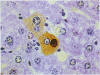
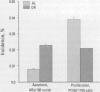
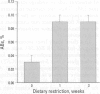
Abstract
The maintenance of cell number homeostasis in normal tissues reflects a highly regulated balance between the rates of cell proliferation and cell death. Under pathologic conditions such as exposure to cytotoxic, genotoxic, or nongenotoxic agents, an imbalance in these rates may indicate subsequent risk of carcinogenesis. Apoptotic cell death, as opposed to necrotic cell death, provides a protective mechanism by selective elimination of senescent, preneoplastic, or superfluous cells that could negatively affect normal function and/or promote cell transformation. The relative efficiency or dysfunction of the cell death program could therefore have a direct impact on the risk of degenerative or neoplastic disease. Dietary restriction of rodents is a noninvasive intervention that has been reproducibly shown to retard tumor development and most physiologic indices of aging relative to ad libitum-fed animals. As such, it provides a powerful model in which to study common mechanistic processes associated with both aging and cancer. In a recent study we established that chronic dietary restriction (DR) induces an increase in spontaneous apoptotic rate and a decrease in cell proliferation rate in hepatocytes of 12-month-old B6C3F1 DR mice relative to ad libitum (AL)-fed mice. This diet-induced shift in cell death/proliferation rates was associated with a marked reduction in subsequent development of spontaneous hepatoma and a marked increase in disease-free life span in DR relative to AL-fed mice. These results suggest that total caloric intake may modulate the rates of cell death and proliferation in a direction consistent with a cancer-protective effect in DR mice and a cancer-promoting effect in AL mice. To determine whether the increase in spontaneous apoptotic rate was maintained over the life span of DR mice, apoptotic rates were quantified in 12-, 18-, 24- and 30-month-old DR and AL mice. The rate of apoptosis was elevated with age in both diet groups; however, the rate of apoptosis was significantly and consistently higher in DR mice regardless of age. In double-labeling experiments, an age-associated increase in the glutathione S-transferase-II expression in putative preneoplastic hepatocytes in AL mice was rapidly reduced by apoptosis upon initiation of DR. Thus, intervention that promote a low-level increase in apoptotic cell death may be expected to protect genotypic and phenotypic stability with age. If during tumor promotion an adaptive increase in apoptosis effectively balances the dysregulated increase proliferation, the risk of permanent genetic error and carcinogenesis would be minimized.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bursch W., Lauer B., Timmermann-Trosiener I., Barthel G., Schuppler J., Schulte-Hermann R. Controlled death (apoptosis) of normal and putative preneoplastic cells in rat liver following withdrawal of tumor promoters. Carcinogenesis. 1984 Apr;5(4):453–458. doi: 10.1093/carcin/5.4.453. [DOI] [PubMed] [Google Scholar]
- Carthew P., Nolan B. M., Edwards R. E., Smith L. L. The role of cell death and cell proliferation in the promotion of rat liver tumours by tamoxifen. Cancer Lett. 1996 Sep 10;106(2):163–169. doi: 10.1016/0304-3835(96)04310-8. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Perkins G. R., Rodriguez-Tarduchy G., Nieto M. A., López-Rivas A. Growth factors as survival factors: regulation of apoptosis. Bioessays. 1994 Feb;16(2):133–138. doi: 10.1002/bies.950160210. [DOI] [PubMed] [Google Scholar]
- Columbano A., Endoh T., Denda A., Noguchi O., Nakae D., Hasegawa K., Ledda-Columbano G. M., Zedda A. I., Konishi Y. Effects of cell proliferation and cell death (apoptosis and necrosis) on the early stages of rat hepatocarcinogenesis. Carcinogenesis. 1996 Mar;17(3):395–400. doi: 10.1093/carcin/17.3.395. [DOI] [PubMed] [Google Scholar]
- Constan A. A., Benjamin S. A., Tessari J. D., Baker D. C., Yang R. S. 1995 STP Young Investigator Award recipient. Increased rate of apoptosis correlates with hepatocellular proliferation in Fischer-344 rats following long-term exposure to a mixture of groundwater contaminants. Toxicol Pathol. 1996 May-Jun;24(3):315–322. doi: 10.1177/019262339602400307. [DOI] [PubMed] [Google Scholar]
- Farber E. Cell proliferation as a major risk factor for cancer: a concept of doubtful validity. Cancer Res. 1995 Sep 1;55(17):3759–3762. [PubMed] [Google Scholar]
