Abstract
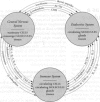
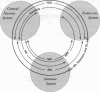
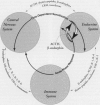
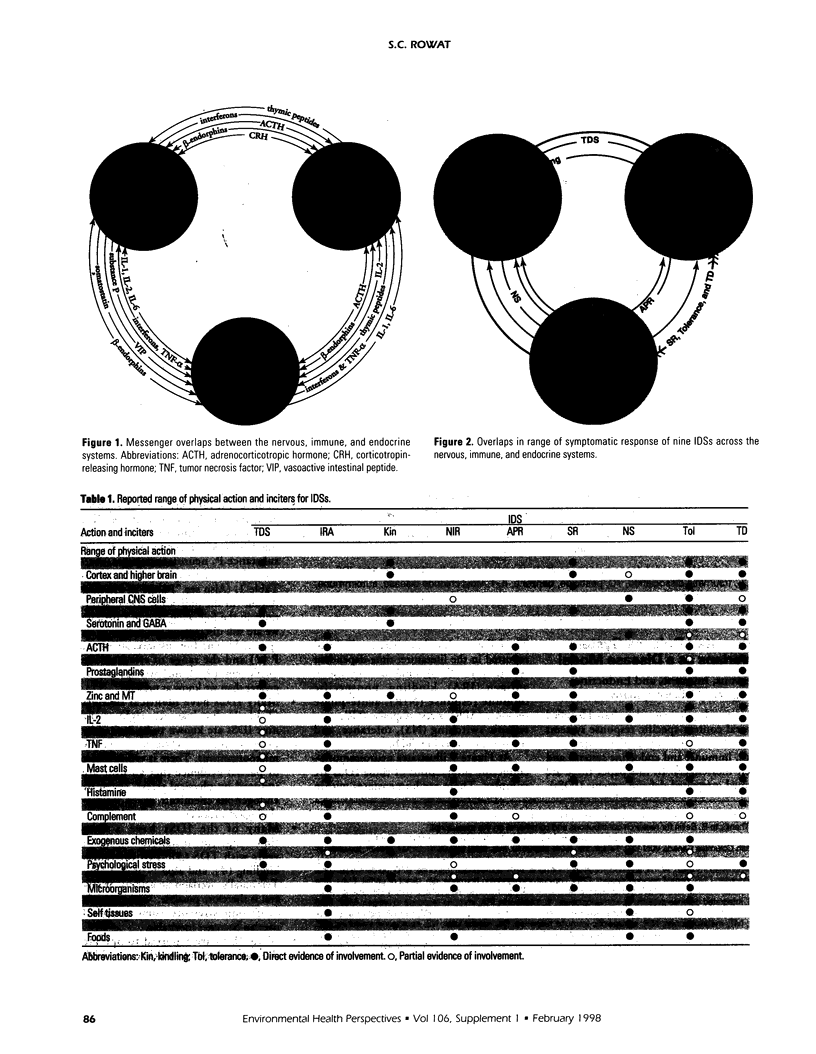
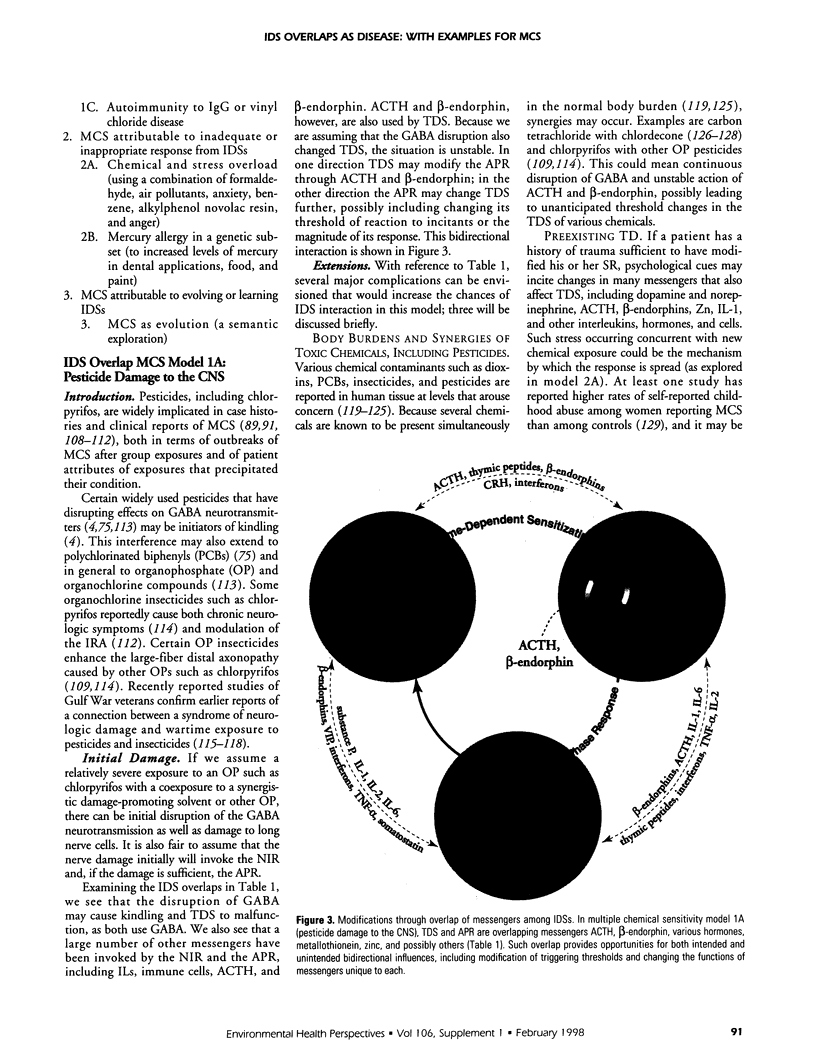
The central nervous, immune, and endocrine systems communicate through multiple common messengers. Over evolutionary time, what may be termed integrated defense system(s) (IDS) have developed to coordinate these communications for specific contexts; these include the stress response, acute-phase response, nonspecific immune response, immune response to antigen, kindling, tolerance, time-dependent sensitization, neurogenic switching, and traumatic dissociation (TD). These IDSs are described and their overlap is examined. Three models of disease production are generated: damage, in which IDSs function incorrectly; inadequate/inappropriate, in which IDS response is outstripped by a changing context; and evolving/learning, in which the IDS learned response to a context is deemed pathologic. Mechanisms of multiple chemical sensitivity (MCS) are developed from several IDS disease models. Model 1A is pesticide damage to the central nervous system, overlapping with body chemical burdens, TD, and chronic zinc deficiency; model 1B is benzene disruption of interleukin-1, overlapping with childhood developmental windows and hapten-antigenic spreading; and model 1C is autoimmunity to immunoglobulin-G (IgG), overlapping with spreading to other IgG-inducers, sudden spreading of inciters, and food-contaminating chemicals. Model 2A is chemical and stress overload, including comparison with the susceptibility/sensitization/triggering/spreading model; model 2B is genetic mercury allergy, overlapping with: heavy metals/zinc displacement and childhood/gestational mercury exposures; and model 3 is MCS as evolution and learning. Remarks are offered on current MCS research. Problems with clinical measurement are suggested on the basis of IDS models. Large-sample patient self-report epidemiology is described as an alternative or addition to clinical biomarker and animal testing.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamec R. Modelling anxiety disorders following chemical exposures. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):391–420. [PubMed] [Google Scholar]
- Ader R., Cohen N. Conditioned immunopharmacologic effects on cell-mediated immunity. Int J Immunopharmacol. 1992 Apr;14(3):323–327. doi: 10.1016/0192-0561(92)90161-d. [DOI] [PubMed] [Google Scholar]
- Ader R., Cohen N. Psychoneuroimmunology: conditioning and stress. Annu Rev Psychol. 1993;44:53–85. doi: 10.1146/annurev.ps.44.020193.000413. [DOI] [PubMed] [Google Scholar]
- Ader R., Kelly K., Moynihan J. A., Grota L. J., Cohen N. Conditioned enhancement of antibody production using antigen as the unconditioned stimulus. Brain Behav Immun. 1993 Dec;7(4):334–343. doi: 10.1006/brbi.1993.1033. [DOI] [PubMed] [Google Scholar]
- Agocs M. M., Etzel R. A., Parrish R. G., Paschal D. C., Campagna P. R., Cohen D. S., Kilbourne E. M., Hesse J. L. Mercury exposure from interior latex paint. N Engl J Med. 1990 Oct 18;323(16):1096–1101. doi: 10.1056/NEJM199010183231603. [DOI] [PubMed] [Google Scholar]
- Ahmed F. E., Hattis D., Wolke R. E., Steinman D. Risk assessment and management of chemical contaminants in fishery products consumed in the USA. J Appl Toxicol. 1993 Nov-Dec;13(6):395–410. doi: 10.1002/jat.2550130606. [DOI] [PubMed] [Google Scholar]
- Albright J. F., Goldstein R. A. Is there evidence of an immunologic basis for multiple chemical sensitivity? Toxicol Ind Health. 1992 Jul-Aug;8(4):215–219. [PubMed] [Google Scholar]
- Alexander P. C. Application of attachment theory to the study of sexual abuse. J Consult Clin Psychol. 1992 Apr;60(2):185–195. doi: 10.1037//0022-006x.60.2.185. [DOI] [PubMed] [Google Scholar]
- Allen J. I., Kay N. E., McClain C. J. Severe zinc deficiency in humans: association with a reversible T-lymphocyte dysfunction. Ann Intern Med. 1981 Aug;95(2):154–157. doi: 10.7326/0003-4819-95-2-154. [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Coevolution of hosts and parasites. Parasitology. 1982 Oct;85(Pt 2):411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Ansel J. C., Brown J. R., Payan D. G., Brown M. A. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993 May 15;150(10):4478–4485. [PubMed] [Google Scholar]
- Antelman S. M., Kocan D., Knopf S., Edwards D. J., Caggiula A. R. One brief exposure to a psychological stressor induces long-lasting, time-dependent sensitization of both the cataleptic and neurochemical responses to haloperidol. Life Sci. 1992;51(4):261–266. doi: 10.1016/0024-3205(92)90084-3. [DOI] [PubMed] [Google Scholar]
- Antelman S. M. Time-dependent sensitization in animals: a possible model of multiple chemical sensitivity in humans. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):335–342. [PubMed] [Google Scholar]
- Arce G. T., Vincent D. R., Cunningham M. J., Choy W. N., Sarrif A. M. In vitro and in vivo genotoxicity of 1,3-butadiene and metabolites. Environ Health Perspect. 1990 Jun;86:75–78. doi: 10.1289/ehp.908675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghurst P. A., McMichael A. J., Wigg N. R., Vimpani G. V., Robertson E. F., Roberts R. J., Tong S. L. Environmental exposure to lead and children's intelligence at the age of seven years. The Port Pirie Cohort Study. N Engl J Med. 1992 Oct 29;327(18):1279–1284. doi: 10.1056/NEJM199210293271805. [DOI] [PubMed] [Google Scholar]
- Balazs T. Immunogenetically controlled autoimmune reactions induced by mercury, gold and D-penicillamine in laboratory animals: a review from the vantage point of premarketing safety studies. Toxicol Ind Health. 1987 Sep;3(3):331–336. doi: 10.1177/074823378700300305. [DOI] [PubMed] [Google Scholar]
- Ballieux R. E. Bidirectional communication between the brain and the immune system. Eur J Clin Invest. 1992 Oct;22 (Suppl 1):6–9. [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Durham D. A. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 1989 Dec;23(6):433–437. doi: 10.1016/0361-9230(89)90185-8. [DOI] [PubMed] [Google Scholar]
- Barlow S. M. The role of the Scientific Committee for Food in evaluating plastics for packaging. Food Addit Contam. 1994 Mar-Apr;11(2):249–259. doi: 10.1080/02652039409374223. [DOI] [PubMed] [Google Scholar]
- Barton S. Chaos, self-organization, and psychology. Am Psychol. 1994 Jan;49(1):5–14. doi: 10.1037//0003-066x.49.1.5. [DOI] [PubMed] [Google Scholar]
- Bascom R., Meggs W. J., Frampton M., Hudnell K., Killburn K., Kobal G., Medinsky M., Rea W. Neurogenic inflammation: with additional discussion of central and perceptual integration of nonneurogenic inflammation. Environ Health Perspect. 1997 Mar;105 (Suppl 2):531–537. doi: 10.1289/ehp.97105s2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassingthwaighte J. B. Chaos in cardiac signals. Adv Exp Med Biol. 1993;346:207–218. doi: 10.1007/978-1-4615-2946-0_20. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol Today. 1994 Feb;15(2):74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Beckman D. A., Brent R. L. Mechanisms of teratogenesis. Annu Rev Pharmacol Toxicol. 1984;24:483–500. doi: 10.1146/annurev.pa.24.040184.002411. [DOI] [PubMed] [Google Scholar]
- Bell I. R., Peterson J. M., Schwartz G. E. Medical histories and psychological profiles of middle-aged women with and without self-reported illness from environmental chemicals. J Clin Psychiatry. 1995 Apr;56(4):151–160. [PubMed] [Google Scholar]
