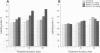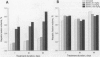Abstract
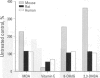
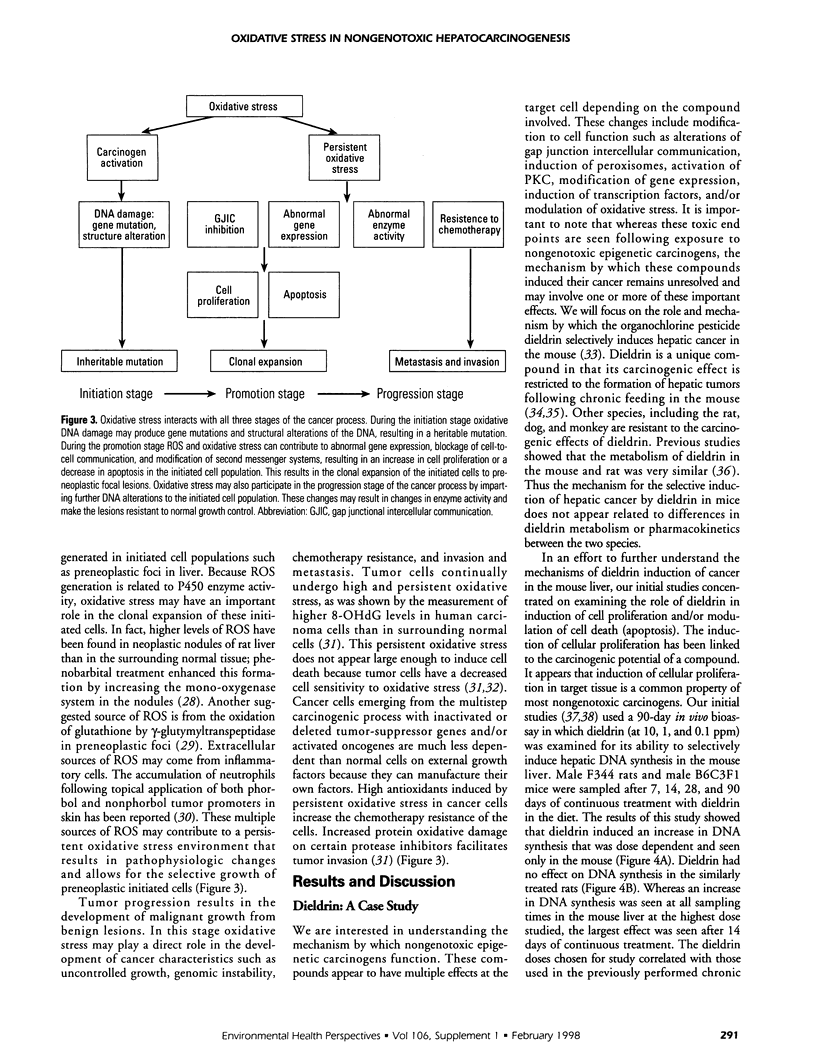
Oxidative stress results when the balance between the production of reactive oxygen species (ROS) overrides the antioxidant capability of the target cell; oxidative damage from the interaction of reactive oxygen with critical cellular macromolecules may occur. ROS may interact with and modify cellular protein, lipid, and DNA, which results in altered target cell function. The accumulation of oxidative damage has been implicated in both acute and chronic cell injury including possible participation in the formation of cancer. Acute oxidative injury may produce selective cell death and a compensatory increase in cell proliferation. This stimulus may result in the formation of newly initiated preneoplastic cells and/or enhance the selective clonal expansion of latent initiated preneoplastic cells. Similarly, sublethal acute oxidative injury may produce unrepaired DNA damage and result in the formation of new mutations and, potentially, new initiated cells. In contrast, sustained chronic oxidative injury may lead to a nonlethal modification of normal cellular growth control mechanisms. Cellular oxidative stress can modify intercellular communication, protein kinase activity, membrane structure and function, and gene expression, and result in modulation of cell growth. We examined the role of oxidative stress as a possible mechanism by which nongenotoxic carcinogens may function. In studies with the selective mouse liver carcinogen dieldrin, a species-specific and dose-dependent decrease in liver antioxidant concentrations with a concomitant increase in ROS formation and oxidative damage was seen. This increase in oxidative stress correlated with an increase in hepatocyte DNA synthesis. Antioxidant supplementation prevented the dieldrin-induced cellular changes. Our findings suggest that the effect of nongenotoxic carcinogens (if they function through oxidative mechanisms) may be amplified in rodents but not in primates because of rodents' greater sensitivity to ROS. These results and findings reported by others support a potential role for oxidative-induced injury in the cancer process specifically during the promotion stage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Gold L. S. Animal cancer tests and cancer prevention. J Natl Cancer Inst Monogr. 1992;(12):125–132. [PubMed] [Google Scholar]
- Baker T. K., Bachowski S., Stevenson D. E., Walborg E. F., Jr, Klaunig J. E. Modulation of gap junctional intercellular communication in rodent, monkey and human hepatocyte by nongenotoxic compounds. Prog Clin Biol Res. 1995;391:71–80. [PubMed] [Google Scholar]
- Baldwin M. K., Robinson J., Parke D. V. A comparison of the metabolism of HEOD (dieldrin) in the CF1 mouse with that in the CFE rat. Food Cosmet Toxicol. 1972 Jun;10(3):333–351. doi: 10.1016/s0015-6264(72)80252-9. [DOI] [PubMed] [Google Scholar]
- Barber D. A., Harris S. R. Oxygen free radicals and antioxidants: a review. Am Pharm. 1994 Sep;NS34(9):26–35. doi: 10.1016/s0160-3450(15)30310-x. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Mirabelli F., Richelmi P., Orrenius S. Critical role of sulfhydryl group(s) in ATP-dependent Ca2+ sequestration by the plasma membrane fraction from rat liver. FEBS Lett. 1983 Oct 31;163(1):136–139. doi: 10.1016/0014-5793(83)81180-6. [DOI] [PubMed] [Google Scholar]
