2381, 2000). The mechanism of this synergism is unknown. CY has several cellular targets including multidrug efflux transporters. The hypothesis that CY might inhibit FLC efflux was investigated by comparing the effect of FLC-CY in FLC-susceptible parent CAF2-1 (FLC MIC, 0.25 mg/liter) and in FLC-hypersusceptible mutant DSY1024 (FLC MIC, 0.03 mg/liter), in which the CDR1, CDR2, CaMDR1, and FLU1 transporter genes have been selectively deleted. We postulated that a loss of the fungicidal effect of FLC-CY in DSY1024 would confirm the roles of these efflux pumps. Time-kill curve studies showed a more potent fungistatic effect of FLC (P = 0.05 at 48 h with an inoculum of 103 CFU/ml) and a more rapid fungicidal effect of FLC-CY (P = 0.05 at 24 h with an inoculum of 103 CFU/ml) in the FLC-hypersusceptible mutant compared to those in the parent. Rats with experimental endocarditis were treated for 2 or 5 days with high-dose FLC, high-dose CY, or both drugs combined. FLC monotherapy for 5 days was more effective against the hypersusceptible mutant than against the parent. However, the addition of CY to FLC still conferred a therapeutic advantage in animals infected with mutant DSY1024, as indicated by better survival (P = 0.04 versus the results obtained with FLC) and sterilization of valves and kidneys after a very short (2-day) treatment (P = 0.009 and 0.002, respectively, versus the results obtained with FLC). Both in vitro and in vivo experiments consistently showed that the deletion of the four membrane transporters in DSY1024 did not result in loss of the fungicidal effect of FLC-CY. Yet, the accelerated killing in the mutant suggested a “dual-hit” mechanism involving FLC hypersusceptibility due to the efflux pump elimination and fungicidal activity conferred by CY. Thus, inhibition of multidrug efflux transporters encoded by CDR1, CDR2, CaMDR1, and FLU1 genes is not responsible for the fungicidal synergism of FLC-CY. Other cellular targets must be considered.
Progress in modern medicine has led to a worldwide increase in the incidence of Candida infections (2, 25). Amphotericin B, a fungicidal agent, has been the standard treatment for these infections for decades, but the toxicity of its conventional form and the costs of its lipid forms limit its use. Other antifungal agents, such as azoles, have excellent efficacy-toxicity profiles and play an important role in the treatment of candidal infections in nonneutropenic patients, although they have mostly fungistatic activities (13, 18, 19). However, the treatment of candidiasis during neutropenia and the emergence of azole-resistant Candida species continue to represent major challenges (1, 17, 19, 25-27). Thus, new therapeutic strategies need to be developed. In Candida albicans, efflux pumps play a major role in azole susceptibility and may represent a new therapeutic target (22). Promising results were reported in studies in which efflux of cytotoxic agents was inhibited in multiresistant human cancer cells (10, 24). As mammalian and fungal multidrug efflux transporters (METs) have strong structural homologies, human efflux pump inhibitors were screened in vitro to determine whether they have synergistic activities with fluconazole (FLC) against C. albicans. We found that the combination of FLC and cyclosporine (CY) is fungicidal against FLC-susceptible C. albicans strains (13). This powerful synergism was confirmed in experimental endocarditis. The association of FLC and CY was fungicidal against infection in aortic valve vegetations, a model of localized neutropenia, as well as in the kidney, an organ with a neutrophil-phagocytic host response (11). Although this phenomenon was discovered during the screening of inhibitors of efflux pumps in cancer cells, the mechanism of the synergism of FLC and CY in C. albicans is unknown. CY has several cellular targets including the cell membrane, METs, and the cyclophilin-calmodulin-calcineurin pathway. Thus, its interaction with FLC might intervene at different sites (9). The objective of the present work was to investigate the involvement of METs encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes, which have been found to mediate FLC efflux in C. albicans. We postulated that their deletion would result in the loss of the fungicidal synergism of FLC-CY. For this purpose, the in vitro and in vivo activities of the combination of FLC and CY were compared against FLC-susceptible parent strain C. albicans CAF2-1 and FLC-hypersusceptible mutant C. albicans DSY1024, obtained by targeted deletion of the MET genes CDR1, CDR2, CaMDR1, and FLU1 (3, 21).