- Bell I. R., Rossi J., 3rd, Gilbert M. E., Kobal G., Morrow L. A., Newlin D. B., Sorg B. A., Wood R. W. Testing the neural sensitization and kindling hypothesis for illness from low levels of environmental chemicals. Environ Health Perspect. 1997 Mar;105 (Suppl 2):539–547. doi: 10.1289/ehp.97105s2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell I. R., Schwartz G. E., Peterson J. M., Amend D. Self-reported illness from chemical odors in young adults without clinical syndromes or occupational exposures. Arch Environ Health. 1993 Jan-Feb;48(1):6–13. doi: 10.1080/00039896.1993.9938387. [DOI] [PubMed] [Google Scholar]
- Bell I. R. White paper: Neuropsychiatric aspects of sensitivity to low-level chemicals: a neural sensitization model. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):277–312. [PubMed] [Google Scholar]
- Bemer V., Rovira P., Truffa-Bachi P. T-cell activation, anergy and immunomodulation by molecules of viral, fungal and vegetal origin. Res Immunol. 1995 May-Jun;146(4-5):249–262. doi: 10.1016/0923-2494(96)80260-4. [DOI] [PubMed] [Google Scholar]
- Beusterien K. M., Etzel R. A., Agocs M. M., Egeland G. M., Socie E. M., Rouse M. A., Mortensen B. K. Indoor air mercury concentrations following application of interior latex paint. Arch Environ Contam Toxicol. 1991 Jul;21(1):62–64. doi: 10.1007/BF01055557. [DOI] [PubMed] [Google Scholar]
- Biondi M. The application of the human stress model to psychoneuroimmunology. Acta Neurol (Napoli) 1991 Aug;13(4):328–334. [PubMed] [Google Scholar]
- Bolla-Wilson K., Wilson R. J., Bleecker M. L. Conditioning of physical symptoms after neurotoxic exposure. J Occup Med. 1988 Sep;30(9):684–686. [PubMed] [Google Scholar]
- Boosalis M. G., Solem L. D., McCall J. T., Ahrenholz D. H., McClain C. J. Serum zinc response in thermal injury. J Am Coll Nutr. 1988 Feb;7(1):69–76. doi: 10.1080/07315724.1988.10720222. [DOI] [PubMed] [Google Scholar]
- Brayton C. F. Dimethyl sulfoxide (DMSO): a review. Cornell Vet. 1986 Jan;76(1):61–90. [PubMed] [Google Scholar]
- Broughton A., Thrasher J. D., Gard Z. Immunological evaluation of four arc welders exposed to fumes from ignited polyurethane (isocyanate) foam: antibodies and immune profiles. Am J Ind Med. 1988;13(4):463–472. doi: 10.1002/ajim.4700130406. [DOI] [PubMed] [Google Scholar]
- Bütz M. Practical applications from chaos theory to the psychotherapeutic process, a basic consideration of dynamics. Psychol Rep. 1993 Oct;73(2):543–554. doi: 10.2466/pr0.1993.73.2.543. [DOI] [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Calabrese E. J., Baldwin L. A., Mehendale H. M. G2 subpopulation in rat liver induced into mitosis by low-level exposure to carbon tetrachloride: an adaptive response. Toxicol Appl Pharmacol. 1993 Jul;121(1):1–7. doi: 10.1006/taap.1993.1121. [DOI] [PubMed] [Google Scholar]
- Castle L., Kelly M., Gilbert J. Migration of mineral hydrocarbons into foods. 2. Polystyrene, ABS, and waxed paperboard containers for dairy products. Food Addit Contam. 1993 Mar-Apr;10(2):167–174. doi: 10.1080/02652039309374140. [DOI] [PubMed] [Google Scholar]
- Cocke R., Moynihan J. A., Cohen N., Grota L. J., Ader R. Exposure to conspecific alarm chemosignals alters immune responses in BALB/c mice. Brain Behav Immun. 1993 Mar;7(1):36–46. doi: 10.1006/brbi.1993.1004. [DOI] [PubMed] [Google Scholar]
- Cohen N., Kehrl H., Berglund B., O'Leary A., Ross G., Seltzer J., Weisel C. Psychoneuroimmunology. Environ Health Perspect. 1997 Mar;105 (Suppl 2):527–529. doi: 10.1289/ehp.97105s2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conacher H. B., Page B. D., Ryan J. J. Industrial chemical contamination of foods. Food Addit Contam. 1993 Jan-Feb;10(1):129–143. doi: 10.1080/02652039309374136. [DOI] [PubMed] [Google Scholar]
- Cone J. E., Sult T. A. Acquired intolerance to solvents following pesticide/solvent exposure in a building: a new group of workers at risk for multiple chemical sensitivities? Toxicol Ind Health. 1992 Jul-Aug;8(4):29–39. [PubMed] [Google Scholar]
- Constantinidis J. The hypothesis of zinc deficiency in the pathogenesis of neurofibrillary tangles. Med Hypotheses. 1991 Aug;35(4):319–323. doi: 10.1016/0306-9877(91)90277-6. [DOI] [PubMed] [Google Scholar]
- Cooper E. L., Rinkevich B., Uhlenbruck G., Valembois P. Invertebrate immunity: another viewpoint. Scand J Immunol. 1992 Mar;35(3):247–266. doi: 10.1111/j.1365-3083.1992.tb02857.x. [DOI] [PubMed] [Google Scholar]
- Cooper P. A whiff of petrol. Food Cosmet Toxicol. 1980 Aug;18(4):433–434. doi: 10.1016/0015-6264(80)90209-6. [DOI] [PubMed] [Google Scholar]
- Corrigan F. M., Coulter F. Neuroleptic malignant syndrome, amitriptyline, and thioridazine. Biol Psychiatry. 1988 Feb 1;23(3):320–321. doi: 10.1016/0006-3223(88)90044-3. [DOI] [PubMed] [Google Scholar]
- Corrigan F. M., MacDonald S., Brown A., Armstrong K., Armstrong E. M. Neurasthenic fatigue, chemical sensitivity and GABAa receptor toxins. Med Hypotheses. 1994 Oct;43(4):195–200. doi: 10.1016/0306-9877(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Coto J. A., Hadden E. M., Sauro M., Zorn N., Hadden J. W. Interleukin 1 regulates secretion of zinc-thymulin by human thymic epithelial cells and its action on T-lymphocyte proliferation and nuclear protein kinase C. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7752–7756. doi: 10.1073/pnas.89.16.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. L., Connor J. D. Zinc in maturing rat brain: hippocampal concentration and localization. J Neurochem. 1972 Jun;19(6):1451–1458. doi: 10.1111/j.1471-4159.1972.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Cross S. S., Cotton D. W. Chaos and antichaos in pathology. Hum Pathol. 1994 Jul;25(7):630–637. doi: 10.1016/0046-8177(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Cunningham E. T., Jr, De Souza E. B. Interleukin 1 receptors in the brain and endocrine tissues. Immunol Today. 1993 Apr;14(4):171–176. doi: 10.1016/0167-5699(93)90281-o. [DOI] [PubMed] [Google Scholar]
- Dafny N., Lee J. R., Dougherty P. M. Immune response products alter CNS activity: interferon modulates central opioid functions. J Neurosci Res. 1988;19(1):130–139. doi: 10.1002/jnr.490190118. [DOI] [PubMed] [Google Scholar]
- Dantzer R., Kelley K. W. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44(26):1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Pléau J. M., Savino W., Prasad A. S., Bach J. F. Biochemical and biological aspects of the interaction between thymulin and zinc. Prog Clin Biol Res. 1993;380:23–32. [PubMed] [Google Scholar]
- Davis J. M., Svendsgaard D. J. U-shaped dose-response curves: their occurrence and implications for risk assessment. J Toxicol Environ Health. 1990 Jun;30(2):71–83. doi: 10.1080/15287399009531412. [DOI] [PubMed] [Google Scholar]
- Depue R. A., Spoont M. R. Conceptualizing a serotonin trait. A behavioral dimension of constraint. Ann N Y Acad Sci. 1986;487:47–62. doi: 10.1111/j.1749-6632.1986.tb27885.x. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Divac-Jovanovic M., Svrakic D., Lecic-Tosevski D. Personality disorders: model for conceptual approach and classification. Part I: General model. Am J Psychother. 1993 Fall;47(4):558–571. doi: 10.1176/appi.psychotherapy.1993.47.4.558. [DOI] [PubMed] [Google Scholar]
- Drasch G. A. An increase of cadmium body burden for this century--an investigation on human tissues. Sci Total Environ. 1983 Jan;26(2):111–119. doi: 10.1016/0048-9697(83)90105-5. [DOI] [PubMed] [Google Scholar]
- Dreosti I. E., Manuel S. J., Buckley R. A., Fraser F. J., Record I. R. The effect of late prenatal and/or early postnatal zinc deficiency on the development and some biochemical aspects of the cerebellum and hippocampus in rats. Life Sci. 1981 May 11;28(19):2133–2141. doi: 10.1016/0024-3205(81)90620-2. [DOI] [PubMed] [Google Scholar]
