Abstract
Resveratrol mimics calorie restriction to extend lifespan of Caenorhabditis elegans, yeast and Drosophila, possibly through activation of Sir2 (silent information regulator 2), a NAD+-dependent histone deacetylase. In the present study, resveratrol is shown to inhibit the insulin signalling pathway in several cell lines and rat primary hepatocytes in addition to its broad-spectrum inhibition of several signalling pathways. Resveratrol effectively inhibits insulin-induced Akt and MAPK (mitogen-activated protein kinase) activation mainly through disruption of the interactions between insulin receptor substrates and its downstream binding proteins including p85 regulatory subunit of phosphoinositide 3-kinase and Grb2 (growth factor receptor-bound protein 2). The inhibitory effect of resveratrol on insulin signalling is also demonstrated at mRNA level, where resveratrol reverses insulin effects on phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, fatty acid synthase and glucokinase. In addition, RNA interference experiment shows that the inhibitory effect of resveratrol on insulin signalling pathway is not weakened in cells with reduced expression of SirT1, the mammalian counterpart of Sir2. These observations raise the possibility that resveratrol may additionally modulate lifespan through inhibition of insulin signalling pathway, independently of its activation of SirT1 histone deacetylase. Furthermore, the present study may help to explain a wide range of biological effects of resveratrol, and provides further insight into the molecular basis of calorie restriction.
Keywords: insulin receptor substrate (IRS), insulin signalling pathway, lifespan, p85, resveratrol, SirT1 histone deacetylase
Abbreviations: ApoE, apolipoprotein E; DMEM, Dulbecco's modified Eagle's medium; EGF, epidermal growth factor; EGFR, EGF receptor; ERK, extracellular-signal-regulated kinase; FAS, fatty acid synthase; GK, glucokinase; Grb2, growth factor receptor-bound protein 2; HEK-293 cell, human embryonic kidney cell; IGF-1, insulin-like growth factor type I; IGFBP-1, IGF-binding protein 1; IRS-1, insulin receptor substrate 1; MAPK, mitogen-activated protein kinase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; NGF, neuronal growth factor; PEPCK, phosphoenolpyruvate carboxykinase; PI3K, phosphoinositide 3-kinase; RNAi, RNA interference; Sir2, silent information regulator 2
INTRODUCTION
The pleiotropic effects of calorie restriction have long been noticed and are linked to extended lifespan in Caenorhabditis elegans, Drosophila, yeast, mice, rats and monkeys [1,2]. Recently, Sir2 (silent information regulator 2), a NAD+-dependent histone deacetylase, has been recognized as an essential factor to calorie restriction-induced lifespan extension [3]. This enzyme is conserved in C. elegans, yeast, Drosophila and mammals [4–6]. Deletion of Sir2 eliminates the effect of calorie restriction on lifespan in both yeast and Drosophila [2,7].
Insulin signalling pathway has also been strongly linked to aging process. Mutations at several key components of insulin signalling pathway, including daf-2 (insulin receptor homologue), age-1 [PI3K (phosphoinositide 3-kinase) homologue] and daf-16 (forkhead family transcription factor homologue), all significantly modify lifespan of C. elegans [8,9]. Insulin signalling pathway is strongly associated with lifespan regulation in mammals as well. Conditional deletion of insulin receptor in adipose tissue results in significant extension of lifespan in mice [10]. Several other rodent aging models, meanwhile, demonstrate extensive alterations of insulin signalling pathway [8,11–16].
Resveratrol is an active ingredient of Polygonum capsidatum, a plant known for its medical use. It is also present in peanuts and grapevines. The presence of resveratrol in red wine at lower micromolar range also raises interest in this compound, as consumption of red wine is know to reduce the risk of cardiovascular diseases [17]. The biological effects of resveratrol include protection of cells from lipid accumulation, chemoprevention, immunomodulation, antiproliferation and promotion of differentiation [17]. Recently, resveratrol has been demonstrated to activate NAD+-dependent Sir2 family deacetylase activities [2,18,19]. The rate of SirT1 deacetylation is doubled by resveratrol at approx. 11 μM, and is saturated at 100–200 μM resveratrol [18]. Consistent with the essential role of Sir2 in calorie restriction, treatment of resveratrol between 50 and 500 μM mimics calorie restriction to extend lifespan in C. elegans, Drosophila and yeast. Deletion of Sir2, on the other hand, eliminates resveratrol effect on lifespan extension in these species [2,3].
The tight connection of insulin signalling pathway and lifespan regulation suggests that insulin signalling pathway may also play an active role in the regulatory effect of resveratrol on lifespan. To test this hypothesis, the effect of resveratrol on insulin signalling pathway was investigated at cellular level in the present study. Resveratrol was shown to actively inhibit insulin responses through disruption of the insulin-induced IRS (insulin receptor substrate) protein complexes. The possible involvement of SirT1 in resveratrol effect on insulin signalling pathway was also explored in this study.
EXPERIMENTAL
General reagents
Various cell-culture reagents, including DMEM (Dulbecco's modified Eagle's medium), M199 medium, penicillin/streptomycin, fetal bovine serum and MEM (minimum essential medium), were obtained from Fisher Scientific. Liver perfusion buffer and liver digest buffer were obtained from Invitrogen (Carlsbad, CA, U.S.A.). Dexamethasone and resveratrol were obtained from BioMol (Plymouth Meeting, PA, U.S.A.). Insulin, 3,3′,5-tri-iodothyronine, RNA loading buffer and formamide were obtained from Sigma. Antibodies against p44/42 MAPK (mitogen-activated protein kinase), phospho-Akt (Ser473), Akt and p85 were obtained from Cell Signaling Technology (Beverly, MA, U.S.A.). Phospho-p44/42 MAPK monoclonal antibody was obtained from Sigma. Antibodies against IRS-1, IRS-2, human SirT1 and anti-phosphotyrosine 4G10 were obtained from Upstate Biotechnology (Lake Placid, NY, U.S.A.). Antibody against insulin receptor β was obtained from BD Biosciences (San Jose, CA, U.S.A.). Growth factors including EGF (epidermal growth factor), IGF-1 (insulin-like growth factor type I) and NGF (neuronal growth factor) were obtained from Calbiochem (San Diego, CA, U.S.A.). Antibodies against Grb2 (growth factor receptor-bound protein 2), EGFR (EGF receptor) and Protein A/G plus beads were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.).