MATERIALS AND METHODS
Strains, media, and growth conditions.
The following isolates were used: C. albicans CAF2-1 (Ura+ parental strain; FLC MIC, 0.25 mg/liter (6), C. albicans DSY1024 (cdr1Δ::hisG/cdr1Δ::hisG cdr2Δ::hisG/cdr2Δ::hisG camdr1Δ::hisG/camdr1Δ::hisG flu1Δ::hisG/flu1Δ::hisG-URA3-hisG, constructed by making targeted gene deletions in parent strain CAF4-2; FLC MIC, 0.03 mg/liter) (3, 21), and C. albicans ATCC 90028 (a quality control strain used for in vitro susceptibility testing; FLC MIC, 0.25 to 1 mg/liter) (16).
Isolates were stocked at −70°C in liquid medium supplemented with 10% (vol/vol) glycerol, maintained at 4°C, and subcultured twice at 35°C before each experiment.
Sabouraud liquid medium (Diagnostics Pasteur, Marnes la Coquette, France) was used for overnight growth in a shaking incubator at 30°C and 200 rpm.
Plates containing Sabouraud dextrose with 2% agar (Difco, Basel, Switzerland) were used to maintain or subculture the tested strains and for colony-counting studies.
RPMI 1640 with l-glutamine without bicarbonate (Difco) buffered with 0.165 M morpholinepropanesulfonic acid (Fluka, Basel, Switzerland) was used for MIC determinations and time-kill curve studies (16).
Drugs and reagents.
FLC for in vitro testing was kindly provided by Pfizer (Sandwich, United Kingdom). FLC for intravenous administration (Diflucan) for in vivo experiments was purchased from Pfizer (Zürich, Switzerland). CY for in vitro studies was purchased from Sigma (Buchs, Switzerland). CY for intravenous administration (Sandimmun) for in vivo treatments was purchased from Novartis Pharma (Basel, Switzerland).
A CY stock solution was constituted in dimethyl sulfoxide (1 mg/liter) and further diluted in H2O or RPMI 1640, as appropriate. All other chemicals were commercially available reagent-grade products.
Susceptibility testing.
The MICs were determined by broth microdilution according to the recommendations outlined in NCCLS approved standard M27-A (16).
Time-kill curve studies.
C. albicans CAF2-1 and C. albicans DSY1024 were tested in RPMI 1640 by broth macrodilution under experimental conditions identical to those recommended by the NCCLS for MIC testing (16). The strains were grown overnight at 30°C and 200 rpm. Tubes (10 ml) containing CY (0.6 mg/liter), FLC (10 mg/liter), or FLC-CY (10 and 0.6 mg/liter, respectively) at concentrations achievable in vivo and 103 or 105 CFU of the tested isolate per ml were incubated at 35°C. To study the effects of these different treatments on viable counts after 12, 24, and 48 h of incubation, an aliquot (100 μl) from each test tube was subcultured after serial dilution on Sabouraud dextrose agar plates and incubated for 48 h at 35°C (13). Any decrease in the viable counts of the starting inoculum was considered killing. Killing of >99.9% (3 logs) of the starting inoculum was defined as a fungicidal effect (15). Since the limit of detection was 101 CFU/ml, the presence of a fungicidal effect (a >3-log decrease in viable counts) could be evaluated only in experiments with an initial inoculum of 105 CFU/ml. The results were reported as the mean colony counts from triplicate experiments.
Experimental endocarditis.