- Eastmond D. A. Induction of micronuclei and aneuploidy by the quinone-forming agents benzene and o-phenylphenol. Toxicol Lett. 1993 Apr;67(1-3):105–118. doi: 10.1016/0378-4274(93)90049-4. [DOI] [PubMed] [Google Scholar]
- Elbert T., Ray W. J., Kowalik Z. J., Skinner J. E., Graf K. E., Birbaumer N. Chaos and physiology: deterministic chaos in excitable cell assemblies. Physiol Rev. 1994 Jan;74(1):1–47. doi: 10.1152/physrev.1994.74.1.1. [DOI] [PubMed] [Google Scholar]
- Eley B. M., Cox S. W. The release, absorption and possible health effects of mercury from dental amalgam: a review of recent findings. Br Dent J. 1993 Nov 20;175(10):355–362. doi: 10.1038/sj.bdj.4808325. [DOI] [PubMed] [Google Scholar]
- Eneström S., Hultman P. Does amalgam affect the immune system? A controversial issue. Int Arch Allergy Immunol. 1995 Mar;106(3):180–203. doi: 10.1159/000236843. [DOI] [PubMed] [Google Scholar]
- Eriksson P. DDT and pyrethroids--ecotoxicological considerations. Comp Biochem Physiol C. 1991;100(1-2):269–270. doi: 10.1016/0742-8413(91)90166-q. [DOI] [PubMed] [Google Scholar]
- Farmer J. D., Kauffman S. A., Packard N. H., Perelson A. S. Adaptive dynamic networks as models for the immune system and autocatalytic sets. Ann N Y Acad Sci. 1987;504:118–131. doi: 10.1111/j.1749-6632.1987.tb48728.x. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Kilian P. L., Ruff M. R., Hill J. M., Pert C. B. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987 Jul 15;139(2):459–463. [PubMed] [Google Scholar]
- Fiedler N., Kipen H. Chemical sensitivity: the scientific literature. Environ Health Perspect. 1997 Mar;105 (Suppl 2):409–415. doi: 10.1289/ehp.97105s2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald W. F., Clarkson T. W. Mercury and monomethylmercury: present and future concerns. Environ Health Perspect. 1991 Dec;96:159–166. doi: 10.1289/ehp.9196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth D. S., Dabeka R., Sun W. F., Dalglish K. Speciation of organotins in poly(vinyl chloride) products. Food Addit Contam. 1993 Sep-Oct;10(5):531–540. doi: 10.1080/02652039309374176. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., DePasquale-Jardieu P., Zwickl C. M., Luecke R. W. Regeneration of T-cell helper function in zinc-deficient adult mice. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5660–5664. doi: 10.1073/pnas.75.11.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson C. J., Danscher G. Zinc-containing neurons in hippocampus and related CNS structures. Prog Brain Res. 1990;83:71–84. doi: 10.1016/s0079-6123(08)61242-x. [DOI] [PubMed] [Google Scholar]
- Gilbert J., Castle L., Jickells S. M., Sharman M. Current research on food contact materials undertaken by the UK Ministry of Agriculture, Fisheries and Food. Food Addit Contam. 1994 Mar-Apr;11(2):231–240. doi: 10.1080/02652039409374221. [DOI] [PubMed] [Google Scholar]
- Gill J. I., Gulley M. L. Immunoglobulin and T-cell receptor gene rearrangement. Hematol Oncol Clin North Am. 1994 Aug;8(4):751–770. [PubMed] [Google Scholar]
- Glaser R., Kennedy S., Lafuse W. P., Bonneau R. H., Speicher C., Hillhouse J., Kiecolt-Glaser J. K. Psychological stress-induced modulation of interleukin 2 receptor gene expression and interleukin 2 production in peripheral blood leukocytes. Arch Gen Psychiatry. 1990 Aug;47(8):707–712. doi: 10.1001/archpsyc.1990.01810200015002. [DOI] [PubMed] [Google Scholar]
- Goldsmith J. R., Kordysh E. Why dose-response relationships are often non-linear and some consequences. J Expo Anal Environ Epidemiol. 1993 Jul-Sep;3(3):259–276. [PubMed] [Google Scholar]
- Grasso P. Neurotoxic and neurobehavioral effects of organic solvents on the nervous system. Occup Med. 1988 Jul-Sep;3(3):525–539. [PubMed] [Google Scholar]
- Griffiths H. R., Lunec J. Effect of polymorph derived oxidants on IgG in relation to rheumatoid factor binding. Scand J Rheumatol Suppl. 1988;75:148–156. doi: 10.3109/03009748809096756. [DOI] [PubMed] [Google Scholar]
- Grob K., Lanfranchi M., Egli J., Artho A. Determination of food contamination by mineral oil from jute sacks using coupled LC-GC. J Assoc Off Anal Chem. 1991 May-Jun;74(3):506–512. [PubMed] [Google Scholar]
- Grossman Z., Herberman R. B., Livnat S. Neural modulation of immunity: conditioning phenomena and the adaptability of lymphoid cells. Int J Neurosci. 1992 May-Jun;64(1-4):275–290. doi: 10.3109/00207459209000555. [DOI] [PubMed] [Google Scholar]
- Haeney M. R., Goodwin B. J., Barratt M. E., Mike N., Asquith P. Soya protein antibodies in man: their occurrence and possible relevance in coeliac disease. J Clin Pathol. 1982 Mar;35(3):319–322. doi: 10.1136/jcp.35.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn L. J., Kloiber R., Vimy M. J., Takahashi Y., Lorscheider F. L. Dental "silver" tooth fillings: a source of mercury exposure revealed by whole-body image scan and tissue analysis. FASEB J. 1989 Dec;3(14):2641–2646. doi: 10.1096/fasebj.3.14.2636872. [DOI] [PubMed] [Google Scholar]
- Haley R. W., Hom J., Roland P. S., Bryan W. W., Van Ness P. C., Bonte F. J., Devous M. D., Sr, Mathews D., Fleckenstein J. L., Wians F. H., Jr Evaluation of neurologic function in Gulf War veterans. A blinded case-control study. JAMA. 1997 Jan 15;277(3):223–230. [PubMed] [Google Scholar]
- Haley R. W., Kurt T. L., Hom J. Is there a Gulf War Syndrome? Searching for syndromes by factor analysis of symptoms. JAMA. 1997 Jan 15;277(3):215–222. [PubMed] [Google Scholar]
- Haley R. W., Kurt T. L. Self-reported exposure to neurotoxic chemical combinations in the Gulf War. A cross-sectional epidemiologic study. JAMA. 1997 Jan 15;277(3):231–237. [PubMed] [Google Scholar]
- Harrison N. L., Radke H. K., Talukder G., Ffrench-Mullen J. M. Zinc modulates transient outward current gating in hippocampal neurons. Receptors Channels. 1993;1(2):153–163. [PubMed] [Google Scholar]
- Herman J., Russell D., Trocki K. Long-term effects of incestuous abuse in childhood. Am J Psychiatry. 1986 Oct;143(10):1293–1296. doi: 10.1176/ajp.143.10.1293. [DOI] [PubMed] [Google Scholar]
- Holmberg P. C. Central-nervous-system defects in children born to mothers exposed to organic solvents during pregnancy. Lancet. 1979 Jul 28;2(8135):177–179. doi: 10.1016/s0140-6736(79)91438-7. [DOI] [PubMed] [Google Scholar]
- Holmskov U., Malhotra R., Sim R. B., Jensenius J. C. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994 Feb;15(2):67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Hou S., Hyland L., Ryan K. W., Portner A., Doherty P. C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994 Jun 23;369(6482):652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- Hultman P., Johansson U., Turley S. J., Lindh U., Eneström S., Pollard K. M. Adverse immunological effects and autoimmunity induced by dental amalgam and alloy in mice. FASEB J. 1994 Nov;8(14):1183–1190. doi: 10.1096/fasebj.8.14.7958626. [DOI] [PubMed] [Google Scholar]
- Ipsen J., Deane M., Ingenito F. E. Relationships of acute respiratory disease to atmospheric pollution and meteorological conditions. Arch Environ Health. 1969 Apr;18(4):462–472. doi: 10.1080/00039896.1969.10665439. [DOI] [PubMed] [Google Scholar]
- Ishiwata H., Inoue T., Yoshihira K. Tetramethylsuccinonitrile in polyvinyl chloride products for food and its release into food-simulating solvents. Z Lebensm Unters Forsch. 1987 Jul;185(1):39–42. doi: 10.1007/BF01083339. [DOI] [PubMed] [Google Scholar]