Cell culture
H4IIE cells (a rat hepatoma cell line), HepG2 cells (a human hepatoma cell line) and HEK-293 cells (human embryonic kidney cells) were maintained in DMEM supplemented with 10% (v/v) fetal bovine serum. Rat primary hepatocytes were isolated as described previously [20] and set up in M199 medium supplemented with 100 nM dexamethasone, 100 nM 3,3′,5-tri-iodothyronine and 1 nM insulin overnight before they were changed into M199 medium supplemented with 100 nM dexamethasone for further treatment.
Immunoprecipitation and Western-blot analysis
Cells were harvested and lysed in lysis buffer [50 mM Hepes, pH 7.4, 137 mM NaCl, 5 mM EDTA, 5 mM EGTA, 1 mM MgCl2, 10 mM Na2P2O7, 1% Triton X-100 and 10% (v/v) glycerol] supplemented with protease and phosphatase inhibitors (100 mM NaF, 10 mM NaVO4, 0.1 mM PMSF, 5 μg/ml pepstatin, 10 μg/ml leupeptin and 5 μg/ml aprotinin) by pipetting up and down 50 times. Supernatants were collected after 5 min of centrifugation at 12846 g for Western-blot analysis. For immunoprecipitation, equal amounts of cell lysates were pre-incubated with Protein A/G plus beads for 30 min at 4 °C under constant agitation and the resulting supernatants were transferred into fresh tubes and incubated with respective antibodies for another 1 h, followed by addition of 60 μl of Protein A/G plus beads for an additional 1 h. Protein A/G plus beads were collected at 1000 g for 1 min using centrifugation and washed several times with lysis buffer, and subjected to Western-blot analysis. For Western-blot analysis, total cell lysates were resuspended in sample buffer [62.5 mM Tris/HCl, pH 6.8, 2% (w/v) SDS, 10% glycerol and 50 mM dithiothreitol supplemented with Bromophenol Blue], and heated for 5 min at 75 °C. Samples were separated using SDS/PAGE and transferred on to nitrocellulose membranes for immunoblotting.
Northern-blot analysis
Total RNA was extracted using TRIzol® reagent following the manufacturer's instructions. Equal amount of RNA was resuspended into RNA loading buffer (Sigma), separated by 1% agarose–formadehyde gel and transferred on to nylon membrane (Hybond-N+). The cDNA probes were labelled with [α-32P]dCTP using a random primer labelling kit from Amersham. Hybridization was performed at 65 °C in ExpressHyb hybridization solution (BD Bioscience Clontech) according to the manufacturer's instructions. Membranes were exposed to an X-ray film at either 25 °C or –80 °C for 16 h.
RNAi (RNA interference)
The target sequence of human SirT1 for RNAi was described elsewhere [21]. HEK-293 cells were seeded at 5×105/60 mm dish and were transfected with 400 pmol of RNA double-stranded oligonucleotides using Oligofectamine (Invitrogen) according to the manufacturers’ instructions. Transfected cells were serum-starved overnight and changed into serum-free DMEM supplemented with 1 μM dexamethasone, and incubated with 100 μM resveratrol or vehicle control for 10 min before they were challenged with 100 nM insulin for another 10 min. Total cell lysates were prepared for Western-blot analysis using antibodies against human SirT1, p42/44, phospho-p42/44, Akt and phospho-Akt at Ser473 site.
In vitro assay
H4IIE cells were cultured in serum-free DMEM supplemented with 1 μM dexamethasone overnight, and treated with 100 nM insulin for 10 min. IRS-1 was immunoprecipitated from total cell lysates using Protein A/G plus beads and divided equally. The beads were washed once with lysis buffer and incubated with reaction buffer (lysis buffer without Triton X-100) with and without resveratrol for 20 min at 37 °C before they were briefly washed three times with lysis buffer, and prepared for Western-blot analysis using both p85 and IRS-1 antibodies.
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay
H4IIE cells were cultured in serum-free DMEM supplemented with 1 μM dexamethasone overnight, and treated with resveratrol at concentrations indicated in Figure 1(C) for 24 h in triplicate. MTT assay was conducted using a CGD-1 kit (MTT-based cell growth determination kit) from Sigma. In brief, DMEM was removed from culture dishes and replaced with 1 ml of PBS/dish. MTT solution was added at 100 μl/dish, and incubated with cells for another 4 h. Culture fluids were removed at the end of incubation and replaced with 1 ml of MTT solvent/dish and then gently stirred in a shaker for another 5 min. The collected MTT solvent from each dish was spectrophotometrically measured at 570 and 690 nm respectively. The corrected readings from triplicates were averaged and expressed as the percentage of those of control cells.
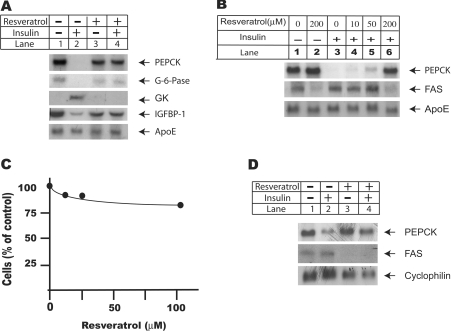
Figure 1. Resveratrol treatment inhibits insulin signalling pathway at transcriptional level.
(A) Resveratrol treatment abolished insulin effects on downstream target genes in rat primary hepatocytes. Rat primary hepatocytes were isolated and set up as described in the Experimental section and treated as indicated in the Figure. For combined treatments of insulin and resveratrol, cells were pre-incubated with 100 nM resveratrol for 30 min before they were challenged with insulin (100 nM) for another 4 h. Total RNA was extracted using TRIzol® reagents according to the manufacturer's instructions. Equal amount of RNA (16 μg/lane) was used for Northern-blot analysis using probes for PEPCK, GK, glucose-6-phosphatase and IGFBP-1. The ApoE mRNA level was used as the loading control. (B) Dose–response study of resveratrol effect on insulin signalling pathway. Rat primary hepatocytes were pre-incubated with resveratrol at 0, 10, 50 and 200 μM for 30 min before they were challenged with 100 nM insulin for another 16 h. Total RNA was extracted using TRIzol® reagents, and equal amount of RNA (16 μg/lane) was used for Northern-blot analysis using probes for PEPCK and FAS. Again, ApoE mRNA level was measured as the loading control. (C) MTT study of H4IIE cells treated with resveratrol. H4IIE cells were seeded at 2×106/60 mm dish in DMEM supplemented with 5% fetal bovine serum on day 1. Cells were serum-starved overnight before they were changed into serum-free DMEM supplemented with 1 μM dexamethasone and treated with resveratrol at 0, 10, 50 and 100 μM for another 24 h. The amount of cells was measured using an MTT based CGD-1 kit from Sigma. Results shown are representative of three independent experiments. (D) Resveratrol reversed insulin effects on PEPCK and FAS mRNA levels in H4IIE cells. H4IIE cells were set up as in (C), and treated with insulin (100 nM) and resveratrol (100 μM) for another 16 h. Total RNA was extracted using TRIzol® reagent according to the manufacturer's instruction. The expression levels of FAS and PEPCK were analysed using Northern-blot analysis. The expression level of cyclophilin was used as the loading control.