Female Wistar rats (weight, 200 g; Iffa Credo, Lyon, France) with sterile catheter-induced aortic valve vegetations were challenged intravenously, as reported previously (8, 11), with 6 × 105 CFU of C. albicans CAF2-1 or DSY1024. Treatment was started at 12 h postchallenge and was administered for 2 or 5 days. To assess the baseline infectious load, control animals were killed at the 12-h time point. The following high-dose regimens were administered once daily to rats infected with CAF2-1 or DSY1024: FLC at 20 mg/kg of body weight/day intraperitoneally (n = 12), CY at 10 mg/kg/day subcutaneously (n = 6), or FLC at 20 mg/kg/day intraperitoneally plus CY at 10 mg/kg/day subcutaneously (n = 9). To monitor the natural course of infection, untreated animals infected with CAF2-1 or DSY1024 were studied (n = 6 animals for each strain). Survival in the different groups was recorded daily. Day 0 corresponded to treatment onset; day 5 corresponded to the end of observation. Moribund animals were killed, and the following day was recorded as the time of death. Rats receiving FLC, CY, or FLC-CY were killed 24 h after the second drug administration (2-day treatment) or 48 h after the fifth drug administration (5-day treatment) in order to ensure drug washout and avoid carryover onto the plates for viable count studies, as shown previously (11). The data for animals undergoing scheduled killing were censored for survival analysis. Aortic valve vegetations and kidneys were dissected, weighed, homogenized in 1 and 2 ml of saline, respectively, serially diluted, and plated. Candida colony counts were recorded after a 3-day incubation at 35°C. Fungal densities were calculated by a previously used formula (11) and expressed as log10 CFU per gram of tissue. Each treatment experiment with animals infected with either the parent or the mutant was performed in duplicate.
The susceptibilities to FLC of both the parent and the mutant were tested ex vivo by using single colonies growing from persistently infected vegetations and kidneys. The FLC and CY concentrations in the plasma of treated rats were determined on the first and last days of therapy and at the time of killing, as described previously (11, 12).
Statistical analysis.
The Mann-Whitney test was used to compare viable counts in time-kill curve studies. Survival curves were compared by the log-rank test. Fungal densities in tissues were compared by the Mann-Whitney test or the Kruskal-Wallis analysis of variance on ranks followed by pairwise comparison, as appropriate. The rates of persistently infected organs were compared by Fisher's exact test. All tests were two tailed, and the significance level was set at P < 0.05. The Bonferroni correction was used for multiple testing.
RESULTS
Time-kill (in vitro) experiments.
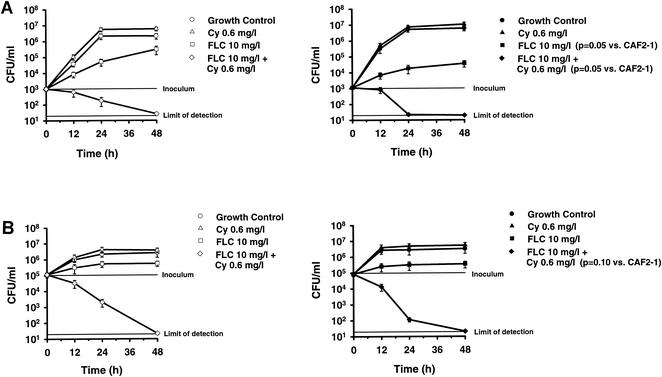
Time-kill experiments (Fig. 1A) were performed with 103 CFU of C. albicans CAF2-1 or DSY1024 per ml, the initial inoculum recommended by present guidelines for antifungal susceptibility testing (16). The viable counts for the growth controls of both strains were comparable. The fungistatic effect of FLC was more marked against the hypersusceptible mutant than against the parent. The mean viable counts after 48 h of incubation were 3.7 × 104 CFU/ml (range, 2.3 × 104 to 5.5 × 104 CFU/ml) and 3.06 × 105 CFU/ml (range, 1.8 × 104 to 5 × 105 CFU/ml), respectively (P = 0.05). In contrast, CY alone had no antifungal activity against either the parent or the mutant. In both strains, the combination of FLC and CY was fungicidal. However, the killing effect was accelerated in the mutant compared to that in the parent. The mean viable counts after 24 h of incubation were 2.2 × 10 CFU/ml (range, 1.9 × 10 to 2.7 × 10 CFU/ml) and 1.83 × 102 CFU/ml (range 9 × 10 to 3 × 102 CFU/ml), respectively (P = 0.05). The results of time-kill curve studies with an initial inoculum of 105 CFU/ml, a baseline condition more close to that of the in vivo model of experimental endocarditis, are shown in Fig. 1B. In contrast to experiments with a lower inoculum, no difference in growth inhibition by FLC alone was found after 48 h of incubation. Killing induced by the combination of FLC and CY was similar in strains DSY1024 and CAF2-1 after 48 h of incubation (>99.9% decrease in viable counts in both strains). Yet, a trend for a more rapid decrease in viable counts was found in the mutant compared to that in the parent. The mean viable counts at 24 h were 1.16 × 102 CFU/ml (range, 8.6 × 10 to 1.5 × 102 CFU/ml) and 2 × 103 CFU/ml (range, 1 × 103 to 3 × 103 CFU/ml), respectively (P = 0.10).
