Abstract
The cDNA encoding trehalose phosphorylase, a family GT-4 glycosyltransferase from the fungus Schizophyllum commune, was isolated and expressed in Escherichia coli to yield functional recombinant protein in its full length of 737 amino acids. Unlike the natural phosphorylase that was previously obtained as a truncated 61 kDa monomer containing one tightly bound Mg2+, the intact enzyme produced in E. coli is a dimer and not associated with metal ions [Eis, Watkins, Prohaska and Nidetzky (2001) Biochem. J. 356, 757–767]. MS analysis of the slow spontaneous conversion of the full-length enzyme into a 61 kDa fragment that is fully active revealed that critical elements of catalysis and specificity of trehalose phosphorylase reside entirely in the C-terminal protein part. Intact and truncated phosphorylases thus show identical inhibition constants for the transition state analogue orthovanadate and α,α-trehalose (Ki≈1 μM). Structure-based sequence comparison with retaining glycosyltransferases of fold family GT-B reveals a putative active centre of trehalose phosphorylase, and results of site-directed mutagenesis confirm the predicted crucial role of Asp379, His403, Arg507 and Lys512 in catalysis and also delineate a function of these residues in determining the large preference of the wild-type enzyme for the phosphorolysis compared with hydrolysis of α,α-trehalose. The pseudo-disaccharide validoxylamine A was identified as a strong inhibitor of trehalose phosphorylase (Ki=1.7±0.2 μM) that displays 350-fold tighter binding to the enzyme–phosphate complex than the non-phosphorolysable substrate analogue α,α-thio-trehalose. Structural and electronic features of the inhibitor that may be responsible for high-affinity binding and their complementarity to an anticipated glucosyl oxocarbenium ion-like transition state are discussed.
Keywords: α-retaining mechanism, glycosyltransferase, fold family GT-B, internal return mechanism, Schizophyllum commune, trehalose phosphorylase
Abbreviations: α-D-Glc 1-P, α-D-glucose 1-phosphate; EcMalP, maltodextrin phosphorylase from Escherichia coli; GPGT, glycogen phosphorylase glycosyltransferase; LC-ESI-Q-TOF MS, liquid chromatography–electrospray ionization–quadrupole–time-of-flight MS; OtsA, trehalose 6-phosphate synthase from E. coli; ScTPase, trehalose phosphorylase from Schizophyllum commune; SNi-like mechanism, internal return-like mechanism; UDP-Glc, UDP-α-D-glucose. Numbering of ScTPase starts with the initiator methionine as 1 and does not consider the N-terminal 11-amino-acid-long fusion peptide that is used for recombinant protein production
INTRODUCTION
Glycosyltransferases catalyse the transfer of a glycosyl moiety from a donor sugar to an acceptor molecule. In the present postgenomic era, there is much interest in elucidating their mechanism and specificity as the molecular determinants of the complexity of natural glycan structures [1,2]. Eighty-three glycosyltransferase families (as on 13 April 2006) have been categorized in a sequence-derived classification system ([3]; see also http://www.cazy.org/CAZY/). Two basic protein topologies, each of the Rossman-fold type, have been observed so far for glycosyltransferases and are commonly termed GT-A and GT-B [1–3].
Mechanistically, the glycosyltransferases can be classified according to whether they retain or invert the anomeric configuration of the donor substrate in the glycosidic product. Enzymatic glycosyl transfer with inversion is thought to occur by a single nucleophilic displacement mechanism that features an oxocarbenium ion-like transition state [4]. The catalytic scenario for retaining glycosyltransferases is conceptually much less clear [5]. Enzymatic hydrolysis of glycosides with retention of configuration involves the formation and subsequent breakdown of an inverted covalent glycosyl enzyme intermediate [4,6,7]. A similar two-step (double-displacement) mechanism was considered probable for retaining glycosyltransferases, but in spite of the attempts to delineate the catalytic steps, no convincing support for a covalent intermediate has been obtained so far ([4] and references therein; [8–10]). This has led to the consideration of alternative scenarios [4,11,12] among which the SNi-like mechanism (internal return-like mechanism) [13] seems to be a strong possible choice.