Data analysis
All the experiments were repeated at least three times. In Figures 2, 4, 5 and 6, the band intensity was also quantified using ImageJ (NIH Image). Statistics were done using either Student's t test or ANOVA as noted in the Figure legends.
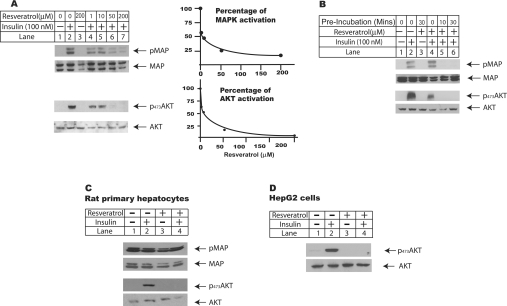
Figure 2. Resveratrol reverses insulin effects on MAPK and Akt phosphorylation.
(A) Dose–response study of resveratrol effects on insulin-induced MAPK and Akt phosphorylation. H4IIE cells were seeded at 2×106/60 mm dish in DMEM supplemented with 5% fetal bovine serum, and serum-deprived overnight before they were changed into plain DMEM supplemented with 1 μM dexamethasone for further treatments. Cells were pre-incubated with resveratrol ranging from 1 to 200 μM or DMSO for 30 min and then treated with 100 nM insulin for another 10 min. Total cell lysates were prepared by harvesting cells in ice-cold PBS and resuspended in lysis buffer supplemented with protease and phosphatase inhibitors. Equal amounts of whole cell lysates were subjected to Western-blot analysis using antibodies against phosphorylated forms of ERK p42/44 and Akt (Ser473). Total protein amounts of Akt and MAPK were also measured as the loading control. In the right panel, the band intensity of MAPK and Akt phosphorylation was quantified from three independent experiments, and the average of three experiments was plotted against the concentration of resveratrol. (B) Pre-incubation of resveratrol was required for its maximum inhibition of insulin-stimulated Akt and MAPK activation. H4IIE cells were set up as described in (A), with or without pre-incubation of 100 μM resveratrol as indicated in the Figure before they were treated with 100 nM insulin for another 10 min. Whole cell lysates were prepared for Western-blot analysis. (C) Resveratrol reversed insulin effects on Akt phosphorylation in rat primary hepatocytes. Rat primary hepatocytes were isolated and set up as described in Figure 1(A); cells were changed into M199 supplemented with 100 nM dexamethasone and pre-incubated with 100 μM resveratrol for 30 min before they were challenged with 100 nM insulin for another 10 min. Total cell lysates were prepared for Western-blot analysis. (D) HepG2 cells were set up at 50% confluence in DMEM supplemented with 5% fetal bovine serum for 1 day before they were serum-starved overnight and changed into plain DMEM supplemented with 1 μM dexamethasone. Cells were pre-incubated with 100 μM resveratrol for 30 min before they were treated with 100 nM insulin for another 10 min. Total cell lysates were prepared for Western-blot analysis.
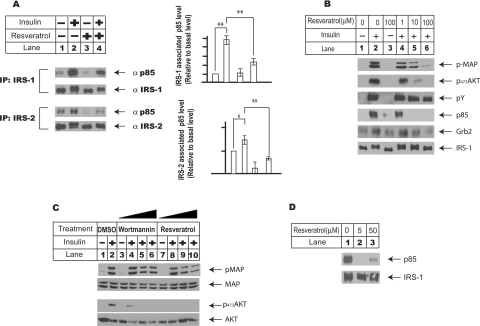
Figure 4. Resveratrol inhibits insulin signalling pathway through disruption of insulin-induced IRS complexes.
(A) Resveratrol disrupts insulin-induced association between p85 regulatory unit of PI3K and IRSs (IRS-1 and IRS-2). H4IIE cells were set up and treated as described in the legend of Figure 3, and both IRS-1 and IRS-2 were immunoprecipitated from total cell lysates using their respective antibodies. The protein levels of p85 associated with IRSs were analysed using p85 antibody from Cell Signaling Technology. The left panel shows a typical Western-blot analysis, and the right panel shows the protein levels of p85 associated with either IRS-1 or IRS-2 respectively under conditions indicated in the Figure. The results shown are averages of three independent experiments, quantified by ImageJ (NIH Image) program. The results were also analysed using ANOVA analysis. *P<0.05, **P<0.01. (B) Dose–response study of resveratrol effect on insulin-induced IRS-1 complex. H4IIE cells were set up as in (A), and pretreated with resveratrol at 1, 10 and 100 μM for 10 min before they were challenged with 100 nM insulin for another 10 min. IRS-1 was immunoprecipitated from total cell lysates to measure the protein levels of tyrosine-phosphorylated form of IRS-1, total IRS-1, p85 and Grb2 using their respective antibodies. In the meantime, the total cell lysates were also used to measure the phosphorylated Akt and MAPK levels under these conditions. (C) Insulin-induced MAPK activation is partially dependent on the PI3K activation. H4IIE cells were set up as described in Figure 2, and pre-incubated with either wortmannin at 5, 25 and 100 nM for 2 h or resveratrol at 10, 50 and 200 μM for 10 min, before they were treated with insulin for another 10 min. Total cell lysates were prepared for Western-blot analysis. (D) Resveratrol directly inhibits insulin-induced association between IRS-1 and p85 subunit of PI3K in vitro. Insulin-induced IRS-1 complex was immunoprecipitated from total cell lysates prepared using insulin-treated H4IIE cells and divided equally, and washed with lysis buffer before they were incubated with DMSO or resveratrol at 5 and 50 μM for 20 min at 37 °C in lysis buffer without Triton X-100. These treated IRS-1 complexes were washed briefly three times with lysis buffer and the p85 protein levels were analysed using Western-blot analysis.