- Israel Y., Hurwitz E., Niemelä O., Arnon R. Monoclonal and polyclonal antibodies against acetaldehyde-containing epitopes in acetaldehyde-protein adducts. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7923–7927. doi: 10.1073/pnas.83.20.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y., MacDonald A., Niemelä O., Zamel D., Shami E., Zywulko M., Klajner F., Borgono C. Hypersensitivity to acetaldehyde-protein adducts. Mol Pharmacol. 1992 Oct;42(4):711–717. [PubMed] [Google Scholar]
- Ito K., Thurston G. D., Hayes C., Lippmann M. Associations of London, England, daily mortality with particulate matter, sulfur dioxide, and acidic aerosol pollution. Arch Environ Health. 1993 Jul-Aug;48(4):213–220. doi: 10.1080/00039896.1993.9940362. [DOI] [PubMed] [Google Scholar]
- Itoh T., Saito T., Fujimura M., Watanabe S., Saito K. Restraint stress-induced changes in endogenous zinc release from the rat hippocampus. Brain Res. 1993 Aug 6;618(2):318–322. doi: 10.1016/0006-8993(93)91283-x. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Jacobson S. W., Humphrey H. E. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J Pediatr. 1990 Jan;116(1):38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- Jameson S. Zinc status in pregnancy: the effect of zinc therapy on perinatal mortality, prematurity, and placental ablation. Ann N Y Acad Sci. 1993 Mar 15;678:178–192. doi: 10.1111/j.1749-6632.1993.tb26121.x. [DOI] [PubMed] [Google Scholar]
- Jensen G. E., Clausen J. Organochlorine compounds in adipose tissue of Greenlanders and southern Danes. J Toxicol Environ Health. 1979 Jul;5(4):617–629. doi: 10.1080/15287397909529774. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Jonakait G. M., Schotland S., Hart R. P. Interleukin-1 specifically increases substance P in injured sympathetic ganglia. Ann N Y Acad Sci. 1990;594:222–230. doi: 10.1111/j.1749-6632.1990.tb40482.x. [DOI] [PubMed] [Google Scholar]
- Jones M. M., Cherian M. G. The search for chelate antagonists for chronic cadmium intoxication. Toxicology. 1990 May 14;62(1):1–25. doi: 10.1016/0300-483x(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Kalf G. F., Renz J. F., Niculescu R. p-Benzoquinone, a reactive metabolite of benzene, prevents the processing of pre-interleukins-1 alpha and -1 beta to active cytokines by inhibition of the processing enzymes, calpain, and interleukin-1 beta converting enzyme. Environ Health Perspect. 1996 Dec;104 (Suppl 6):1251–1256. doi: 10.1289/ehp.961041251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. G., Kessler J., Rosenberg N., Pack D., Schaumburg H. H. Sensory neuropathy associated with Dursban (chlorpyrifos) exposure. Neurology. 1993 Nov;43(11):2193–2196. doi: 10.1212/wnl.43.11.2193. [DOI] [PubMed] [Google Scholar]
- Kavelaars A., Ballieux R. E., Heijnen C. J. The role of IL-1 in the corticotropin-releasing factor and arginine- vasopressin-induced secretion of immunoreactive beta-endorphin by human peripheral blood mononuclear cells. J Immunol. 1989 Apr 1;142(7):2338–2342. [PubMed] [Google Scholar]
- Keen C. L., Taubeneck M. W., Daston G. P., Rogers J. M., Gershwin M. E. Primary and secondary zinc deficiency as factors underlying abnormal CNS development. Ann N Y Acad Sci. 1993 Mar 15;678:37–47. doi: 10.1111/j.1749-6632.1993.tb26108.x. [DOI] [PubMed] [Google Scholar]
- Kenna J. G., Neuberger J., Williams R. Evidence for expression in human liver of halothane-induced neoantigens recognized by antibodies in sera from patients with halothane hepatitis. Hepatology. 1988 Nov-Dec;8(6):1635–1641. doi: 10.1002/hep.1840080627. [DOI] [PubMed] [Google Scholar]
- Kenney J. S., Baker C., Welch M. R., Altman L. C. Synthesis of interleukin-1 alpha, interleukin-6, and interleukin-8 by cultured human nasal epithelial cells. J Allergy Clin Immunol. 1994 Jun;93(6):1060–1067. doi: 10.1016/s0091-6749(94)70055-9. [DOI] [PubMed] [Google Scholar]
- Keplinger H. Patient statement: chemically sensitive. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):313–317. [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Marucha P. T., Malarkey W. B., Mercado A. M., Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995 Nov 4;346(8984):1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Fanselow M. S. Modality-specific retrograde amnesia of fear. Science. 1992 May 1;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kluft R. P. Clinical presentations of multiple personality disorder. Psychiatr Clin North Am. 1991 Sep;14(3):605–629. [PubMed] [Google Scholar]
- Kolachana P., Subrahmanyam V. V., Meyer K. B., Zhang L., Smith M. T. Benzene and its phenolic metabolites produce oxidative DNA damage in HL60 cells in vitro and in the bone marrow in vivo. Cancer Res. 1993 Mar 1;53(5):1023–1026. [PubMed] [Google Scholar]
- Koshte V. L., Aalbers M., Calkhoven P. G., Aalberse R. C. The potent IgG4-inducing antigen in banana is a mannose-binding lectin, BanLec-I. Int Arch Allergy Immunol. 1992;97(1):17–24. doi: 10.1159/000236090. [DOI] [PubMed] [Google Scholar]
- Kuhnert B. R., Kuhnert P. M., Debanne S., Williams T. G. The relationship between cadmium, zinc, and birth weight in pregnant women who smoke. Am J Obstet Gynecol. 1987 Nov;157(5):1247–1251. doi: 10.1016/s0002-9378(87)80303-4. [DOI] [PubMed] [Google Scholar]
- Kuhnert B. R., Kuhnert P. M., Groh-Wargo S. L., Webster S., Erhard P., Lazebnik N. Smoking alters the relationship between maternal zinc intake and biochemical indices of fetal zinc status. Am J Clin Nutr. 1992 May;55(5):981–984. doi: 10.1093/ajcn/55.5.981. [DOI] [PubMed] [Google Scholar]
- Kutz F. W., Cook B. T., Carter-Pokras O. D., Brody D., Murphy R. S. Selected pesticide residues and metabolites in urine from a survey of the U.S. general population. J Toxicol Environ Health. 1992 Oct;37(2):277–291. doi: 10.1080/15287399209531670. [DOI] [PubMed] [Google Scholar]
- Kutz F. W., Wood P. H., Bottimore D. P. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev Environ Contam Toxicol. 1991;120:1–82. doi: 10.1007/978-1-4612-3080-9_1. [DOI] [PubMed] [Google Scholar]
- La Via M. F., Workman E. A. Psychoneuroimmunology: where are we, where are we going? Recenti Prog Med. 1991 Dec;82(12):637–641. [PubMed] [Google Scholar]
- LaMarte F. P., Merchant J. A., Casale T. B. Acute systemic reactions to carbonless copy paper associated with histamine release. JAMA. 1988 Jul 8;260(2):242–243. [PubMed] [Google Scholar]
- Lambert B., He S. M. DNA and chromosome damage induced by acetaldehyde in human lymphocytes in vitro. Ann N Y Acad Sci. 1988;534:369–376. doi: 10.1111/j.1749-6632.1988.tb30124.x. [DOI] [PubMed] [Google Scholar]
- Landrigan P. J. Critical assessment of epidemiologic studies on the human carcinogenicity of 1,3-butadiene. Environ Health Perspect. 1990 Jun;86:143–147. doi: 10.1289/ehp.9086143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. L., Jamieson B. D., Somasundaram T., Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994 Jun 23;369(6482):648–652. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- Levin E. D., Uemura E., Bowman R. E. Neurobehavioral toxicology of halothane in rats. Neurotoxicol Teratol. 1991 Jul-Aug;13(4):461–470. doi: 10.1016/0892-0362(91)90096-f. [DOI] [PubMed] [Google Scholar]
- Licastro F., Davis L. J., Morini M. C. Lectins and superantigens: membrane interactions of these compounds with T lymphocytes affect immune responses. Int J Biochem. 1993 Jun;25(6):845–852. doi: 10.1016/0020-711x(93)90239-b. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Biochemical and molecular basis of alcohol-induced injury to liver and other tissues. N Engl J Med. 1988 Dec 22;319(25):1639–1650. doi: 10.1056/NEJM198812223192505. [DOI] [PubMed] [Google Scholar]
