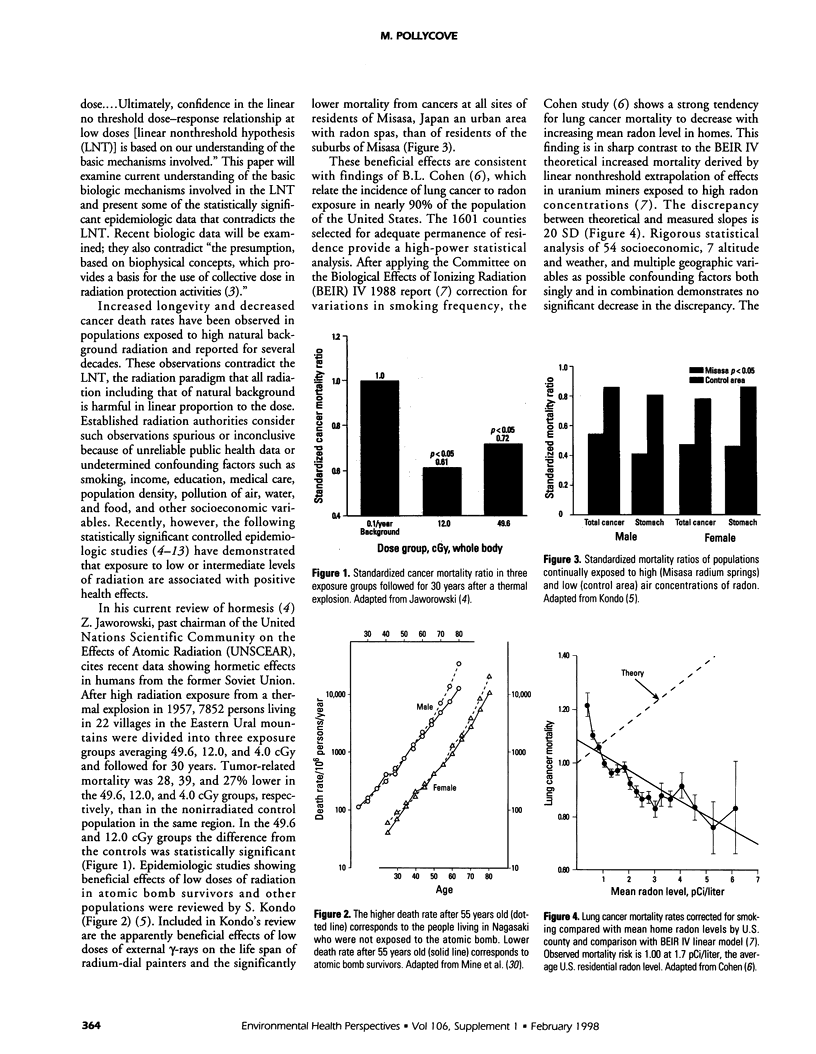
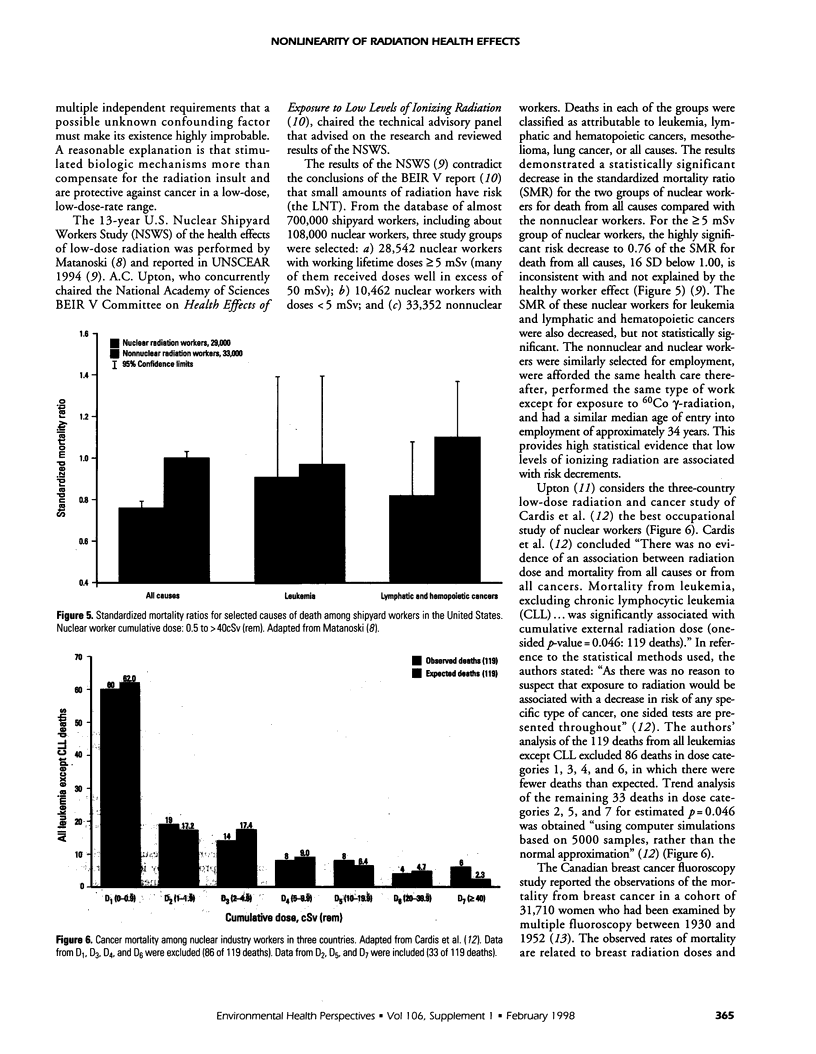
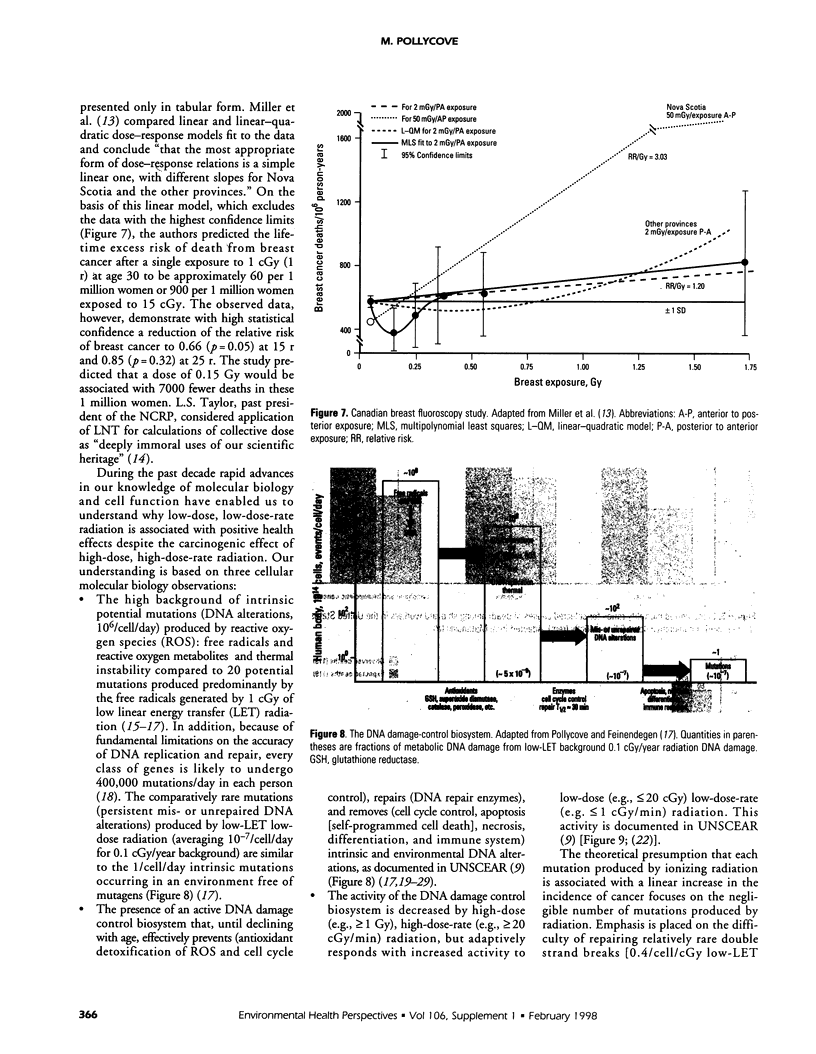
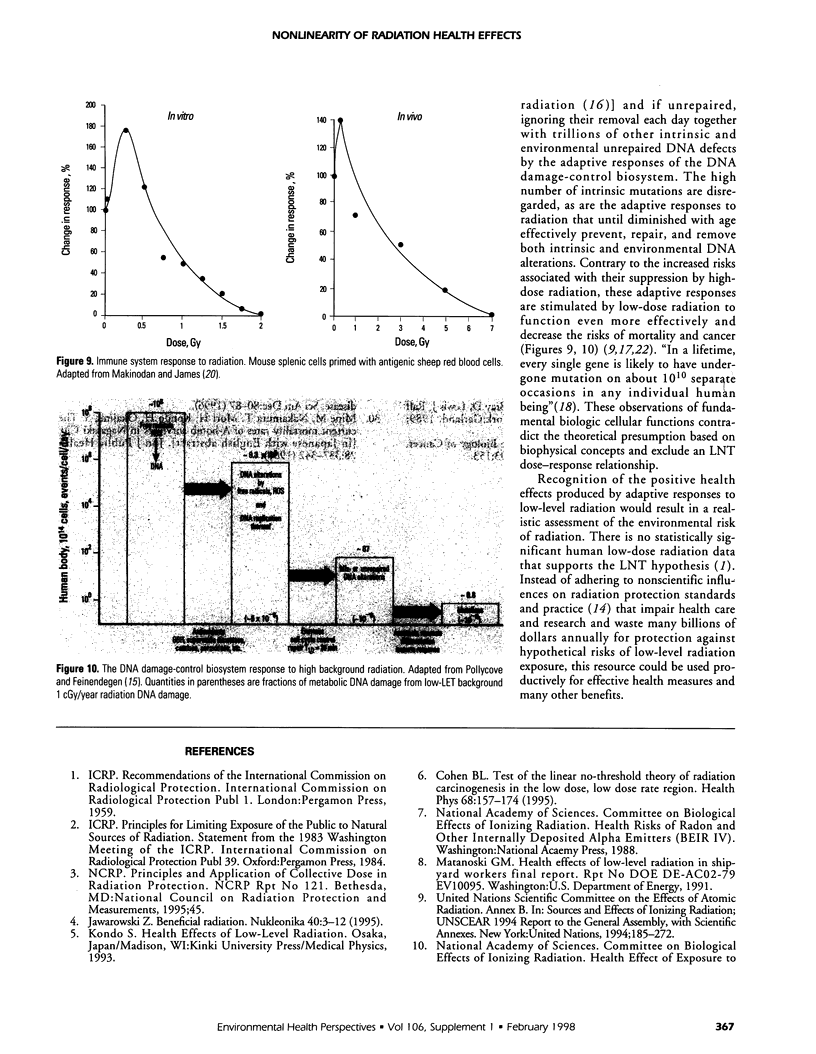
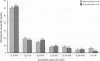Abstract
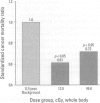
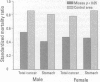
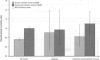
The prime concern of radiation protection policy since 1959 has been to protect DNA from damage. In 1994 the United Nations Scientific Community on the Effects of Atomic Radiation focused on biosystem response to radiation with its report Adaptive Responses to Radiation of Cells and Organisms. The 1995 National Council on Radiation Protection and Measurements report Principles and Application of Collective Dose in Radiation Protection states that because no human data provides direct support for the linear nonthreshold hypothesis (LNT), confidence in LNT is based on the biophysical concept that the passage of a single charged particle could cause damage to DNA that would result in cancer. Several statistically significant epidemiologic studies contradict the validity of this concept by showing risk decrements, i.e., hormesis, of cancer mortality and mortality from all causes in populations exposed to low-dose radiation. Unrepaired low-dose radiation damage to DNA is negligible compared to metabolic damage. The DNA damage-control biosystem is physiologically operative on both metabolic and radiation damage and effected predominantly by free radicals. The