- Fernandes R. S., McGowan A. J., Cotter T. G. Mutant H-ras overexpression inhibits drug and U.V. induced apoptosis. Anticancer Res. 1996 Jul-Aug;16(4A):1691–1705. [PubMed] [Google Scholar]
- Feuers R. J., Weindruch R., Hart R. W. Caloric restriction, aging, and antioxidant enzymes. Mutat Res. 1993 Dec;295(4-6):191–200. doi: 10.1016/0921-8734(93)90020-4. [DOI] [PubMed] [Google Scholar]
- Gill J. H., Molloy C. A., Shoesmith K. J., Bayly A. C., Roberts R. A. The rodent non-genotoxic hepatocarcinogen Nafenopin and EGF alter the mitosis/apoptosis balance promoting hepatoma cell clonal growth. Cell Death Differ. 1995 Jul;2(3):211–217. [PubMed] [Google Scholar]
- Goldsworthy T. L., Conolly R. B., Fransson-Steen R. Apoptosis and cancer risk assessment. Mutat Res. 1996 Sep;365(1-3):71–90. doi: 10.1016/s0165-1110(96)90013-5. [DOI] [PubMed] [Google Scholar]
- Green D. R., Martin S. J. The killer and the executioner: how apoptosis controls malignancy. Curr Opin Immunol. 1995 Oct;7(5):694–703. doi: 10.1016/0952-7915(95)80079-4. [DOI] [PubMed] [Google Scholar]
- Haas-Kogan D. A., Kogan S. C., Levi D., Dazin P., T'Ang A., Fung Y. K., Israel M. A. Inhibition of apoptosis by the retinoblastoma gene product. EMBO J. 1995 Feb 1;14(3):461–472. doi: 10.1002/j.1460-2075.1995.tb07022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Lane D. P. Genetics of growth arrest and cell death: key determinants of tissue homeostasis. Eur J Cancer. 1994;30A(13):2001–2012. doi: 10.1016/0959-8049(94)00394-k. [DOI] [PubMed] [Google Scholar]
- Hart R. W., Turturro A. Overview of cancer and aging: a mechanistic perspective. Exp Gerontol. 1992 Sep-Dec;27(5-6):567–574. doi: 10.1016/0531-5565(92)90011-n. [DOI] [PubMed] [Google Scholar]
- Hoffman B., Liebermann D. A. Molecular controls of apoptosis: differentiation/growth arrest primary response genes, proto-oncogenes, and tumor suppressor genes as positive & negative modulators. Oncogene. 1994 Jul;9(7):1807–1812. [PubMed] [Google Scholar]
- Ishida M., Gomyo Y., Tatebe S., Ohfuji S., Ito H. Apoptosis in human gastric mucosa, chronic gastritis, dysplasia and carcinoma: analysis by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling. Virchows Arch. 1996 Jul;428(4-5):229–235. doi: 10.1007/BF00196695. [DOI] [PubMed] [Google Scholar]
- James S. J., Muskhelishvili L. Rates of apoptosis and proliferation vary with caloric intake and may influence incidence of spontaneous hepatoma in C57BL/6 x C3H F1 mice. Cancer Res. 1994 Nov 1;54(21):5508–5510. [PubMed] [Google Scholar]
- Kerr J. F., Searle J. A suggested explanation for the paradoxically slow growth rate of basal-cell carcinomas that contain numerous mitotic figures. J Pathol. 1972 May;107(1):41–44. doi: 10.1002/path.1711070107. [DOI] [PubMed] [Google Scholar]
- King K. L., Cidlowski J. A. Cell cycle and apoptosis: common pathways to life and death. J Cell Biochem. 1995 Jun;58(2):175–180. doi: 10.1002/jcb.240580206. [DOI] [PubMed] [Google Scholar]
- Kolaja K. L., Stevenson D. E., Walborg E. F., Jr, Klaunig J. E. Dose dependence of phenobarbital promotion of preneoplastic hepatic lesions in F344 rats and B6C3F1 mice: effects on DNA synthesis and apoptosis. Carcinogenesis. 1996 May;17(5):947–954. doi: 10.1093/carcin/17.5.947. [DOI] [PubMed] [Google Scholar]
- Kong J., Ringer D. P. Quantitative analysis of changes in cell proliferation and apoptosis during preneoplastic and neoplastic stages of hepatocarcinogenesis in rat. Cancer Lett. 1996 Aug 2;105(2):241–248. doi: 10.1016/0304-3835(96)04291-7. [DOI] [PubMed] [Google Scholar]
- Lagopoulos L., Sunahara G. I., Würzner H., Dombrowsky I., Stalder R. The effects of alternating dietary restriction and ad libitum feeding of mice on the development of diethylnitrosamine-induced liver tumours and its correlation to insulinaemia. Carcinogenesis. 1991 Feb;12(2):311–315. doi: 10.1093/carcin/12.2.311. [DOI] [PubMed] [Google Scholar]
- Luan X., Zhao W., Chandrasekar B., Fernandes G. Calorie restriction modulates lymphocyte subset phenotype and increases apoptosis in MRL/lpr mice. Immunol Lett. 1995 Sep;47(3):181–186. doi: 10.1016/0165-2478(95)00091-5. [DOI] [PubMed] [Google Scholar]
- Marsman D. S., Barrett J. C. Apoptosis and chemical carcinogenesis. Risk Anal. 1994 Jun;14(3):321–326. doi: 10.1111/j.1539-6924.1994.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Green D. R. Apoptosis and cancer: the failure of controls on cell death and cell survival. Crit Rev Oncol Hematol. 1995 Feb;18(2):137–153. doi: 10.1016/1040-8428(94)00124-c. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J. Cell division versus cell death: a functional model of multistep neoplasia. Mol Carcinog. 1993;8(4):209–213. doi: 10.1002/mc.2940080402. [DOI] [PubMed] [Google Scholar]