- Lisk D. J. Environmental implications of incineration of municipal solid waste and ash disposal. Sci Total Environ. 1988 Aug 1;74:39–66. doi: 10.1016/0048-9697(88)90128-3. [DOI] [PubMed] [Google Scholar]
- Litovitz T., Greene A. E. Health implications of petroleum distillate ingestion. Occup Med. 1988 Jul-Sep;3(3):555–568. [PubMed] [Google Scholar]
- Lonie I. Borderline disorder and post-traumatic stress disorder: an equivalence? Aust N Z J Psychiatry. 1993 Jun;27(2):233–245. doi: 10.1080/00048679309075772. [DOI] [PubMed] [Google Scholar]
- Lorscheider F. L., Vimy M. J. Evaluation of the safety issue of mercury release from dental fillings. FASEB J. 1993 Dec;7(15):1432–1433. doi: 10.1096/fasebj.7.15.8262327. [DOI] [PubMed] [Google Scholar]
- Lorscheider F. L., Vimy M. J. Mercury exposure from interior latex paint. N Engl J Med. 1991 Mar 21;324(12):851–852. doi: 10.1056/NEJM199103213241216. [DOI] [PubMed] [Google Scholar]
- MacFarland H. N. Toxicology of petroleum hydrocarbons. Occup Med. 1988 Jul-Sep;3(3):445–454. [PubMed] [Google Scholar]
- MacQueen G., Marshall J., Perdue M., Siegel S., Bienenstock J. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science. 1989 Jan 6;243(4887):83–85. doi: 10.1126/science.2911721. [DOI] [PubMed] [Google Scholar]
- Marks J. G., Jr, Trautlein J. J., Zwillich C. W., Demers L. M. Contact urticaria and airway obstruction from carbonless copy paper. JAMA. 1984 Aug 24;252(8):1038–1040. [PubMed] [Google Scholar]
- Marsh D. O., Myers G. J., Clarkson T. W., Amin-Zaki L., Tikriti S., Majeed M. A. Fetal methylmercury poisoning: clinical and toxicological data on 29 cases. Ann Neurol. 1980 Apr;7(4):348–353. doi: 10.1002/ana.410070412. [DOI] [PubMed] [Google Scholar]
- May R. M. Parasitic infections as regulators of animal populations. Am Sci. 1983 Jan-Feb;71(1):36–45. [PubMed] [Google Scholar]
- McCoy J. J., Mann B. J., Petri W. A., Jr Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect Immun. 1994 Aug;62(8):3045–3050. doi: 10.1128/iai.62.8.3045-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern J. J., Jr, Lazaroni J. A., Hicks M. F., Adler J. C., Cleary P. Food and chemical sensitivity. Clinical and immunologic correlates. Arch Otolaryngol. 1983 May;109(5):292–297. doi: 10.1001/archotol.1983.00800190014004. [DOI] [PubMed] [Google Scholar]
- Meggs W. J. Neurogenic inflammation and sensitivity to environmental chemicals. Environ Health Perspect. 1993 Aug;101(3):234–238. doi: 10.1289/ehp.93101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggs W. J. Neurogenic switching: a hypothesis for a mechanism for shifting the site of inflammation in allergy and chemical sensitivity. Environ Health Perspect. 1995 Jan;103(1):54–56. doi: 10.1289/ehp.9510354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggs W. J. RADS and RUDS--the toxic induction of asthma and rhinitis. J Toxicol Clin Toxicol. 1994;32(5):487–501. doi: 10.3109/15563659409011053. [DOI] [PubMed] [Google Scholar]
- Mehendale H. M. Potentiation of halomethane hepatotoxicity by chlordecone: a hypothesis for the mechanism. Med Hypotheses. 1990 Dec;33(4):289–299. doi: 10.1016/0306-9877(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Miller A. C., Schattenberg D. G., Malkinson A. M., Ross D. Decreased content of the IL1 alpha processing enzyme calpain in murine bone marrow-derived macrophages after treatment with the benzene metabolite hydroquinone. Toxicol Lett. 1994 Nov;74(2):177–184. doi: 10.1016/0378-4274(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Miller C. S. Chemical sensitivity: symptom, syndrome or mechanism for disease? Toxicology. 1996 Jul 17;111(1-3):69–86. doi: 10.1016/0300-483x(96)03393-8. [DOI] [PubMed] [Google Scholar]
- Miller C. S., Mitzel H. C. Chemical sensitivity attributed to pesticide exposure versus remodeling. Arch Environ Health. 1995 Mar-Apr;50(2):119–129. doi: 10.1080/00039896.1995.9940889. [DOI] [PubMed] [Google Scholar]
- Miller C. S. Possible models for multiple chemical sensitivity: conceptual issues and role of the limbic system. Toxicol Ind Health. 1992 Jul-Aug;8(4):181–202. [PubMed] [Google Scholar]
- Miller C. S. Toxicant-induced loss of tolerance--an emerging theory of disease? Environ Health Perspect. 1997 Mar;105 (Suppl 2):445–453. doi: 10.1289/ehp.97105s2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. S. White paper: Chemical sensitivity: history and phenomenology. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):253–276. [PubMed] [Google Scholar]
- Miller C., Ashford N., Doty R., Lamielle M., Otto D., Rahill A., Wallace L. Empirical approaches for the investigation of toxicant-induced loss of tolerance. Environ Health Perspect. 1997 Mar;105 (Suppl 2):515–519. doi: 10.1289/ehp.97105s2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington G., Buckingham J. C. Thymic peptides and neuroendocrine-immune communication. J Endocrinol. 1992 May;133(2):163–168. doi: 10.1677/joe.0.1330163. [DOI] [PubMed] [Google Scholar]
- Montgomery M. R., Reasor M. J. A toxicologic approach for evaluating cases of sick building syndrome or multiple chemical sensitivity. J Allergy Clin Immunol. 1994 Aug;94(2 Pt 2):371–375. [PubMed] [Google Scholar]
- Moretto A., Lotti M. Promotion of peripheral axonopathies by certain esterase inhibitors. Toxicol Ind Health. 1993 Nov-Dec;9(6):1037–1046. doi: 10.1177/074823379300900604. [DOI] [PubMed] [Google Scholar]
- Mori N., Wada J. A., Watanabe M., Kumashiro H. Increased activity of superoxide dismutase in kindled brain and suppression of kindled seizure following intra-amygdaloid injection of superoxide dismutase in rats. Brain Res. 1991 Aug 23;557(1-2):313–315. doi: 10.1016/0006-8993(91)90151-k. [DOI] [PubMed] [Google Scholar]
- Morrissey R. E., Schwetz B. A., Hackett P. L., Sikov M. R., Hardin B. D., McClanahan B. J., Decker J. R., Mast T. J. Overview of reproductive and developmental toxicity studies of 1,3-butadiene in rodents. Environ Health Perspect. 1990 Jun;86:79–84. doi: 10.1289/ehp.908679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlebach S., Wyss P. A., Bickel M. H. The use of 2,4,5,2',4',5'-hexachlorobiphenyl (6-CB) as an unmetabolizable lipophilic model compound. Pharmacol Toxicol. 1991 Dec;69(6):410–415. doi: 10.1111/j.1600-0773.1991.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Nerín C., Cacho J., Gancedo P. Plasticizers from printing inks in a selection of food packagings and their migration to food. Food Addit Contam. 1993 Jul-Aug;10(4):453–460. doi: 10.1080/02652039309374168. [DOI] [PubMed] [Google Scholar]
- Newlin D. B. Drug sensitization, substance abuse, and chemical sensitivity. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):463–480. [PubMed] [Google Scholar]
- Niculescu R., Bradford H. N., Colman R. W., Kalf G. F. Inhibition of the conversion of pre-interleukins-1 alpha and 1 beta to mature cytokines by p-benzoquinone, a metabolite of benzene. Chem Biol Interact. 1995 Dec 22;98(3):211–222. doi: 10.1016/0009-2797(95)03647-4. [DOI] [PubMed] [Google Scholar]
- Niculescu R., Kalf G. F. A morphological analysis of the short-term effects of benzene on the development of the hematological cells in the bone marrow of mice and the effects of interleukin-1 alpha on the process. Arch Toxicol. 1995;69(3):141–148. doi: 10.1007/s002040050150. [DOI] [PubMed] [Google Scholar]
- Niemelä O. Acetaldehyde adducts of proteins: diagnostic and pathogenic implications in diseases caused by excessive alcohol consumption. Scand J Clin Lab Invest Suppl. 1993;213:45–54. doi: 10.3109/00365519309090673. [DOI] [PubMed] [Google Scholar]
- Niemelä O., Israel Y. Hemoglobin-acetaldehyde adducts in human alcohol abusers. Lab Invest. 1992 Aug;67(2):246–252. [PubMed] [Google Scholar]