FIG. 1.
Time-kill curves obtained by using an initial inoculum of 103 CFU/ml (A) or 105 CFU/ml (B). The effects of FLC, CY, and FLC-CY against C. albicans CAF2-1 (susceptible parent; FLC MIC, 0.25 mg/liter [left panels]) and DSY1024 (hypersusceptible efflux-pump deleted mutant; FLC MIC, 0.03 mg/liter [right panels]) were tested. Mean colony counts and error bars for triplicate experiments are shown.
In vivo experiments. (i) Survival studies with rats with experimental endocarditis.
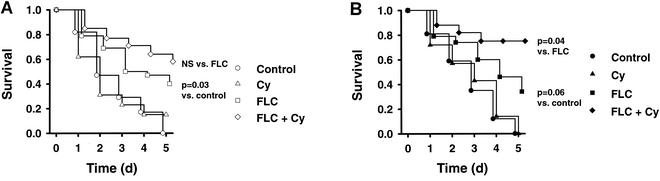
Figure 2 shows the survival curves for untreated animals and animals treated with FLC, CY, or FLC-CY after infection with FLC-susceptible parent strain CAF2-1 or FLC-hypersusceptible mutant DSY1024. In rats infected with CAF2-1, 5-day mortality rates with no treatment (n = 12) and with CY treatment (n = 12) were 100 and 85%, respectively. The results for animals infected with DSY1024 were identical: no treatment (n = 12) and CY treatment (n = 12) both resulted in 100% mortality at 5 days. FLC (n = 24 for the group infected with the parent strain, n = 24 for the group infected with the mutant) and FLC-CY (n = 18 for the group infected with the parent strain, n = 18 for the group infected with the mutant) improved rates of survival significantly compared to those for untreated or CY-treated animals infected with either strain (for the group infected with the parent strain, for FLC and FLC-CY versus the control, P = 0.03 and P = 0.008, respectively, and versus CY, P = 0.06 and P = 0.01, respectively; for the group infected with the mutant, for FLC and FLC-CY versus the control P = 0.06 and P = 0.0008, respectively, and versus CY, P = 0.09 and P = 0.0008, respectively). However, no statistically significant survival advantage was found between FLC and FLC-CY in animals infected with CAF2-1. In contrast, in animals infected with DSY1024, treatment with the combination of FLC and CY significantly improved the survival rate compared to that after treatment with FLC alone (P = 0.04).
FIG. 2.
Survival studies in rats with experimental endocarditis due to FLC-susceptible parent CAF2-1 (A) and FLC-hypersusceptible mutant DSY1024 (B). The results for untreated controls and animals receiving CY, FLC, and FLC-CY were compared. The pooled results for duplicate experiments are shown. Day 0 corresponds to the onset of treatment, and day 5 corresponds to the end of treatment. NS, not significant.
(ii) Therapeutic results for experimental endocarditis after a 2-day treatment.