- Meikrantz W., Schlegel R. Apoptosis and the cell cycle. J Cell Biochem. 1995 Jun;58(2):160–174. doi: 10.1002/jcb.240580205. [DOI] [PubMed] [Google Scholar]
- Monti D., Grassilli E., Troiano L., Cossarizza A., Salvioli S., Barbieri D., Agnesini C., Bettuzzi S., Ingletti M. C., Corti A. Senescence, immortalization, and apoptosis. An intriguing relationship. Ann N Y Acad Sci. 1992 Dec 26;673:70–82. doi: 10.1111/j.1749-6632.1992.tb27438.x. [DOI] [PubMed] [Google Scholar]
- Muskhelishvili L., Hart R. W., Turturro A., James S. J. Age-related changes in the intrinsic rate of apoptosis in livers of diet-restricted and ad libitum-fed B6C3F1 mice. Am J Pathol. 1995 Jul;147(1):20–24. [PMC free article] [PubMed] [Google Scholar]
- Muskhelishvili L., Turturro A., Hart R. W., James S. J. Pi-class glutathione-S-transferase-positive hepatocytes in aging B6C3F1 mice undergo apoptosis induced by dietary restriction. Am J Pathol. 1996 Nov;149(5):1585–1591. [PMC free article] [PubMed] [Google Scholar]
- Naik P., Karrim J., Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev. 1996 Sep 1;10(17):2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Barres B. A., Burne J. F., Coles H. S., Ishizaki Y., Jacobson M. D. Programmed cell death and the control of cell survival. Philos Trans R Soc Lond B Biol Sci. 1994 Aug 30;345(1313):265–268. doi: 10.1098/rstb.1994.0104. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Miyashita T., Takayama S., Wang H. G., Sato T., Krajewski S., Aimé-Sempé C., Bodrug S., Kitada S., Hanada M. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996 Jan;60(1):23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Resnicoff M., Abraham D., Yutanawiboonchai W., Rotman H. L., Kajstura J., Rubin R., Zoltick P., Baserga R. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995 Jun 1;55(11):2463–2469. [PubMed] [Google Scholar]
- Resnicoff M., Burgaud J. L., Rotman H. L., Abraham D., Baserga R. Correlation between apoptosis, tumorigenesis, and levels of insulin-like growth factor I receptors. Cancer Res. 1995 Sep 1;55(17):3739–3741. [PubMed] [Google Scholar]
- Ruggeri B. A., Klurfeld D. M., Kritchevsky D., Furlanetto R. W. Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 1989 Aug 1;49(15):4130–4134. [PubMed] [Google Scholar]
- Schulte-Hermann R., Bursch W., Grasl-Kraupp B., Müllauer L., Ruttkay-Nedecky B. Apoptosis and multistage carcinogenesis in rat liver. Mutat Res. 1995 Dec;333(1-2):81–87. doi: 10.1016/0027-5107(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R., Bursch W., Kraupp-Grasl B., Oberhammer F., Wagner A., Jirtle R. Cell proliferation and apoptosis in normal liver and preneoplastic foci. Environ Health Perspect. 1993 Dec;101 (Suppl 5):87–90. doi: 10.1289/ehp.93101s587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hermann R., Grasl-Kraupp B., Bursch W. Tumor development and apoptosis. Int Arch Allergy Immunol. 1994 Dec;105(4):363–367. doi: 10.1159/000236784. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996 Jul 5;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson C. M., Rogers S. A., Korsmeyer S. J., Hammerman M. R. Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. Am J Physiol. 1995 Jan;268(1 Pt 2):F73–F81. doi: 10.1152/ajprenal.1995.268.1.F73. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Goodman J. I. Intercellular communication may facilitate apoptosis: implications for tumor promotion. Mol Carcinog. 1994 Sep;11(1):8–12. doi: 10.1002/mc.2940110103. [DOI] [PubMed] [Google Scholar]
- Urao M., Ueda G., Abe M., Kanno K., Hirose S., Shirai T. Food restriction inhibits an autoimmune disease resembling systemic lupus erythematosus in (NZB x NZW) F1 mice. J Nutr. 1995 Sep;125(9):2316–2324. doi: 10.1093/jn/125.9.2316. [DOI] [PubMed] [Google Scholar]
- Weindruch R., Albanes D., Kritchevsky D. The role of calories and caloric restriction in carcinogenesis. Hematol Oncol Clin North Am. 1991 Feb;5(1):79–89. [PubMed] [Google Scholar]
- Weindruch R. Caloric restriction and aging. Sci Am. 1996 Jan;274(1):46–52. doi: 10.1038/scientificamerican0196-46. [DOI] [PubMed] [Google Scholar]
- Weindruch R., Walford R. L., Fligiel S., Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986 Apr;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Worner W., Schrenk D. Influence of liver tumor promoters on apoptosis in rat hepatocytes induced by 2-acetylaminofluorene, ultraviolet light, or transforming growth factor beta 1. Cancer Res. 1996 Mar 15;56(6):1272–1278. [PubMed] [Google Scholar]