- Niemelä O., Juvonen T., Parkkila S. Immunohistochemical demonstration of acetaldehyde-modified epitopes in human liver after alcohol consumption. J Clin Invest. 1991 Apr;87(4):1367–1374. doi: 10.1172/JCI115141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä O., Klajner F., Orrego H., Vidins E., Blendis L., Israel Y. Antibodies against acetaldehyde-modified protein epitopes in human alcoholics. Hepatology. 1987 Nov-Dec;7(6):1210–1214. doi: 10.1002/hep.1840070607. [DOI] [PubMed] [Google Scholar]
- Olness K., Ader R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. J Dev Behav Pediatr. 1992 Apr;13(2):124–125. doi: 10.1097/00004703-199204000-00008. [DOI] [PubMed] [Google Scholar]
- Oppelt E. T. Air emissions from the incineration of hazardous waste. Toxicol Ind Health. 1990 Oct;6(5):23–51. [PubMed] [Google Scholar]
- Ottaway C. A., Husband A. J. Central nervous system influences on lymphocyte migration. Brain Behav Immun. 1992 Jun;6(2):97–116. doi: 10.1016/0889-1591(92)90011-c. [DOI] [PubMed] [Google Scholar]
- Ottaway C. A. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol Clin North Am. 1991 Sep;20(3):511–529. [PubMed] [Google Scholar]
- Page B. D., Lacroix G. M. The occurrence of phthalate ester and di-2-ethylhexyl adipate plasticizers in Canadian packaging and food sampled in 1985-1989: a survey. Food Addit Contam. 1995 Jan-Feb;12(1):129–151. doi: 10.1080/02652039509374287. [DOI] [PubMed] [Google Scholar]
- Patterson R., Dykewicz M. S., Evans R., 3rd, Grammer L. C., Greenberger P. A., Harris K. E., Lawrence I. D., Pruzansky J. J., Roberts M., Shaughnessy M. A. IgG antibody against formaldehyde human serum proteins: a comparison with other IgG antibodies against inhalant proteins and reactive chemicals. J Allergy Clin Immunol. 1989 Sep;84(3):359–366. doi: 10.1016/0091-6749(89)90421-1. [DOI] [PubMed] [Google Scholar]
- Patterson R., Pateras V., Grammer L. C., Harris K. E. Human antibodies against formaldehyde-human serum albumin conjugates or human serum albumin in individuals exposed to formaldehyde. Int Arch Allergy Appl Immunol. 1986;79(1):53–59. doi: 10.1159/000233942. [DOI] [PubMed] [Google Scholar]
- Payan D. G., Goetzl E. J. Dual roles of substance P: modulator of immune and neuroendocrine functions. Ann N Y Acad Sci. 1987;512:465–475. doi: 10.1111/j.1749-6632.1987.tb24981.x. [DOI] [PubMed] [Google Scholar]
- Payan D. G. Substance P: a modulator of neuroendocrine-immune function. Hosp Pract (Off Ed) 1989 Feb 15;24(2):67-73, 76, 78-80. doi: 10.1080/21548331.1989.11703658. [DOI] [PubMed] [Google Scholar]
- Pearlman M. E., Finklea J. F., Creason J. P., Shy C. M., Young M. M., Horton R. J. Nitrogen dioxide and lower respiratory illness. Pediatrics. 1971 Feb;47(2):391–398. [PubMed] [Google Scholar]
- Plata-Salamán C. R. Immunoregulators in the nervous system. Neurosci Biobehav Rev. 1991 Summer;15(2):185–215. doi: 10.1016/s0149-7634(05)80001-6. [DOI] [PubMed] [Google Scholar]
- Prasad A. S., Meftah S., Abdallah J., Kaplan J., Brewer G. J., Bach J. F., Dardenne M. Serum thymulin in human zinc deficiency. J Clin Invest. 1988 Oct;82(4):1202–1210. doi: 10.1172/JCI113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler H., Raza A. An overview of some studies of chronic myelogenous leukemia: biological-clinical observations and viewing the disease as a chaotic system. Leuk Lymphoma. 1993;11 (Suppl 1):145–150. doi: 10.3109/10428199309047878. [DOI] [PubMed] [Google Scholar]
- Pusztai A. Dietary lectins are metabolic signals for the gut and modulate immune and hormone functions. Eur J Clin Nutr. 1993 Oct;47(10):691–699. [PubMed] [Google Scholar]
- Putnam F. W., Guroff J. J., Silberman E. K., Barban L., Post R. M. The clinical phenomenology of multiple personality disorder: review of 100 recent cases. J Clin Psychiatry. 1986 Jun;47(6):285–293. [PubMed] [Google Scholar]
- Ragan H. A., Mast T. J. Cadmium inhalation and male reproductive toxicity. Rev Environ Contam Toxicol. 1990;114:1–22. doi: 10.1007/978-1-4612-3368-8_1. [DOI] [PubMed] [Google Scholar]
- Rao S. B., Mehendale H. M. Halomethane-chlordecone (CD) interactive hepatotoxicity--current concepts on the mechanism. Indian J Biochem Biophys. 1993 Aug;30(4):191–198. [PubMed] [Google Scholar]
- Ratcliffe H. E., Swanson G. M., Fischer L. J. Human exposure to mercury: a critical assessment of the evidence of adverse health effects. J Toxicol Environ Health. 1996 Oct 25;49(3):221–270. doi: 10.1080/713851079. [DOI] [PubMed] [Google Scholar]
- Rauch S. L., van der Kolk B. A., Fisler R. E., Alpert N. M., Orr S. P., Savage C. R., Fischman A. J., Jenike M. A., Pitman R. K. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996 May;53(5):380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Raucy J. L., Kraner J. C., Lasker J. M. Bioactivation of halogenated hydrocarbons by cytochrome P4502E1. Crit Rev Toxicol. 1993;23(1):1–20. doi: 10.3109/10408449309104072. [DOI] [PubMed] [Google Scholar]
- Reid W. D., Ilett K. F., Glick J. M., Krishna G. Metabolism and binding of aromatic hydrocarbons in the lung. Relationship to experimental bronchiolar necrosis. Am Rev Respir Dis. 1973 Apr;107(4):539–551. doi: 10.1164/arrd.1973.107.4.539. [DOI] [PubMed] [Google Scholar]
- Reidbord S. P., Redington D. J. Psychophysiological processes during insight-oriented therapy. Further investigations into nonlinear psychodynamics. J Nerv Ment Dis. 1992 Oct;180(10):649–657. doi: 10.1097/00005053-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Reinhardt J. W. Side-effects: mercury contribution to body burden from dental amalgam. Adv Dent Res. 1992 Sep;6:110–113. doi: 10.1177/08959374920060010201. [DOI] [PubMed] [Google Scholar]
- Renz J. F., Kalf G. F. Role for interleukin-1 (IL-1) in benzene-induced hematotoxicity: inhibition of conversion of pre-IL-1 alpha to mature cytokine in murine macrophages by hydroquinone and prevention of benzene-induced hematotoxicity in mice by IL-1 alpha. Blood. 1991 Aug 15;78(4):938–944. [PubMed] [Google Scholar]
- Riihimäki V., Savolainen K. Human exposure to m-xylene. Kinetics and acute effects on the central nervous system. Ann Occup Hyg. 1980;23(4):411–422. doi: 10.1093/annhyg/23.4.411. [DOI] [PubMed] [Google Scholar]
- Robertson M. L., Eastmond D. A., Smith M. T. Two benzene metabolites, catechol and hydroquinone, produce a synergistic induction of micronuclei and toxicity in cultured human lymphocytes. Mutat Res. 1991 Jul;249(1):201–209. doi: 10.1016/0027-5107(91)90147-g. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. E., Cameron C. L. Exaggerated sensitivity to an organophosphate pesticide. Am J Psychiatry. 1991 Feb;148(2):270–270. [PubMed] [Google Scholar]
- Ross C. A. Epidemiology of multiple personality disorder and dissociation. Psychiatr Clin North Am. 1991 Sep;14(3):503–517. [PubMed] [Google Scholar]
- Ross C. A., Joshi S., Currie R. Dissociative experiences in the general population: a factor analysis. Hosp Community Psychiatry. 1991 Mar;42(3):297–301. doi: 10.1176/ps.42.3.297. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J. CNS regulation of thermogenesis. Crit Rev Neurobiol. 1994;8(1-2):1–10. [PubMed] [Google Scholar]
- Russek L. G., Schwartz G. E. Narrative descriptions of parental love and caring predict health status in midlife: a 35-year follow-up of the Harvard Mastery of Stress Study. Altern Ther Health Med. 1996 Nov;2(6):55–62. [PubMed] [Google Scholar]
- Sandstead H. H. Is zinc deficiency a public health problem? Nutrition. 1995 Jan-Feb;11(1 Suppl):87–92. [PubMed] [Google Scholar]