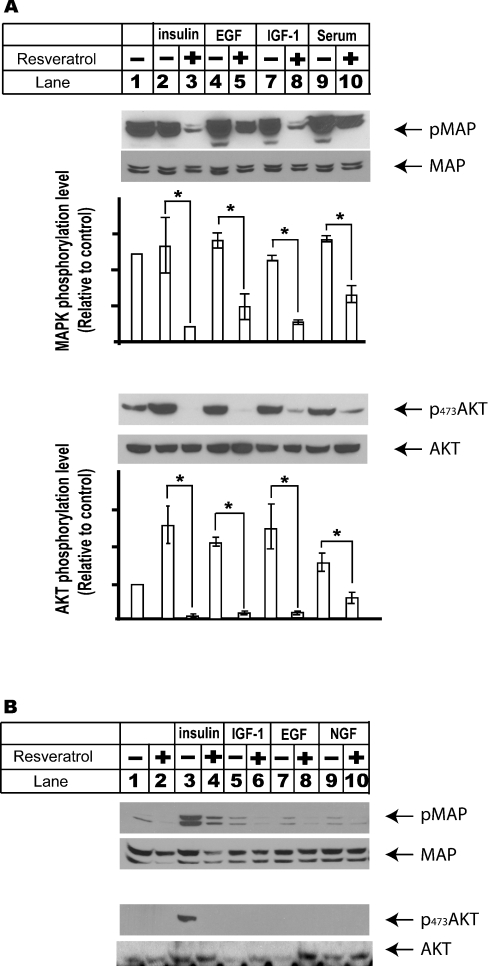
Figure 5. Biological effects of resveratrol result mainly from its inhibition of insulin signalling pathway in liver cells.
(A) HEK-293 cells were set up in DMEM supplemented with 5% fetal bovine serum for 1 day and serum-deprived overnight prior to the treatments. Cells were then changed into DMEM supplemented with 1 μM dexamethasone and incubated with 100 μM resveratrol for 30 min before they were challenged with insulin (100 nM, 10 min), IGF-1 (50 ng/ml, 15 min), EGF (50 ng/ml, 30 min) and 5% fetal bovine serum (30 min). Total cell lysates were prepared for Western-blot analysis using antibodies against both total and phosphorylated forms of Akt and MAPK. The protein amount of phosphorylated MAPK and Akt were also quantified using ImageJ (NIH Image) program, and the results from three independent experiments were averaged and plotted relative to the basal level. The significance of resveratrol inhibition of MAPK and Akt activations elicited by these growth factors was analysed using Student's t test. *P<0.05. (B) Insulin signalling pathway is an essential pathway leading to MAPK and Akt activations in liver cells. H4IIE cells were set up as described in Figure 2(A). Cells were changed into DMEM supplemented with 1 μM dexamethasone and pre-incubated with 100 μM resveratrol for 20 min and then challenged with various growth factors, including insulin (100 nm, 10 min), IGF-1 (100 ng/ml, 30 min), EGF (50 ng/ml, 30 min) and NGF (50 ng/ml, 30 min). Whole cell lysates were prepared for Western-blot analysis under these conditions.
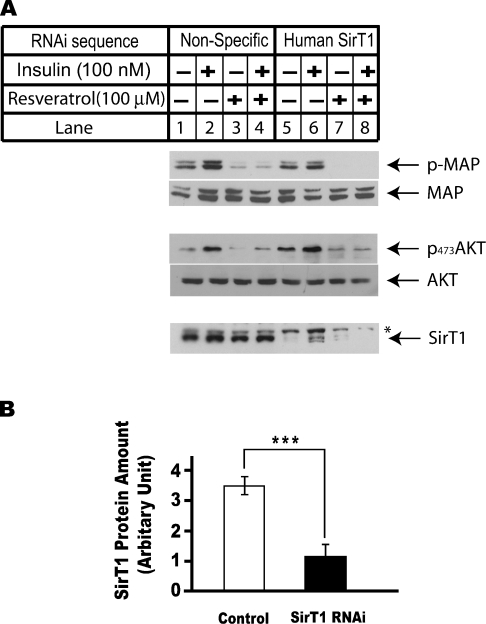
Figure 6. Resveratrol inhibits insulin-induced MAPK and Akt phosphorylations in a SirT1-independent pathway.
HEK-293 cells were seeded at 5×105/60 mm dish on day 0. Cells were transfected with 400 pmol of RNA oligonucleotide sequence against luciferase sequence in control group and same amount of RNA oligonucleotides against human SirT1 sequence in anti-SirT1 group using Oligofectamine reagent. Cells were transfected with RNA oligonucleotides for 30 h before they were serum-starved overnight. Both control and anti-SirT1 groups were treated with either DMSO or 100 μM resveratrol for 10 min before they were treated with 100 nM insulin for another 10 min. Total cell lysates were prepared for Western-blot analysis. (A) Western-blot analysis of the phosphorylation statuses of MAPK and Akt in response to insulin treatment in control cells or cells transfected with RNA oligonucleotides against SirT1. (B) The protein levels of SirT1 in cells described in (A) were analysed using Western-blot analysis. The results were also quantified using ImageJ (NIH Image) program. The average amount of SirT1 in control cells (lanes 1–4) were compared with those in cells transfected with RNA oligonucleotides against SirT1 (lanes 5–8) using Student's t test. ***P<0.001.
RESULTS
Liver is the key organ in energy homoeostasis. The putative effect of resveratrol on insulin signalling pathway was therefore first investigated in liver cells. Rat primary hepatocytes were pretreated with 100 μM resveratrol for 30 min before they were challenged with 100 nM insulin for another 4 h (Figure 1A). Northern-blot analysis showed that resveratrol treatment alone had no effect on PEPCK (phosphoenolpyruvate carboxykinase) mRNA level, whereas insulin treatment clearly reduced PEPCK mRNA to undetectable level within 4 h. The combined treatments with insulin and resveratrol, however, restored PEPCK mRNA to basal level. Co-treatments with resveratrol and insulin also reversed insulin effects on mRNA levels of several other insulin target genes, including glucose-6-phosphatase, IGFBP-1 (IGF-binding protein 1) and GK (glucokinase). As the loading control, the mRNA level of ApoE (apolipoprotein E) was not affected by these treatments.