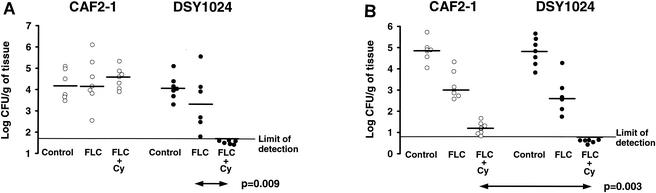
Figure 3 summarizes the results observed in the vegetations and kidneys of untreated animals and animals receiving FLC or FLC-CY after infection with parent strain CAF2-1 or mutant DSY1024. The baseline fungal load in both target organs after infection with CAF2-1 was similar to that in the organs of rats infected with DSY1024. Among the animals with endocarditis, only the group infected with DSY1024 and treated with FLC-CY was successfully cured: all rats had sterile aortic valve vegetations (P = 0.009 for comparison of fungal densities, P = 0.0008 for comparison of infection eradication rates to those for animals infected with DSY1024 and treated with FLC alone). In all other groups, the fungal densities in the aortic valves were not different from those in the aortic valves of the controls at the baseline. In the kidneys of animals infected with strain CAF2-1, FLC was partially effective (P = 0.003 versus the counts for the controls at the baseline) and FLC-CY was better than FLC alone (P = 0.0008). In rats infected with mutant DSY1024, the results of treatment with FLC were similar to those obtained with strain CAF2-1 (P = 0.004 versus the counts for the controls at the baseline). However, the effect of the combination of FLC and CY was powerful, with the combination sterilizing all organs in DSY1024-infected rats (P = 0.002 for comparison of fungal densities or eradication rates to those for FLC-treated animals, P = 0.003 for comparison of fungal densities and P = 0.0006 for comparison of infection eradication rates to those for CAF2-1-infected rats treated with FLC-CY). CY alone had no antifungal activity: as for the untreated controls, the fungal densities in the vegetations and kidneys of animals infected with either the parent or the mutant were comparable to those observed at the baseline (data not shown). The ex vivo FLC MICs for isolates from all animals with persistently infected valves and/or kidneys despite FLC or FLC-CY treatment were identical to those measured before inoculation (0.25 mg/liter for CAF2-1 and 0.03 mg/liter for DSY1024).
FIG. 3.
Fungal densities in aortic valve vegetations (A) and kidneys (B) after 2 days of treatment for experimental endocarditis due to CAF2-1 or DSY1024. The pooled results of duplicate experiments are shown. Each point represents the fungal density observed in a single animal. Horizontal bars indicate the median fungal densities for each group.
(iii) Therapeutic results for experimental endocarditis after a 5-day treatment.
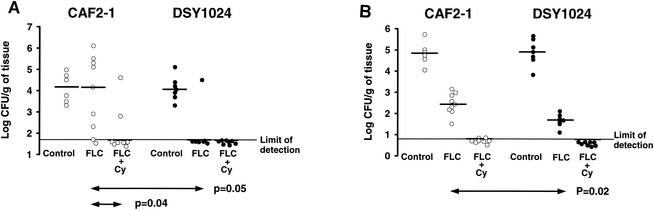
The results for the 5-day treatments are shown in Fig. 4. Among the animals with endocarditis, FLC was effective only against DSY1024, sterilizing the valves of six of seven (86%) of the animals (P = 0.04 for comparison of fungal densities and P = 0.003 for comparison of infection eradication rates to those for the controls at the baseline, P = 0.05 for comparison of fungal densities to those for rats infected with CAF2-1 and treated with FLC). FLC-CY sterilized most vegetations in rats infected with either CAF2-1 (six of eight rats; 75%) or DSY1024 (eight of eight rats; 100%). Significant differences between FLC-CY-treated rats and the corresponding controls at the baseline were found in comparisons of fungal densities (P = 0.008 for CAF2-1 and P = 0.0007 for DSY1024, respectively) and infection eradication rates (P = 0.03 for CAF2-1 and P = 0.0009 for DSY1024, respectively). Moreover, when FLC-CY was compared to FLC in CAF2-1-infected rats, differences in fungal densities (P = 0.04) and infection eradication rates (six of eight [75%] versus two of nine [22%] rats [P = 0.06]) were found. Although treatment with FLC was successful in kidneys infected with either strain (P = 0.0006 for comparison of the fungal densities to those for the controls at the baseline), this regimen was more effective against DSY1024 than against CAF2-1 (P = 0.02). The combination of FLC and CY was the most effective treatment, sterilizing the majority of the organs infected with CAF2-1 (seven of eight; 88%) or DSY1024 (eight of eight; 100%). For both strains, significant differences were found in a comparison of the fungal densities to those for the controls at the baseline (P = 0.0007) or to those for FLC-treated rats (P = 0.0006). When infection eradication rates were compared to those for the controls at the baseline, similar differences were found (P = 0.003 for CAF2-1, P = 0.0007 for DSY1024). CY alone did not display any activity against the organisms in the vegetations or in the kidneys of animals infected with either strain: as for the untreated controls, fungal densities were higher than those measured at the baseline (data not shown). The ex vivo FLC MIC for isolates from all animals with persistently infected valves and/or kidneys despite FLC or FLC-CY treatment were identical to those measured before inoculation (0.25 mg/liter for CAF2-1 and 0.03 mg/liter for DSY1024).
FIG. 4.
Fungal densities in aortic valve vegetations (A) and kidneys (B) after 5 days of treatment for experimental endocarditis due to CAF2-1 or DSY1024. The pooled results of duplicate experiments are shown. Each point represents the fungal density observed in a single animal. Horizontal bars indicate the median fungal densities for each group.
DISCUSSION
Efflux pumps belonging to two different classes, the ATP-binding cassette and the major facilitators, play a major role in the susceptibilities of yeasts to azole antifungals. Their upregulation results in resistance, and their targeted deletion results in increased susceptibility (22). The fungicidal synergism of FLC and CY in FLC-susceptible C. albicans was found during the screening of mammalian MET inhibitors for potentiation of the antifungal effect of FLC. Interestingly, despite the lack of intrinsic antifungal activity, CY conferred a killing effect on FLC, leaving its MIC unchanged (13). This unusual synergism of an azole antifungal and an immunosuppressive agent was confirmed in vivo. The combination of FLC and CY was successful in eradicating infection in aortic valve vegetations and the kidneys in a model of experimental endocarditis (11). However, the mechanism underlying this observation is unknown. In other eukaryotic cells, CY has several targets such as membrane-bound multidrug transporters, the cell membrane itself, and the cyclophilin-calmodulin-calcineurin pathway (7, 14). The interaction of CY with calcineurin results, for example, in the inhibition of interleukin-1 synthesis in human T cells and in cell death in some parasites (23). Four METs encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes, respectively, have been identified in C. albicans. As these transporters mediate FLC efflux, C. albicans strains from which the genes for METs are deleted show increased susceptibilities to FLC (21). Moreover, according to the initial working hypothesis of FLC efflux inhibition by CY, a loss of the killing effect conferred by CY on FLC would be expected in a C. albicans mutant lacking these METs. The goal of the present work was to compare the effects of FLC and CY in this FLC-hypersusceptible mutant (DYS1024; FLC MIC, 0.03 mg/liter) and in the parent strain (CAF2-1; FLC MIC, 0.25 mg/liter).