- Brawn M. K., Chiou W. J., Leach K. L. Oxidant-induced activation of protein kinase C in UC11MG cells. Free Radic Res. 1995 Jan;22(1):23–37. doi: 10.3109/10715769509147525. [DOI] [PubMed] [Google Scholar]
- Breimer L. H. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3(4):188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A., Trump B. F. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991 Jan;3(1):1–7. [PubMed] [Google Scholar]
- Chaudhary A. K., Nokubo M., Marnett L. J., Blair I. A. Analysis of the malondialdehyde-2'-deoxyguanosine adduct in rat liver DNA by gas chromatography/electron capture negative chemical ionization mass spectrometry. Biol Mass Spectrom. 1994 Aug;23(8):457–464. doi: 10.1002/bms.1200230802. [DOI] [PubMed] [Google Scholar]
- Davies K. J. Intracellular proteolytic systems may function as secondary antioxidant defenses: an hypothesis. J Free Radic Biol Med. 1986;2(3):155–173. doi: 10.1016/s0748-5514(86)80066-6. [DOI] [PubMed] [Google Scholar]
- Feig D. I., Reid T. M., Loeb L. A. Reactive oxygen species in tumorigenesis. Cancer Res. 1994 Apr 1;54(7 Suppl):1890s–1894s. [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Guyton K. Z., Kensler T. W. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993 Jul;49(3):523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Mechanisms involved in the generation of free radicals. Pathol Biol (Paris) 1996 Jan;44(1):6–13. [PubMed] [Google Scholar]
- Hutson D. H. Comparative metabolism of dieldrin in the rat (CFE) and in two strains of mouse (CF1 and LACG). Food Cosmet Toxicol. 1976 Dec;14(6):577–591. doi: 10.1016/s0015-6264(76)80012-0. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Verma I. M. Signal transduction: the nuclear target. Curr Opin Cell Biol. 1992 Jun;4(3):496–501. doi: 10.1016/0955-0674(92)90017-7. [DOI] [PubMed] [Google Scholar]
- Kolaja K. L., Stevenson D. E., Johnson J. T., Walborg E. F., Jr, Klaunig J. E. Hepatic effects of dieldrin and phenobarbital in male B6C3F1 mice and Fisher 344 rats: species selective induction of DNA synthesis. Prog Clin Biol Res. 1995;391:397–408. [PubMed] [Google Scholar]
- Kolaja K. L., Stevenson D. E., Johnson J. T., Walborg E. F., Jr, Klaunig J. E. Subchronic effects of dieldrin and phenobarbital on hepatic DNA synthesis in mice and rats. Fundam Appl Toxicol. 1996 Feb;29(2):219–228. doi: 10.1006/faat.1996.0025. [DOI] [PubMed] [Google Scholar]
- Kolaja K. L., Stevenson D. E., Walborg E. F., Jr, Klaunig J. E. Selective dieldrin promotion of hepatic focal lesions in mice. Carcinogenesis. 1996 Jun;17(6):1243–1250. doi: 10.1093/carcin/17.6.1243. [DOI] [PubMed] [Google Scholar]
- Nelson R. L. Dietary iron and colorectal cancer risk. Free Radic Biol Med. 1992;12(2):161–168. doi: 10.1016/0891-5849(92)90010-e. [DOI] [PubMed] [Google Scholar]
- Palozza P., Agostara G., Piccioni E., Bartoli G. M. Different role of lipid peroxidation in oxidative stress-induced lethal injury in normal and tumor thymocytes. Arch Biochem Biophys. 1994 Jul;312(1):88–94. doi: 10.1006/abbi.1994.1284. [DOI] [PubMed] [Google Scholar]