Next, rat primary hepatocytes were treated with insulin alongside resveratrol ranging from 10 to 200 μM for 16 h, and total RNA was extracted for Northern-blot analysis (Figure 1B). Resveratrol showed inhibitory effect at 10 μM, and at 200 μM, restored PEPCK mRNA to basal level. The mRNA level of FAS (fatty acid synthase) was also analysed in the same experiment. FAS is the rate-limiting enzyme in lipogenesis. Compared with mRNA level measured in control cells, resveratrol alone significantly reduced basal level of FAS mRNA. Insulin treatment for 16 h significantly increased FAS mRNA level. Co-treatment of cells with insulin and resveratrol, up to 50 μM, had minimal effects on insulin-stimulated FAS mRNA level. At 200 μM, resveratrol reduced insulin-stimulated FAS mRNA below basal level. The ApoE mRNA level was again used as the loading control and was not affected by these treatments.
The inhibitory effect of resveratrol on insulin signalling pathway was also investigated using a rat hepatoma cell line H4IIE, as this cell line has been widely used in studies related to insulin signalling events [22]. Resveratrol treatment has been reported to cause apoptosis in multiple cell lines [17]. The toxicity of resveratrol was evaluated in H4IIE cells in Figure 1(C). H4IIE cells were serum-starved overnight before they were incubated with increasing doses of resveratrol for another 24 h, and cell growth was measured using MTT assay. MTT measures the activity of mitochondrial reductase enzymes; therefore it becomes an effective indicator of the amount of cells. Compared with cells treated with vehicle alone, resveratrol treatment decreased the amount of cells by 10% at 10 μM, and at 100 μM, the amount of cells was decreased by 15%. The slightly decreased cell amount may be caused by growth inhibition rather than cell death, as there was no appreciable cell death under microscopic observations.
Consistent with observations in rat primary hepatocytes, resveratrol treatment significantly reversed insulin effects on PEPCK and FAS mRNA levels. Resveratrol alone also significantly reduced basal level expression of FAS mRNA. The mRNA level of cyclophilin, as the loading control, was not affected by these treatments (Figure 1D).
The putative target(s) of resveratrol in insulin signalling path-way was explored in the next several experiments. MAPK and Akt pathways are the two major signalling pathways mediating insulin effects on cell metabolism, growth and differentiation [23]. Therefore resveratrol effects on insulin-induced MAPK and Akt activations were investigated subsequently.
In Figure 2(A), H4IIE cells were pre-incubated with resveratrol at concentrations ranging from 1 to 200 μM for 30 min before they were challenged with insulin for another 10 min. While total MAPK protein level remained constant in all the treatments, resveratrol at 1 μM already showed inhibitory effect on insulin-induced MAPK activation, demonstrated here by the reduced levels of phosphorylated forms of p42/44 ERK (extracellular-signal-regulated kinase) proteins. At 50 μM, resveratrol treatment reduced ERK protein phosphorylation to undetectable level. Similarly, while insulin treatment led to strong Akt activation, demonstrated by strong serine phosphorylation at Ser473 site of Akt protein, resveratrol at 1 μM considerably inhibited Akt phosphorylation, and at 50 μM reduced Akt phosphorylation to undetectable level. The phosphorylation levels of ERK and Akt proteins in this and two other experiments were also quantified using NIH Image program, and the average of these experiments was plotted against resveratrol concentration on the right panel in Figure 2(A).
In all the previous experiments, cells were pre-incubated with resveratrol for 30 min before they were treated with insulin. To investigate whether pre-incubation is required for the maximum inhibitory effect of resveratrol on insulin signalling, insulin-induced MAPK and Akt activations in cells pre-incubated with resveratrol for either 10 or 30 min respectively were compared with those in cells treated with insulin and resveratrol simultaneously (Figure 2B). Pre-incubation with resveratrol for 10 min already sufficiently inhibited insulin-induced MAPK and Akt activations in H4IIE cells. For those cells without pre-incubation, although resveratrol appreciably inhibited insulin-induced Akt activation, it showed minimal effect on MAPK activation. These results showed that pre-incubation with resveratrol was necessary for its maximal inhibitory effect on insulin-induced MAPK and Akt activations.
The resveratrol effects on insulin-induced MAPK and Akt activations were also examined using rat primary hepatocytes (Figure 2C) and HepG2 human hepatoma cells (Figure 2D). Unlike H4IIE cells, these cells showed high basal level activation of MAPK, which was not affected by either insulin or resveratrol treatment. Insulin treatment activated Akt in both rat primary hepatocytes and HepG2 cells, and this effect was abolished by cotreatment with resveratrol. Resveratrol also inhibited insulin-induced MAPK and Akt activations in HEK-293 and HeLa cells (Figure 4A and results not shown). Consistent with its effect on insulin signalling pathway at transcriptional level, these observations clearly demonstrated that resveratrol potently inhibited insulin signalling pathway in a variety of cells including liver cells.
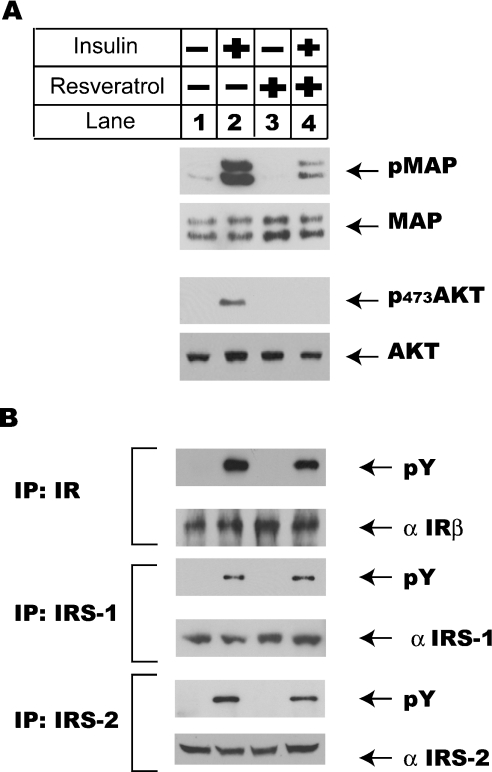
Insulin-induced activation of MAPK and Akt kinase cascades are mediated by a group of adaptor proteins downstream of insulin receptor tyrosine kinase. Insulin treatment activates insulin receptor tyrosine kinase, which leads to its autophosphorylation and the phosphorylation of IRSs. These phosphorylated IRSs recruit p85 subunit of PI3K and Grb2 to form IRS complexes to further activate downstream kinase cascades including Akt pathway (mediated by p85) and MAPK pathway (mediated by Grb2) [23]. To further define the molecular mechanism underlying the inhibitory effect of resveratrol on insulin signalling pathway, the impact of resveratrol treatment on the tyrosine phosphorylation status of insulin receptor, IRS-1 and IRS-2 in insulin signalling pathway was analysed next. In Figure 3(A), consistent with the results in Figure 2, resveratrol potently inhibited insulin-stimulated MAPK and Akt phosphorylations in H4IIE cells. Analysis of immunoprecipitated insulin receptor β, IRS-1 and IRS-2 from insulin-treated cells showed strong tyrosine phosphorylations of insulin receptor β, IRS-1 and IRS-2, regardless of the presence of resveratrol (Figure 3B). These results indicated that resveratrol acted downstream of insulin receptor β, IRS-1 and IRS-2 tyrosine phosphorylations to inhibit insulin signalling pathway. These results also ruled out the possibility that resveratrol inhibited insulin signalling pathway through direct inactivation of insulin.