- Sato M., Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993 Mar;14(3):325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- Saunders E. A., Arnold F. A critique of conceptual and treatment approaches to borderline psychopathology in light of findings about childhood abuse. Psychiatry. 1993 May;56(2):188–203. doi: 10.1080/00332747.1993.11024633. [DOI] [PubMed] [Google Scholar]
- Savolainen H. Some aspects of the mechanisms by which industrial solvents produce neurotoxic effects. Chem Biol Interact. 1977 Jul;18(1):1–10. doi: 10.1016/0009-2797(77)90136-3. [DOI] [PubMed] [Google Scholar]
- Saxe G. N., van der Kolk B. A., Berkowitz R., Chinman G., Hall K., Lieberg G., Schwartz J. Dissociative disorders in psychiatric inpatients. Am J Psychiatry. 1993 Jul;150(7):1037–1042. doi: 10.1176/ajp.150.7.1037. [DOI] [PubMed] [Google Scholar]
- Scheepers P. T., Bos R. P. Combustion of diesel fuel from a toxicological perspective. II. Toxicity. Int Arch Occup Environ Health. 1992;64(3):163–177. doi: 10.1007/BF00380905. [DOI] [PubMed] [Google Scholar]
- Schwarz R. H. Considerations of antibiotic therapy during pregnancy. Obstet Gynecol. 1981 Nov;58(5 Suppl):95S–99S. [PubMed] [Google Scholar]
- Schwope A. D., Till D. E., Ehntholt D. J., Sidman K. R., Whelan R. H., Schwartz P. S., Reid R. C. Migration of BHT and Irganox 1010 from low-density polyethylene (LDPE) to foods and food-simulating liquids. Food Chem Toxicol. 1987 Apr;25(4):317–326. doi: 10.1016/0278-6915(87)90129-3. [DOI] [PubMed] [Google Scholar]
- Seaton A., MacNee W., Donaldson K., Godden D. Particulate air pollution and acute health effects. Lancet. 1995 Jan 21;345(8943):176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Shuman R. M., Leech R. W., Alvord E. C., Jr Neurotoxicity of hexachlorophene in humans. II. A clinicopathological study of 46 premature infants. Arch Neurol. 1975 May;32(5):320–325. doi: 10.1001/archneur.1975.00490470064009. [DOI] [PubMed] [Google Scholar]
- Shuman R. M., Leech R. W., Alvord E. C., Jr Neurotoxicity of hexachlorophene in the human: I. A clinicopathologic study of 248 children. Pediatrics. 1974 Dec;54(6):689–695. [PubMed] [Google Scholar]
- Siblerud R. L. A comparison of mental health of multiple sclerosis patients with silver/mercury dental fillings and those with fillings removed. Psychol Rep. 1992 Jun;70(3 Pt 2):1139–1151. doi: 10.2466/pr0.1992.70.3c.1139. [DOI] [PubMed] [Google Scholar]
- Siblerud R. L., Kienholz E. Evidence that mercury from silver dental fillings may be an etiological factor in multiple sclerosis. Sci Total Environ. 1994 Mar 15;142(3):191–205. doi: 10.1016/0048-9697(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Siblerud R. L., Motl J., Kienholz E. Psychometric evidence that mercury from silver dental fillings may be an etiological factor in depression, excessive anger, and anxiety. Psychol Rep. 1994 Feb;74(1):67–80. doi: 10.2466/pr0.1994.74.1.67. [DOI] [PubMed] [Google Scholar]
- Siblerud R. L. The relationship between mercury from dental amalgam and oral cavity health. Ann Dent. 1990 Winter;49(2):6–10. [PubMed] [Google Scholar]
- Siblerud R. L. The relationship between mercury from dental amalgam and the cardiovascular system. Sci Total Environ. 1990 Dec 1;99(1-2):23–35. doi: 10.1016/0048-9697(90)90207-b. [DOI] [PubMed] [Google Scholar]
- Siegel S., Kreutzer R. Pavlovian conditioning and multiple chemical sensitivity. Environ Health Perspect. 1997 Mar;105 (Suppl 2):521–526. doi: 10.1289/ehp.97105s2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G. E., Daniell W., Stockbridge H., Claypoole K., Rosenstock L. Immunologic, psychological, and neuropsychological factors in multiple chemical sensitivity. A controlled study. Ann Intern Med. 1993 Jul 15;119(2):97–103. doi: 10.7326/0003-4819-119-2-199307150-00001. [DOI] [PubMed] [Google Scholar]
- Simon T. R., Hickey D. C., Fincher C. E., Johnson A. R., Ross G. H., Rea W. J. Single photon emission computed tomography of the brain in patients with chemical sensitivities. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):573–577. [PubMed] [Google Scholar]
- Slevin J. T., Kasarskis E. J. Effects of zinc on markers of glutamate and aspartate neurotransmission in rat hippocampus. Brain Res. 1985 May 20;334(2):281–286. doi: 10.1016/0006-8993(85)90219-7. [DOI] [PubMed] [Google Scholar]
- Sorg B. A., Hooks M. S., Kalivas P. W. Neuroanatomy and neurochemical mechanisms of time-dependent sensitization. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):369–386. [PubMed] [Google Scholar]
- Sparrow G. P. A connective tissue disorder similar to vinyl chloride disease in a patient exposed to perchlorethylene. Clin Exp Dermatol. 1977 Mar;2(1):17–22. doi: 10.1111/j.1365-2230.1977.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Staudenmayer H., Selner J. C., Buhr M. P. Double-blind provocation chamber challenges in 20 patients presenting with "multiple chemical sensitivity". Regul Toxicol Pharmacol. 1993 Aug;18(1):44–53. doi: 10.1006/rtph.1993.1043. [DOI] [PubMed] [Google Scholar]
- Staudenmayer H., Selner M. E., Selner J. C. Adult sequelae of childhood abuse presenting as environmental illness. Ann Allergy. 1993 Dec;71(6):538–546. [PubMed] [Google Scholar]
- Stead R. H. Innervation of mucosal immune cells in the gastrointestinal tract. Reg Immunol. 1992 Mar-Apr;4(2):91–99. [PubMed] [Google Scholar]
- Stephens R. J., Freeman G., Evans M. J. Early response of lungs to low levels of nitrogen dioxide. Light and electron microscopy. Arch Environ Health. 1972 Mar;24(3):160–179. doi: 10.1080/00039896.1972.10666066. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Chrousos G. P., Wilder R. L., Gold P. W. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992 Nov 15;117(10):854–866. doi: 10.7326/0003-4819-117-10-854. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Chrousos G. P., Wilder R. L., Gold P. W. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992 Nov 15;117(10):854–866. doi: 10.7326/0003-4819-117-10-854. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Chrousos G. P., Wilder R. L., Gold P. W. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992 Nov 15;117(10):854–866. doi: 10.7326/0003-4819-117-10-854. [DOI] [PubMed] [Google Scholar]
- Strobel S. Dietary manipulation and induction of tolerance. J Pediatr. 1992 Nov;121(5 Pt 2):S74–S79. doi: 10.1016/s0022-3476(05)81411-8. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Wireman J., Vimy M. J., Lorscheider F. L., Marshall B., Levy S. B., Bennett S., Billard L. Mercury released from dental "silver" fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob Agents Chemother. 1993 Apr;37(4):825–834. doi: 10.1128/aac.37.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B. G., Schütz A., Nilsson A., Akesson I., Akesson B., Skerfving S. Fish as a source of exposure to mercury and selenium. Sci Total Environ. 1992 Sep 11;126(1-2):61–74. doi: 10.1016/0048-9697(92)90484-a. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. E. The possible relationship between mercury from dental amalgam and diseases. I: Effects within the oral cavity. Med Hypotheses. 1993 Jul;41(1):31–34. doi: 10.1016/0306-9877(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Taubeneck M. W., Daston G. P., Rogers J. M., Keen C. L. Altered maternal zinc metabolism following exposure to diverse developmental toxicants. Reprod Toxicol. 1994 Jan-Feb;8(1):25–40. doi: 10.1016/0890-6238(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Thaler D. S. The evolution of genetic intelligence. Science. 1994 Apr 8;264(5156):224–225. doi: 10.1126/science.8146652. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity. 1995 Nov;3(5):531–539. doi: 10.1016/1074-7613(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Thrasher J. D., Madison R., Broughton A. Immunologic abnormalities in humans exposed to chlorpyrifos: preliminary observations. Arch Environ Health. 1993 Mar-Apr;48(2):89–93. doi: 10.1080/00039896.1993.9938400. [DOI] [PubMed] [Google Scholar]