DNA damage-control biosystem is suppressed by high dose and stimulated by low-dose radiation. The hormetic effect of low-dose radiation may be explained by its increase of biosystem efficiency. Improved DNA damage control reduces persistent mis- or unrepaired DNA damage i.e., the number of mutations that accumulate during a lifetime. This progressive accumulation of gene mutations in stem cells is associated with decreasing DNA damage control, aging, and malignancy. Recognition of the positive health effects produced by adaptive responses to low-dose radiation would result in a realistic assessment of the environmental risk of radiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S., Willett W. C. The causes and prevention of cancer. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D. Spontaneous DNA damage and its significance for the "negligible dose" controversy in radiation protection. Radiat Res. 1990 Nov;124(2):242–245. [PubMed] [Google Scholar]
- Cardis E., Gilbert E. S., Carpenter L., Howe G., Kato I., Armstrong B. K., Beral V., Cowper G., Douglas A., Fix J. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995 May;142(2):117–132. [PubMed] [Google Scholar]
- Cohen B. L. Test of the linear-no threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995 Feb;68(2):157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Ojcius D. M., Young J. D. Cell suicide in health and disease. Sci Am. 1996 Dec;275(6):80–87. doi: 10.1038/scientificamerican1296-80. [DOI] [PubMed] [Google Scholar]