Figure 3. Resveratrol treatment has no effect on insulin-induced tyrosine phosphorylations of insulin receptor, IRS-1 and IRS-2.
Cells were set up as described in the legend of Figure 2(A) and pre-incubated with 100 μM resveratrol for 10 min before they were challenged with 100 nM insulin for 10 min. Total cell lysates were prepared for both Western-blot analysis and immunoprecipitation. (A) Resveratrol treatment inhibited insulin-induced MAPK and Akt activation. (B) Resveratrol treatment had no effect on insulin-induced tyrosine phosphorylations of insulin receptor (IR) β, IRS-1 and IRS-2. Insulin receptor β, IRS-1 and IRS-2 were immunoprecipitated (IP) from total cell lysates prepared in (A), using their respective antibodies. Tyrosine phosphorylation statuses of insulin receptor β, IRS-1 and IRS-2 were analysed using a phosphotyrosine antibody (4G10; pY) from Upstate Biotechnology. The amounts of immunoprecipitated insulin receptor β, IRS-1 and IRS-2 were also analysed using Western-blot analysis.
The interactions between IRSs (IRS-1 and IRS-2) and p85 regulatory subunit of PI3K is critical to insulin-induced PI3K activation [24,25]. Therefore the impact of resveratrol treatment on the interactions between IRSs and p85 was evaluated next (Figure 4A). H4IIE cells were again treated with or without resveratrol (100 μM) before they were challenged with insulin for another 10 min. Cells were immunoprecipitated with IRS-1 or IRS-2 antibody respectively, and the immunoprecipitated protein complexes were analysed using p85 antibody. While, under basal state, there was only residual interaction between IRS-1 and p85, insulin treatment strongly enhanced this interaction (IRS-1 with p85; 568%). The interaction between IRS-1 and p85 remained low in cells treated with resveratrol alone. Co-treatment with resveratrol and insulin, however, clearly reduced insulin-induced IRS-1 and p85 interaction significantly (256%, P<0.01). Similarly, the interaction between IRS-2 and p85 increased in response to insulin treatment (157%, P<0.05), and this interaction was inhibited by resveratrol significantly (77%, P<0.01). These results showed that resveratrol treatment interrupted insulin-induced association of IRSs with p85.
To rule out the possibility that resveratrol treatment led to rapid degradation of p85 protein, the protein level of p85 was also analysed in the total cell lysates. Treatment of resveratrol at 100 μM for up to 60 min had minimal effect on total protein level of p85 (results not shown).
A dose–response study of the resveratrol effect on insulin-induced IRS protein complexes was also conducted, as demonstrated in Figure 4(B). H4IIE cells were pretreated with resveratrol ranging from 1 to 100 μM for 10 min before they were challenged with 100 nM insulin for another 10 min. Consistent with previous results, resveratrol at 10 μM significantly inhibited both MAPK and Akt activation, yet, even at 100 μM, resveratrol had minimal effect on insulin-induced IRS-1 tyrosine phosphorylation. In the meantime, while resveratrol at 1 μM had no effect on insulin-induced IRS-1/p85 association, it effectively eliminated this association at 10 and 100 μM. This observation further supports the conclusion that the disruption of the insulin-induced complexes between IRSs and p85 subunit of PI3K is critical in mediating the inhibitory effect of resveratrol on insulin signalling pathway.
The recruitment of Grb2 to IRS protein is critical to the transmission of insulin signalling to MAPK cascade. In the same experiment (Figure 4B), the association between IRS-1 and Grb2 was also analysed, and the presence of resveratrol at 10 and 100 μM also led to the disruption of the association between IRS-1 and Grb2. Clearly, although the insulin-induced MAPK activation is partially dependent on PI3K activation in H4IIE cells, as demonstrated using wortmannin, a specific inhibitor of PI3K, in Figure 4(C) and in previous work [26], disruption of Grb2–IRS association by resveratrol contributes significantly to the potent effect of resveratrol on MAPK activation (Figure 4C).
To understand further the disruptive effect of resveratrol on insulin-induced IRS protein complexes, the inhibitory effect of resveratrol on the IRS–p85 association was also investigated directly in vitro (Figure 4D). In this experiment, insulin-induced IRS-1–p85 protein complex was immunoprecipitated and incubated with increased concentration of resveratrol (20 min at 37 °C) to test if the presence of resveratrol in vitro had any direct effect on IRS1–p85 association. Figure 4(D) clearly showed that presence of resveratrol at 5 and 50 μM disrupted the association between IRS-1 protein and p85 subunit of PI3K significantly. This experiment directly indicated interference of resveratrol on the protein interaction between p85 and IRS.
The possible regulatory effects of resveratrol on signalling pathways elicited by several other growth factors, including IGF-1, EGF and NGF, were also examined. HEK-293 cells showed high basal levels of MAPK and Akt activations. Resveratrol, on the other hand, reduced MAPK activation below basal level regardless of the growth factors used under all these conditions (P<0.05).
Growth factors including insulin, IGF-1 and EGF all potently activated Akt (P<0.05), which was significantly repressed by co-treatment with resveratrol (P<0.01). Resveratrol treatment alone also significantly reduced basal level activation of Akt (P<0.01). These results showed that resveratrol had broad inhibitory effects on growth-factor-mediated signalling pathways (Figure 5A). In addition, at least in cells treated with EGF, resveratrol had minimal effect on EGFR tyrosine phosphorylation and the interaction between EGFR and Grb2 (see supplementary data at http://www.BiochemJ.org/bj/397/bj3970519add.htm).