Time-kill curves for DSY1024 and CAF2-1 with an inoculum of 103 CFU/ml showed the enhanced inhibitory effect of FLC induced by the targeted deletion of the CDR1, CDR2, CaMDR1, and FLU1 genes. On the other hand, the fungicidal effect of CY and FLC against DSY1024 not only was maintained but was also more rapid than that against CAF2-1. High-dose treatments with FLC and FLC-CY were tested in rats infected with CAF2-1 or DSY1024. In order to discriminate the effect of the antifungal treatment from that of host defenses, infection was studied in two different targets, aortic valve vegetations, with their neutropenic environment, and the kidneys, with their massive recruitment of polymorphonuclear phagocytes (11). The absence of polymorphonuclear phagocytes in endocarditis provides very stringent experimental conditions, and therapeutic success relies exclusively on the killing effect of the antimicrobial treatment. Given that previous studies of Candida experimental endocarditis described cure only after weeks of antifungal treatment, the sterilization of aortic valve vegetations recently reported in rats infected with parent strain CAF2-1 after a 5-day treatment with high doses of FLC-CY represented a clear proof of concept (4, 11, 28). The present work tested identical regimens, which, as in previous experiments, produced supratherapeutic blood FLC concentrations (i.e., trough levels up to 15 mg/liter, a concentration still compatible with that achieved with high-dose treatment in humans) and CY (i.e., trough levels up to 2.5 mg/liter, which is 5- to 10-fold the concentrations targeted in humans) (data not shown) (11). The virulences of the parent and the mutant from which efflux pumps were deleted were similar, as assessed by the fungal densities at the baseline and the rates of mortality among untreated animals. CY alone had no activity against either fungal strain. The improved antifungal efficacy of FLC against the organisms in both the vegetations and the kidneys after a 5-day treatment in animals infected with the mutant was compatible with the more favorable pharmacodynamics of FLC (higher in vivo FLC levels/MIC ratios resulting from identical dosing schedules and a fourfold lower FLC MIC for DSY1024). The limited survival advantage and the prolonged time to cure for animals infected with either CAF2-1 or DSY1024 and treated with FLC and for those infected with the parent and treated with FLC-CY were not due to the emergence of FLC resistance, as demonstrated by ex vivo susceptibility testing of isolates from persistently infected vegetations and kidneys. The delayed eradication of infection in these groups in vivo was consistent with the fungistatic effect of FLC against either the parent or the mutant and the slow fungicidal activity of FLC-CY against CAF2-1, achieving >99.9% killing only after 48 h in vitro. This is in contrast to the powerful fungicidal activity displayed by the combination of FLC and CY in DSY1024-infected animals: survival was significantly improved compared to that among rats treated with FLC alone, and infection could be eradicated from both the vegetations and the kidneys after a 2-day treatment. These striking results obtained with DSY1024 in vivo are consistent with the accelerated killing observed in vitro, in which the combination of FLC and CY was already fungicidal after 24 h of incubation. Despite the limitations of the present study (use of a single strain in a single in vitro condition and in a single experimental animal model), both in vitro and in vivo experiments showed that METs encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes are not involved in the powerful fungicidal synergism of FLC and CY. Moreover, the accelerated killing effect against DSY1024 suggests a “dual-hit” mechanism involving MET deletion-induced FLC hypersusceptibility and killing conferred by CY. Although the inhibition of other unrecognized METs should be considered, recent observations from other investigators and from our laboratory suggest that calcineurin could play a key role in this phenomenon (5, 20). Thus, the cellular mechanisms underlying this new and promising therapeutic concept deserve further investigation. On the other hand, studies with other azole antifungals and CY analogues lacking immunosuppressive properties are needed.
Acknowledgments
We thank Marlyse Giddey, Françoise Ischer, Marlies Knaup, and Paul Antony Majcherczyk for outstanding technical assistance.