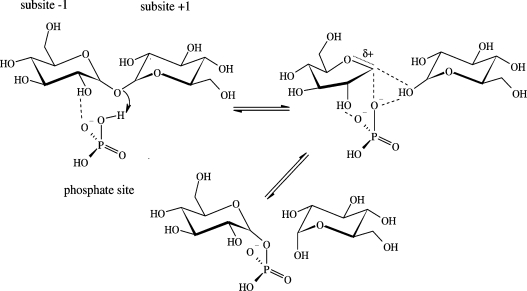
Retaining glycosyl transfer via internal return would involve departure of the leaving group and attack of the nucleophile in a concerted manner from the same side of the glycosyl moiety (Figure 1). A close hydrogen bond between the incoming nucleophile and leaving group would facilitate the SNi-like reaction, providing stabilization to the anticipated oxocarbenium ion-like transition state that is likely to have a strongly dissociative character. Further electrostatic stabilization of this transition state could come from the suitably positioned anionic leaving group/nucleophile. Surrounding the catalytic event, the widely observed kinetic scenario for retaining glycosyltransferases in which donor sugar and acceptor must combine in a ternary enzyme–substrate complex before reaction takes place is a strict requirement of the SNi-like mechanism, whereas glycosyl transfer via covalent intermediate is compatible with other kinetic schemes, especially the Ping Pong mechanism. While trapping of covalent glycosyl enzyme intermediate is conceptually straightforward, it is very difficult to design experiments that probe internal return.
Figure 1. Phosphorolysis of α,α-trehalose by a hypothetical SNi-like mechanism.
For further explanations, see the text. The reaction is thought to proceed through an oxocarbenium ion-like transition state.
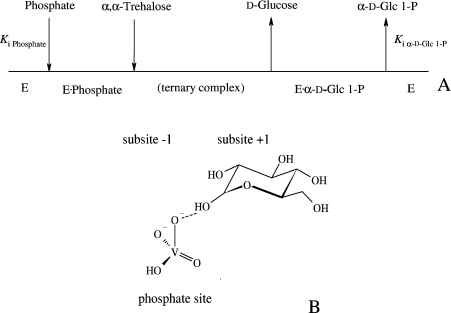
ScTPase (trehalose phosphorylase from Schizophyllum commune; EC 2.4.1.231) catalyses glucosyl transfer to and from phosphate using α,α-trehalose and α-D-Glc 1-P (α-D-glucose 1-phosphate) as the respective donor substrates [14]. Peptide ‘fingerprinting’ analysis revealed that the enzyme belongs to glycosyltransferase family GT-4 [10]. ScTPase has an ordered kinetic mechanism, as indicated in Figure 2(A), and the physiological direction of the reaction is the phosphorolysis of α,α-trehalose [10,14]. Evidence hinting at an SNi-type mechanism of retaining glucosyl transfer by ScTPase is the transition state-like inhibition by the phosphate analogue vanadate in combination with glucose [15]. A non-covalent adduct of the two enzyme-bound ligands, arguably stabilized by a hydrogen bond as shown in Figure 2(B), is the true inhibitor, and this could mimic the ‘effective leaving group’ of the reaction. The proposed interactions would explain the apparently inherent coupling of bond-breaking and bond-forming steps seen in reactions catalysed by ScTPase and other retaining glycosyltransferases. In contrast, they are compatible with, but not clearly required for, glucosyl transfer via a covalent intermediate.
Figure 2. Steady-state kinetic mechanism of ScTPase (A) and suggested interactions leading to the transition-state-like inhibition by orthovanadate and glucose (B).
In (A), Ki is a dissociation constant.
The available biochemical evidence for the wild-type enzyme [10,14–16] strongly promotes studies of the ScTPase catalytic mechanism with site-directed mutagenesis. To provide the required structural basis, we report here on the isolation of the cDNA encoding ScTPase and the efficient heterologous expression thereof in Escherichia coli, as well as the detailed characterization of the purified recombinant enzyme. Results of secondary and three-dimensional structure-based sequence comparisons and the analysis of kinetic consequences in four point mutants of ScTPase suggest a putative active site of the enzyme which shares striking similarity with the proposed catalytic centres of retaining GT-B fold glycosyltransferases.
EXPERIMENTAL
Materials
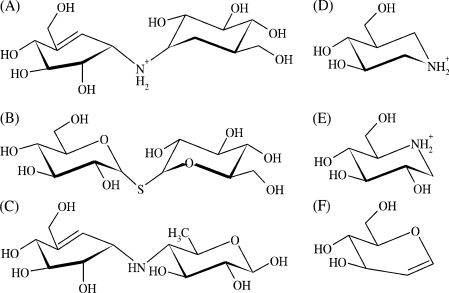
Validoxylamine A [17] was a gift from Dr Naoki Asano (Hokuriku University, Kanazawa, Japan). Its structure is shown in Figure 3 along with other structures discussed below. Unless otherwise mentioned, all materials used have been described elsewhere [10,14].
Figure 3. Structures of common inhibitors of glycosyl hydrolases and glycosyltransferases including ScTPase.
(A) Validoxylamine A (1.7 μM; the present study); (B) α,α-thio-trehalose (560 μM) [10]; (C) acarviosine unit of acarbose (n.a.); (D) isofagomine (56 μM) [15]; (E) 1-deoxynojirimycin (1200 μM) [15]; (F) D-glucal (320 μM) [10]. Values in parentheses show inhibition constants that describe binding of the inhibitor to the complex of ScTPase and phosphate; n.a., not applicable.