The inhibitory effects of resveratrol on growth factor-induced MAPK and Akt activations were also investigated using rat hepatoma H4IIE cells. However, unlike HEK-293 cells, none of the growth factors examined above except insulin showed any striking effects on MAPK and Akt activations (Figure 5B). These results emphasized the importance of insulin signalling pathway in liver cells. Likewise, none of these growth factors other than insulin induced Akt activation in rat primary hepatocytes (results not shown). These results suggested that although resveratrol might have a broad effect on a variety of signalling pathways, the inhibitory effect of resveratrol on insulin signalling pathway might be more relevant to its physiological effects.
Resveratrol has been shown to activate Sir2 family histone deacetylases both in vivo and in vitro [3]. The involvement of SirT1, the mammalian orthologue of Sir2, in resveratrol regulation of insulin signalling pathway was investigated using RNAi technique, a well-developed method to knock-down specific endogenous protein expression without inducing global changes [27]. In Figure 6(B), the protein level of SirT1 was significantly reduced in HEK-293 cells transfected with RNA double-stranded oligonucleotides against human SirT1 (lanes 5–8) compared with that of the cells transfected with non-specific RNA double-stranded oligonucleotides (lanes 1–4) (P<0.001). However, resveratrol suppressed insulin-induced MAPK and Akt activations regardless of the expression level of SirT1. Similar results were also obtained from HeLa and H4IIE cells (results not shown). These results suggested that resveratrol inhibits insulin signalling pathway independent of its effect on SirT1 activation.
DISCUSSION
Beyond the broad biological effects of resveratrol reported previously [17], the newly recognized function of resveratrol in activating a family of key enzymes in calorie restriction, Sir2-family histone deacetylases, promotes more intensive research on this small compound and its analogues. In the present study, the molecular mechanism(s) mediating the broad effects of resveratrol was explored by identifying a new role of resveratrol as the inhibitor of insulin signalling pathway. In addition, the inhibitory effect of resveratrol on insulin signalling pathway is suggested to be independent of its activation of SirT1 histone deacetylase.
The involvement of insulin signalling pathway in aging process has been demonstrated in species ranging from C. elegans to mammals. Inactivation of insulin signalling pathway in C. elegans leads to significantly prolonged lifespan. In mammalian systems, the role of insulin signalling pathway in aging process is more complicated. While aging process is undoubtedly associated with altered insulin signalling pathway, the direct impact of insulin signalling pathway on lifespan is still unclear [10]. Nonetheless, inactivation of insulin receptor in mouse adipose tissues, one of the key organs in energy homoeostasis, leads to significantly increased lifespan [10]. Several other aging models, including Snell mice and Ames mice, are also associated with reduced insulin signalling pathway [15]. Therefore the strong inhibitory effect of resveratrol on insulin signalling pathway demonstrated in the present study is consistent with its reported effect on lifespan extension, and inevitably raised the possibility that the regulatory effect of resveratrol on aging may also derive from its inhibition of insulin signalling pathway.
Resveratrol has been suggested to inhibit a variety of biological responses, including MAPK and Akt activations [17]. Consistent with these studies, the inhibitory effects of resveratrol on insulin-induced MAPK and Akt activations were clearly demonstrated in the present study. Further investigations suggested that resveratrol disrupts insulin-induced IRS protein complexes in mammalian cells. This conclusion was supported by in vitro studies, where incubation of resveratrol with IRS-1–p85 complex directly reduced their interaction. It is still unclear how the interactions between these adaptor proteins and IRS proteins are disrupted, as a similar interaction between phosphorylated receptor protein (EGFR) and Grb2 is not affected by the presence of resveratrol.
One unexpected observation in the present study is the independent regulation of insulin signalling pathway by resveratrol from its activation of SirT1 histone deacetylase. SirT1 is generally regarded as the orthologue of Sir2 in mammals [3]. Resveratrol treatment leads to activation of Sir2 family NAD+-dependent histone deacetylases both in vivo and in vitro [2,6,19,28], and prolongs lifespan in C. elegans, Drosophila and yeast. Deletion of Sir2 in C. elegans and yeast eliminates resveratrol effect on lifespan. All these results implicate that Sir2 family histone deacetylases are mediators of resveratrol effect on aging.
However, the RNAi experiment in the present study indicated that significant reduction of endogenous SirT1 protein level did not lead to diminished inhibitory effect of resveratrol on insulin signalling pathway. This obvious discrepancy cannot be explained at this time. A simple explanation would be that SirT1 is not the only target of resveratrol in mammalian cells. There are at least seven members of Sir2 family of NAD+-dependent histone deacetylases in mammals [3]. Member(s) of this family other than SirT1 might be the true mediators of the resveratrol effect on insulin signalling pathway. The possibility that the RNAi experiment in the present study may not sufficiently reduce endogenous SirT1 level to induce any significant effect also exists.
Another possibility that resveratrol may additionally modulate lifespan from its inhibition of insulin signalling pathway, independently from its activation of Sir2 family histone deacetylases, should also be considered. In fact, there are studies dissociating Sir2 from calorie restriction in lifespan regulation [29,30]. In one study, calorie restriction prolongs lifespan in several yeast strains lacking Sir2 expression. Combined treatments of calorie restriction and Sir2 overexpression extend yeast lifespan longer than any single treatment [30]. The possibility that the inhibitory effect of resveratrol on insulin signalling pathway is only limited to mammalian cells should also be considered, although it is highly unlikely considering that key components of insulin signalling pathway, including MAPK, Akt, PI3K and forkhead family transcriptional factors, are highly conserved across species. Nonetheless, additional studies are required to elucidate the relationship among resveratrol, SirT1 and aging in mammals.
The present study may be also related to other biological properties of resveratrol. Resveratrol has been reported to cause cell-cycle arrest, accumulation of DNA mutations and apoptosis in multiple cell lines and therefore has been proposed as a potential chemotherapeutic agent [17]. The inhibitory effect of resveratrol on signalling pathways induced by various growth factors and hormones, including EGF, IGF-1, insulin and even serum, provides an alternative explanation of these antiproliferative effects of resveratrol. Most of these studies have been conducted using transformed cells, and tumour cell growth depends heavily on various growth factors and serum. Inhibition of downstream signalling pathways of these growth factors by resveratrol presumably leads to the inhibition of cell proliferation. Resveratrol also inhibited basal level kinase activities, including both MAPK and Akt activities in HEK-293 and H4IIE cells, and therefore might deprive the basic survival requirement of these cells. In addition, the inhibitory effect of resveratrol on both basal and insulin-stimulated FAS mRNA level may also suggest a regulatory effect of resveratrol in vivo lipid homoeostasis.