- Parke D. V., Ioannides C. Role of cytochromes P-450 in mouse liver tumor production. Prog Clin Biol Res. 1990;331:215–230. [PubMed] [Google Scholar]
- Rice-Evans C., Burdon R. Free radical-lipid interactions and their pathological consequences. Prog Lipid Res. 1993;32(1):71–110. doi: 10.1016/0163-7827(93)90006-i. [DOI] [PubMed] [Google Scholar]
- Schenk H., Klein M., Erdbrügger W., Dröge W., Schulze-Osthoff K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz W., Schütze K., Kunz W., Schwarz M. Phenobarbital enhances the formation of reactive oxygen in neoplastic rat liver nodules. Cancer Res. 1990 Nov 1;50(21):7015–7022. [PubMed] [Google Scholar]
- Schulte-Hermann R., Timmermann-Trosiener I., Barthel G., Bursch W. DNA synthesis, apoptosis, and phenotypic expression as determinants of growth of altered foci in rat liver during phenobarbital promotion. Cancer Res. 1990 Aug 15;50(16):5127–5135. [PubMed] [Google Scholar]
- Stark A. A. Oxidative metabolism of glutathione by gamma-glutamyl transpeptidase and peroxisome proliferation: the relevance to hepatocarcinogenesis. A hypothesis. Mutagenesis. 1991 Jul;6(4):241–245. doi: 10.1093/mutage/6.4.241. [DOI] [PubMed] [Google Scholar]
- Stevenson D. E., Kehrer J. P., Kolaja K. L., Walborg E. F., Jr, Klaunig J. E. Effect of dietary antioxidants on dieldrin-induced hepatotoxicity in mice. Toxicol Lett. 1995 Jan;75(1-3):177–183. doi: 10.1016/0378-4274(94)03178-a. [DOI] [PubMed] [Google Scholar]
- Storz G., Polla B. S. Transcriptional regulators of oxidative stress-inducible genes in prokaryotes and eukaryotes. EXS. 1996;77:239–254. doi: 10.1007/978-3-0348-9088-5_16. [DOI] [PubMed] [Google Scholar]
- Thorpe E., Walker A. I. The toxicology of dieldrin (HEOD). II. Comparative long-term oral toxicity studies in mice with dieldrin, DDT, phenobarbitone, -BHC and -BHC. Food Cosmet Toxicol. 1973 Jun;11(3):433–442. doi: 10.1016/0015-6264(73)90008-4. [DOI] [PubMed] [Google Scholar]
- Toyokuni S., Okamoto K., Yodoi J., Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995 Jan 16;358(1):1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- Trush M. A., Kensler T. W. An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radic Biol Med. 1991;10(3-4):201–209. doi: 10.1016/0891-5849(91)90077-g. [DOI] [PubMed] [Google Scholar]
- Vellanoweth R. L., Suprakar P. C., Roy A. K. Transcription factors in development, growth, and aging. Lab Invest. 1994 Jun;70(6):784–799. [PubMed] [Google Scholar]
- Vuillaume M. Reduced oxygen species, mutation, induction and cancer initiation. Mutat Res. 1987 Jul;186(1):43–72. doi: 10.1016/0165-1110(87)90014-5. [DOI] [PubMed] [Google Scholar]
- White A. A., Crawford K. M., Patt C. S., Lad P. J. Activation of soluble guanylate cyclase from rat lung by incubation or by hydrogen peroxide. J Biol Chem. 1976 Dec 10;251(23):7304–7312. [PubMed] [Google Scholar]
- Whiteside S. T., Goodbourn S. Signal transduction and nuclear targeting: regulation of transcription factor activity by subcellular localisation. J Cell Sci. 1993 Apr;104(Pt 4):949–955. doi: 10.1242/jcs.104.4.949. [DOI] [PubMed] [Google Scholar]
- Willett W. C., MacMahon B. Diet and cancer--an overview. N Engl J Med. 1984 Mar 8;310(10):633–638. doi: 10.1056/NEJM198403083101006. [DOI] [PubMed] [Google Scholar]