Molecular cloning of full-length ScTPase
Washed mycelia of S. commune BT 2115 (prepared by using reported conditions [14]) were frozen in liquid nitrogen. Total RNA was isolated from the powdered biomass using RNeasy Plant Mini kit (Qiagen). The cDNA of S. commune was constructed with a reverse transcription system from Promega. A pair of degenerated primers (5′-primer: 5′-GCSTTCATYGATGACCKCAGATG-3′; 3′-primer: 5′-GTCGATSACGTTSGGGATRCC-3′, where K=T+G, R=A+G, S=C+G, Y=C+T) were deduced from two peptides that are well conserved in fungal trehalose phosphorylases: A371FIDDPQM378 and G509IPNVID515. {The amino acid numbering of trehalose phosphorylase from Grifola frondosa (GenBank®/GenPept accession no. BAA31350) is used [18]}. An approx. 450-bp PCR product was obtained using Taq DNA polymerase under optimized conditions (see Supplementary Table 1 at http://www.BiochemJ.org/bj/397/bj3970491add.htm), cloned into a pGEM-T-vector (Promega) and sequenced. The 5′- and 3′-ends of the cDNA, containing the full-length gene encoding ScTPase, were obtained using GeneRacer™ kit (Invitrogen) with two pairs of oligonucleotide primers: (i) GeneRacer™ 5′-nested primer and gene-specific 3′-primer (5′-GGGGAGCTCAGGCCGGACTTTCTT-3′) and (ii) gene-specific 5′-primer (5′-CCGAACCGCGAGTACTGCATCCAGAT-3′) and GeneRacer™ 3′-nested primer. The PCR products obtained by amplification with Taq DNA polymerase (see Supplementary Table 1) were cloned into a pCR 4-TOPO vector (Invitrogen) and sequenced. Amino acid sequence analysis was performed with the programs Vector NTI (Suite 8; Informax) and SeqMan (DNASTAR).
For expression cloning, the ScTPase gene was amplified from the cDNA with proofreading Pfu DNA polymerase using two gene-specific primers (5′-primer: 5′-AGCGGATCCTCTCCTCCCCACGGATTCATG-3′; 3′-primer: 5′-ACCCTGCAGCTAGTCCTGCACCTTGATACC-3′, where BamHI and PstI restriction sites are underlined respectively). The obtained 2229-bp fragment (see Supplementary Table 1 for conditions) was digested and placed into a pQE 30 vector (Qiagen). The expression vector (pQE 30-ScTPase) was transferred into E. coli DH10B cells and the cloned insert was sequenced. Recombinant ScTPase contains an N-terminal fusion peptide with the sequence RGSHHHHHHGS.
Production and purification of recombinant ScTPase
Cells of E. coli DH10B harbouring pQE 30-ScTPase were grown in baffled 1 litre shaken flasks (37 °C; 120 rev./min) containing 250 ml of Luria–Bertani media supplemented with 115 mg/l ampicillin. Gene expression was induced with 0.1 mM isopropyl-β-D-thiogalactopyranoside when the culture had reached an attenuance of 0.8–1.0 and continued for 18 h at 25 °C. Harvested cells were disrupted with an Aminco French press using an FA-030 cell (SLM Instruments) at approx. 150 bar. After removal of cell debris at 80000 g and 4 °C, the supernatant (∼15 ml containing 450 mg of protein) was applied on to an XK 26/40 column packed with 50 ml of Macro-Prep DEAE Support gel (Bio-Rad) and equilibrated with 50 mM potassium phosphate buffer (pH 7.0). (Note that isolation of the His-tagged ScTPase using immobilized copper affinity chromatography on an XK 16/20 Chelating Sepharose Fast Flow column was not successful, because the enzyme lost most of its activity during elution with imidazole.) Proteins were eluted from the anion exchange column with a linear gradient from 0 to 250 mM NaCl, followed by a step gradient at 500 and 1000 mM NaCl in the same phosphate buffer. ScTPase was eluted at 160 mM NaCl. After change of buffer to 5 mM potassium phosphate (pH 7.0) using a HiPrep 26/10 Desalting column (GE Healthcare Biosciences), the concentrated ScTPase-containing fractions (2.7 mg/ml) were loaded on to an XK 16/20 column packed with 22 ml of Hydroxyapatite High-resolution resin (Merck). Proteins were eluted with a linear gradient from 5 to 150 mM potassium phosphate, followed by a final washing step at 500 mM phosphate. ScTPase was eluted at approx. 40 mM phosphate. Pooled fractions containing phosphorylase activity were concentrated to approx. 3 mg/ml using 10 kDa cut-off ultra-filtration concentrator tubes (Vivascience) in 50 mM potassium phosphate buffer (pH 7.0) and stored at −21 °C. Protein concentration was determined using the Bio-Rad dye-binding method with BSA as the standard. Purification was monitored by SDS/PAGE, performed with a PhastSystem (GE Healthcare Biosciences) using precast gradient gels (PhastGel 8-25) as well as determination of specific enzyme activity. Before further use, the enzyme storage solution was gel-filtered using a combination of NAP-5 and NAP-10 columns (GE Healthcare Biosciences), equilibrated with 50 mM Mes buffer (pH 6.6).