Overall, the present study showed that resveratrol potently inhibited insulin signalling pathway in several cell lines and rat primary hepatocytes through disruption of insulin-induced IRS protein complexes. SirT1 was not required in the resveratrol regulation of insulin signalling pathway. These observations may explain a wide range of biological effects of resveratrol, including its regulatory effect on aging. Furthermore, the newly identified inhibitory effect of a compound known to modulate lifespan on insulin signalling further highlights the critical role of insulin signalling pathway in the aging process.
Metabolic syndrome, also called syndrome X, describes a collection of health disorders including high blood pressure, obesity, insulin resistance, glucose intolerance and dyslipidaemia. Metabolic syndrome increases risks for stroke, cardiovascular diseases and diabetes, and has become a major health threat in the United States and the world [31]. Recently, calorie restriction has been shown to significantly improve several risk factors including lipid profiles, blood pressure, fasting glucose and insulin levels and body weight in human studies [32]. These observations imply a possible treatment of metabolic syndrome through activation of the genetic programme mediating the pleiotropic effects of calorie restriction. Considering that resveratrol effectively mimics calorie restriction in animals ranging from C. elegans to Drosophila and the beneficial effects of calorie restriction on metabolic syndrome, the continuing investigation of the molecular mechanism of resveratrol inhibition of insulin signalling pathway may greatly facilitate our investigation and intervention of metabolic syndrome and other aging-related diseases.
Online Data
Acknowledgments
I express my deep appreciation and gratitude to Dr Scott M. Grundy (University of Texas Southwestern Medical Center at Dallas) for his constant support and critical reading of this paper, to editors and reviewers for their constructive comments, and to Dr Mike Brown (University of Texas Southwestern Medical Center at Dallas) for his invaluable advice. This project is supported by the Center for Human Nutrition Endowment and the Moss Heart Center, University of Texas Southwestern Medical Center (Dallas, TX, U.S.A.).
References
- 1.Guarente L., Picard F. Calorie restriction – the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Wood J. G., Rogina B., Lavu S., Howitz K., Helfand S. L., Tatar M., Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature (London) 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 3.Blander G., Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 4.Tissenbaum H. A., Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature (London) 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 5.Lin S. J., Defossez P. A., Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 6.Rogina B., Helfand S. L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamming D. W., Wood J. G., Sinclair D. A. Small molecules that regulate lifespan: evidence for xenohormesis. Mol. Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- 8.Tatar M., Bartke A., Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 9.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Bluher M., Kahn B. B., Kahn C. R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 11.Holzenberger M., Dupont J., Ducos B., Leneuve P., Geloen A., Even P. C., Cervera P., Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature (London) 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 12.Barbieri M., Bonafe M., Franceschi C., Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh C. C., DeFord J. H., Flurkey K., Harrison D. E., Papaconstantinou J. Implications for the insulin signaling pathway in Snell dwarf mouse longevity: a similarity with the C. elegans longevity paradigm. Mech. Ageing Dev. 2002;123:1229–1244. doi: 10.1016/s0047-6374(02)00036-2. [DOI] [PubMed] [Google Scholar]
- 14.Bartke A., Brown-Borg H. Life extension in the dwarf mouse. Curr. Top. Dev. Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 15.Liang H., Masoro E. J., Nelson J. F., Strong R., McMahan C. A., Richardson A. Genetic mouse models of extended lifespan. Exp. Gerontol. 2003;38:1353–1364. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh C. C., DeFord J. H., Flurkey K., Harrison D. E., Papaconstantinou J. Effects of the Pit1 mutation on the insulin signaling pathway: implications on the longevity of the long-lived Snell dwarf mouse. Mech. Ageing Dev. 2002;123:1245–1255. doi: 10.1016/s0047-6374(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 17.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 18.Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature (London) 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 19.Yeung F., Hoberg J. E., Ramsey C. S., Keller M. D., Jones D. R., Frye R. A., Mayo M. W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Ou J., Bashmakov Y., Horton J. D., Brown M. S., Goldstein J. L. Insulin inhibits transcription of IRS-2 gene in rat liver through an insulin response element (IRE) that resembles IREs of other insulin-repressed genes. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3756–3761. doi: 10.1073/pnas.071054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Oliveira R. M., Leid M., McBurney M. W., Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature (London) 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granner D., Andreone T., Sasaki K., Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature (London) 1983;305:549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- 23.Kahn C. R., Vicent D., Doria A. Genetics of non-insulin-dependent (type-II) diabetes mellitus. Annu. Rev. Med. 1996;47:509–531. doi: 10.1146/annurev.med.47.1.509. [DOI] [PubMed] [Google Scholar]
- 24.Myers M. G., Jr, Backer J. M., Sun X. J., Shoelson S., Hu P., Schlessinger J., Yoakim M., Schaffhausen B., White M. F. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patti M., Sun X. J., Bruening J. C., Araki E., Lipes M. A., White M. F., Kahn C. R. 4PS/Insulin receptor substrate (IRS)-2 is the alternative substrate of the insulin receptor in IRS-1-deficient mice. J. Biol. Chem. 1995;270:24670–24673. doi: 10.1074/jbc.270.42.24670. [DOI] [PubMed] [Google Scholar]
- 26.Ui M., Okada T., Hazeki K., Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem. Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 27.Huppi K., Martin S. E., Caplen N. J. Defining and assaying RNAi in mammalian cells. Mol. Cell. 2005;17:1–10. doi: 10.1016/j.molcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt M. T., Smith B. C., Jackson M. D., Denu J. M. Co-enzyme specificity of Sir2 protein deacetylases: Implications for physiological regulation. J. Biol. Chem. 2004;279:40122–40129. doi: 10.1074/jbc.M407484200. [DOI] [PubMed] [Google Scholar]
- 29.Kaeberlein M., McDonagh T., Heltweg B., Hixon J., Westman E. A., Caldwell S., Napper A., Curtis R., Distefano P. S., Fields S., et al. Substrate specific activation fo sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 30.Kaeberlein M., Kirkland K. T., Fields S., Kennedy B. K. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmet P., Alberti K. G., Shaw J. Global and societal implications of the diabetes epidemic. Nature (London) 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 32.Fontana L., Meyer T. E., Klein S., Holloszy J. O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.