D.S. is supported by grant 3100-055901 from the Swiss National Found for Scientific Research, and P.M. is supported by grant 3200-044099 from the Swiss National Found for Scientific Research.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Sagué, C. M., W. R. Jarvis, and the National Nosocomial Infection Surveillance System. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 3.Calabrese, D., J. Bille, and D. Sanglard. 2000. A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology 146:2743-2754. [DOI] [PubMed] [Google Scholar]
- 4.Chemlal, K., L. Saint-Julien, V. Joly, R. Farinotti, N. Seta, P. Yeni, and C. Carbon. 1996. Comparison of fluconazole and amphotericin B for treatment of experimental Candida albicans endocarditis in rabbits. Antimicrob. Agents Chemother. 40:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz, M. C., A. L. Goldstein, J. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes, M., L. Fuller, D. H. Haynes, and J. Miller. 1985. Cyclosporin partitions into phospholipid vesicles and disrupts membrane architecture. Immunol. Lett. 11:343-349. [DOI] [PubMed] [Google Scholar]
- 8.Héraïef, E., M. P. Glauser, and L. R. Freedman. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho, S., N. Clipstone, L. Timmermann, J. Northrp, I. Graef, D. Fiorentino, J. Nourse, and G. R. Crabtree. 1996. The mechanism of action of cyclosporin A and FK 506. Clin. Immunol. Immunopathol. 80(Suppl. 3):S40-S45. [DOI] [PubMed] [Google Scholar]
- 10.List, A. F., C. Spier, J. Greer, S. Wolff, J. Hutter, R. Dorr, S. Salmon, B. Futscher, M. Baier, and W. Dalton. 1993. Phase I/II trial of cyclosporine as a chemotherapy-resistance modifier in acute leukemia. J. Clin. Oncol. 11:1652-1660. [DOI] [PubMed] [Google Scholar]
- 11.Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon. 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44:2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchetti, O., P. A. Majcherczyk, M. P. Glauser, J. Bille, P. Moreillon, and D. Sanglard. 2001. Sensitive bioassay for determination of fluconazole concentrations in plasma using a Candida albicans mutant hypersusceptible to azoles. Antimicrob. Agents Chemother. 45:696-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks, A. R. 1996. Cellular functions of immunophilins. Physiol. Rev. 76:631-649. [DOI] [PubMed] [Google Scholar]
- 15.McGinnis, M. R., and M. G. Rinaldi. 1996. Antifungal drugs: mechanisms of action, drug resistance, susceptibility testing, and assays of activity in biologic fluids, p. 176-211. In V. Lorian (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins Co., Baltimore, Md.
- 16.National Committee for Clinical and Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts, vol. 17, no. 9. Approved standard. Document M27-A. National Committee for Clinical and Laboratory Standards, Wayne, Pa.
- 17.Nguyen, M. H., J. E. Peacock, A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 18.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, Jr., R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, M. J. Levenstein, C. D. Webb, and The Candidemia Study Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 19.Rex, J. H., T. J. Walsh, J. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards, Jr. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 20.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol., in press. [DOI] [PubMed]
- 21.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman, J. A., M. L. Hayes, B. J. Luft, and K. A. Joiner. 1997. Characterization of anti-Toxoplasma activity of SDZ 215-918, a cyclosporin derivative lacking immunosuppressive and peptidyl-prolyl-isomerase-inhibiting activity: possible role of a P glycoprotein in Toxoplasma physiology. Antimicrob. Agents Chemother. 41:1859-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonneveld, P., and K. Nooter. 1990. Reversal of drug-resistance by cyclosporin-A in a patient with acute myelocytic leukemia. Br. J. Haematol. 75:208-211. [DOI] [PubMed] [Google Scholar]
- 25.Voss, A., J. A. J. W. Kluytmans, J. G. M. Koeleman, L. Spanjaard, C. M. J. E. Vandenbroucke, H. A. Verbrugh, M. C. Vos, A. Y. L. Weersink, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1996. Occurrence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 15:909-912. [DOI] [PubMed] [Google Scholar]
- 26.Walsh, T. J., R. W. Finberg, C. Arndt, J. Hiemenz, C. Schwartz, D. Bodensteiner, P. Pappas, N. Seibel, R. N. Greenberg, S. Dummer, M. Schuster, J. S. Holcenberg, and the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 340:764-771. [DOI] [PubMed] [Google Scholar]
- 27.Wingard, J. R., P. Kubilis, L. Lee, G. Yee, M. H. White, L. Walshe, R. A. Bowden, E. J. Anaissie, J. Hiemenz, and J. Lister. 1999. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29:1402-1407. [DOI] [PubMed] [Google Scholar]
- 28.Witt, M. D., and A. S. Bayer. 1991. Comparison of fluconazole and amphotericin B for prevention and treatment of experimental Candida endocarditis. Antimicrob. Agents Chemother. 35:2481-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]