- Tollefson L. Multiple chemical sensitivity: controlled scientific studies as proof of causation. Regul Toxicol Pharmacol. 1993 Aug;18(1):32–43. doi: 10.1006/rtph.1993.1042. [DOI] [PubMed] [Google Scholar]
- Unger M., Olsen J. Organochlorine compounds in the adipose tissue of deceased people with and without cancer. Environ Res. 1980 Dec;23(2):257–263. doi: 10.1016/0013-9351(80)90059-6. [DOI] [PubMed] [Google Scholar]
- Varner S. L., Hollifield H. C., Andrzejewski D. Determination of benzene in polypropylene food-packaging materials and food-contact paraffin waxes. J Assoc Off Anal Chem. 1991 Mar-Apr;74(2):367–374. [PubMed] [Google Scholar]
- Vasta G. R., Ahmed H., Fink N. E., Elola M. T., Marsh A. G., Snowden A., Odom E. W. Animal lectins as self/non-self recognition molecules. Biochemical and genetic approaches to understanding their biological roles and evolution. Ann N Y Acad Sci. 1994 Apr 15;712:55–73. doi: 10.1111/j.1749-6632.1994.tb33562.x. [DOI] [PubMed] [Google Scholar]
- Vaughan R. W., Sipes I. G., Brown B. R., Jr Role of biotransformation in the toxicity of inhalation anesthetics. Life Sci. 1978 Dec 18;23(25):2447–2462. doi: 10.1016/0024-3205(78)90168-6. [DOI] [PubMed] [Google Scholar]
- Vautrin J., Schaffner A. E., Barker J. L. Tonic GABA secretion of cultured rat hippocampal neurons rapidly transformed by Zn2+ into quantal release. Neurosci Lett. 1993 Aug 20;158(2):125–129. doi: 10.1016/0304-3940(93)90245-g. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Berton M. T., Burger C., Kepron M., Lee W. T., Yin X. M. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- Vojdani A., Ghoneum M., Brautbar N. Immune alteration associated with exposure to toxic chemicals. Toxicol Ind Health. 1992 Sep-Oct;8(5):239–254. [PubMed] [Google Scholar]
- Waalkes M. P., Coogan T. P., Barter R. A. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit Rev Toxicol. 1992;22(3-4):175–201. doi: 10.3109/10408449209145323. [DOI] [PubMed] [Google Scholar]
- Walsh C. T., Sandstead H. H., Prasad A. S., Newberne P. M., Fraker P. J. Zinc: health effects and research priorities for the 1990s. Environ Health Perspect. 1994 Jun;102 (Suppl 2):5–46. doi: 10.1289/ehp.941025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. M. Evidence of an immune complex disorder in vinyl chloride workers. Proc R Soc Med. 1976 Apr;69(4):289–290. [PMC free article] [PubMed] [Google Scholar]
- Weiner J. A., Nylander M., Berglund F. Does mercury from amalgam restorations constitute a health hazard? Sci Total Environ. 1990 Dec 1;99(1-2):1–22. doi: 10.1016/0048-9697(90)90206-a. [DOI] [PubMed] [Google Scholar]
- Weiss B. Experimental strategies for research on multiple chemical sensitivity. Environ Health Perspect. 1997 Mar;105 (Suppl 2):487–494. doi: 10.1289/ehp.97105s2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Low-level chemical sensitivity: a perspective from behavioral toxicology. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):605–617. [PubMed] [Google Scholar]
- Welch L. S., Sokas R. Development of multiple chemical sensitivity after an outbreak of sick-building syndrome. Toxicol Ind Health. 1992 Jul-Aug;8(4):47–50. [PubMed] [Google Scholar]
- Wenger G. D., O'Dorisio M. S., Goetzl E. J. Vasoactive intestinal peptide. Messenger in a neuroimmune axis. Ann N Y Acad Sci. 1990;594:104–119. doi: 10.1111/j.1749-6632.1990.tb40472.x. [DOI] [PubMed] [Google Scholar]
- Wess J. A. Reproductive toxicity of ethylene glycol monomethyl ether, ethylene glycol monoethyl ether and their acetates. Scand J Work Environ Health. 1992;18 (Suppl 2):43–45. [PubMed] [Google Scholar]
- Whipkey R. R., Paris P. M., Stewart R. D. Drug use in pregnancy. Ann Emerg Med. 1984 May;13(5):346–354. doi: 10.1016/s0196-0644(84)80118-3. [DOI] [PubMed] [Google Scholar]
- Whitacre C. C. Immunology. A state of the art lecture. Ann N Y Acad Sci. 1990;594:1–16. doi: 10.1111/j.1749-6632.1990.tb40463.x. [DOI] [PubMed] [Google Scholar]
- Wilson C. Patient statement: chemical sensitivity--one victim's perspective. Toxicol Ind Health. 1994 Jul-Oct;10(4-5):319–321. [PubMed] [Google Scholar]
- Withey J. R., Collins P. G. Styrene monomer in foods a limited Canadian Survey. Bull Environ Contam Toxicol. 1978 Jan;19(1):86–94. doi: 10.1007/BF01685771. [DOI] [PubMed] [Google Scholar]
- Wolff M. S., Anderson H. A., Rosenman K. D., Selikoff I. J. Equilibrium of polybrominated biphenyl (PBB) residues in serum and fat of Michigan residents. Bull Environ Contam Toxicol. 1979 Apr;21(6):775–781. doi: 10.1007/BF01685504. [DOI] [PubMed] [Google Scholar]
- Wolff S. P. Correlation between car ownership and leukaemia: is non-occupational exposure to benzene from petrol and motor vehicle exhaust a causative factor in leukaemia and lymphoma? Experientia. 1992 Mar 15;48(3):301–304. doi: 10.1007/BF01930480. [DOI] [PubMed] [Google Scholar]
- Workman E. A., La Via M. F. T-lymphocyte polyclonal proliferation: effects of stress and stress response style on medical students taking national board examinations. Clin Immunol Immunopathol. 1987 Jun;43(3):308–313. doi: 10.1016/0090-1229(87)90140-1. [DOI] [PubMed] [Google Scholar]
- Xie X. M., Smart T. G. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991 Feb 7;349(6309):521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- Xie X., Smart T. G. Giant GABAB-mediated synaptic potentials induced by zinc in the rat hippocampus: paradoxical effects of zinc on the GABAB receptor. Eur J Neurosci. 1993 May 1;5(5):430–436. doi: 10.1111/j.1460-9568.1993.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Yardley-Jones A., Anderson D., Parke D. V. The toxicity of benzene and its metabolism and molecular pathology in human risk assessment. Br J Ind Med. 1991 Jul;48(7):437–444. doi: 10.1136/oem.48.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah C. H., Zeanah P. D. Intergenerational transmission of maltreatment: insights from attachment theory and research. Psychiatry. 1989 May;52(2):177–196. doi: 10.1080/00332747.1989.11024442. [DOI] [PubMed] [Google Scholar]
- Ziem G. E., Castleman B. I. Threshold limit values: historical perspectives and current practice. J Occup Med. 1989 Nov;31(11):910–918. doi: 10.1097/00043764-198911000-00014. [DOI] [PubMed] [Google Scholar]
- Ziem G., McTamney J. Profile of patients with chemical injury and sensitivity. Environ Health Perspect. 1997 Mar;105 (Suppl 2):417–436. doi: 10.1289/ehp.97105s2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk B. A., Dreyfuss D., Michaels M., Shera D., Berkowitz R., Fisler R., Saxe G. Fluoxetine in posttraumatic stress disorder. J Clin Psychiatry. 1994 Dec;55(12):517–522. [PubMed] [Google Scholar]
- van der Kolk B. A., Fisler R. E. Childhood abuse and neglect and loss of self-regulation. Bull Menninger Clin. 1994 Spring;58(2):145–168. [PubMed] [Google Scholar]
- van der Kolk B. A., Fisler R. E. The biologic basis of posttraumatic stress. Prim Care. 1993 Jun;20(2):417–432. [PubMed] [Google Scholar]
- van der Kolk B. A., Fisler R. Dissociation and the fragmentary nature of traumatic memories: overview and exploratory study. J Trauma Stress. 1995 Oct;8(4):505–525. doi: 10.1007/BF02102887. [DOI] [PubMed] [Google Scholar]
- van der Kolk B. A., Herron N., Hostetler A. The history of trauma in psychiatry. Psychiatr Clin North Am. 1994 Sep;17(3):583–600. [PubMed] [Google Scholar]