- Lithgow G. J., Kirkwood T. B. Mechanisms and evolution of aging. Science. 1996 Jul 5;273(5271):80–80. doi: 10.1126/science.273.5271.80. [DOI] [PubMed] [Google Scholar]
- Makinodan T., James S. J. T cell potentiation by low dose ionizing radiation: possible mechanisms. Health Phys. 1990 Jul;59(1):29–34. doi: 10.1097/00004032-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Miller A. B., Howe G. R., Sherman G. J., Lindsay J. P., Yaffe M. J., Dinner P. J., Risch H. A., Preston D. L. Mortality from breast cancer after irradiation during fluoroscopic examinations in patients being treated for tuberculosis. N Engl J Med. 1989 Nov 9;321(19):1285–1289. doi: 10.1056/NEJM198911093211902. [DOI] [PubMed] [Google Scholar]
- Miller R. A. The aging immune system: primer and prospectus. Science. 1996 Jul 5;273(5271):70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Ross D. W. Biology of aging. Arch Pathol Lab Med. 1996 Dec;120(12):1148–1148. [PubMed] [Google Scholar]
- Sohal R. S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996 Jul 5;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L. S. Some nonscientific influences on radiation protection standards and practice. The 1980 Sievert Lecture. Health Phys. 1980 Dec;39(6):851–874. [PubMed] [Google Scholar]
- Wei Q., Matanoski G. M., Farmer E. R., Hedayati M. A., Grossman L. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1614–1618. doi: 10.1073/pnas.90.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]