Biochemical characterization of recombinant ScTPase
The continuous coupled-enzyme assay of ScTPase activity was described elsewhere [14]. Initial rates of α,α-trehalose phosphorolysis and synthesis were recorded using discontinuous assays as reported in earlier works [10,14] where the formation of α-D-Glc 1-P and phosphate is measured respectively. Inhibitor binding studies were performed by using methods developed with the native ScTPase [10]. Data processing for the calculation of kinetic parameters and inhibitor binding constants used reported procedures [10] (see the Results section). Molecular-mass determination of ScTPase by gel filtration analysis was carried out with a Superdex 200 10/300 GL High-Performance column (GE Healthcare Biosciences) that was equilibrated with 50 mM potassium phosphate buffer (pH 7.0) containing 150 mM sodium chloride and calibrated with Bio-Rad Gel Filtration standard.
Analysis of alternative reactions catalysed by ScTPase
Enzymatic assays were performed in which the hydrolysis of α,α-trehalose (400 mM) or α-D-Glc 1-P (25 mM) by ScTPase was measured. The enzyme (∼0.3 mg/ml) was incubated in the presence of substrate at 30 °C in 50 mM Mes buffer (pH 6.6) and the release of glucose or phosphate was monitored over time, up to 30 h. UDP-Glc (UDP-α-D-glucose; 25 mM) was tested as a substrate of ScTPase in place of α-D-Glc 1-P, whereby the time-dependent formation of UDP was recorded in the absence or presence of glucose (400 mM) under the conditions described above. The UDP concentration in samples taken from the reaction mixture was determined enzymatically [19]. Appropriate controls lacking ScTPase were always recorded, and the reported values are corrected for blank readings that were typically <10% of the enzymatic rates.
Characterization of recombinant ScTPase by MS
Metal analysis was performed with inductively coupled plasma MS, as previously published [10]. Protein and peptide MS was performed on a Q-TOF Ultima Global (Waters Micromass) mass spectrometer equipped with a standard electrospray unit, a Cap-LC System (Waters Micromass) and a ten-port solvent switch module (Rheodyne). Approximately 200–300 pmol of protein was loaded on to a BioBasic C4 column (Thermo Electron) and eluted with an increasing gradient of acetonitrile. Protein (5 μg) was subjected to SDS/PAGE, destained, carbamidomethylated, digested with trypsin and extracted from gel pieces as described elsewhere [20]. Dried extracts were reconstituted in 0.1% formic acid before LC-ESI-Q-TOF MS (liquid chromatography–electrospray ionization–quadrupole–time-of-flight MS) analysis. Peptides were captured on an Aquasil C18 precolumn (Thermo Electron) before elution from the analytical column with a linear gradient from 5 to 50% of acetonitrile. Samples were analysed in the MS and tandem MS mode, and data analysis was performed with MassLynx 4.0 SP4 and BioLynx software (Waters Micromass).
Site-directed mutagenesis
Site-directed mutations were introduced with a Two-stage PCR method [21], employing pQE 30-ScTPase as the template. In the first step, two separate primer extension reactions were performed using the forward oligonucleotide primer and its reverse complementary respectively. The following mutation primers were used where the mismatched parts are underlined: D379N, 5′-gcattcattgacaacccgcagatgc-3′; H403A, 5′-ACCGCTCGGCC-ATTGAGATCAGG-3′; R507A, 5′-GCATCCAGATCGCGGCG-TTCGACCCGG-3′; K512A, 5′-CGACCCGGCGGCGGGCATCCCGAACG-3′. In the second step, the two reactions were combined and amplification was continued (see Supplementary Table 1 for detailed PCR conditions). After digestion of the template DNA with DpnI, the amplified plasmid vectors were transformed into electro-competent cells of E. coli JM109. Plasmid miniprep DNA from positive clones was subjected to dideoxy sequencing of the entire ScTPase gene to verify that the desired mutation had been introduced and no base misincorporations had occurred because of DNA polymerase errors. Expression and purification of ScTPase mutants were carried out as described above.
RESULTS
Gene cloning, and heterologous expression and purification of recombinant ScTPase
The cDNA encoding the full-length ScTPase was isolated, cloned and sequenced. The entire open reading frame consists of 2247 bp, including the codon for the initiator methionine and the stop codon. The recombinant ScTPase is a protein of 748 amino acids (including the tag of 11 extra amino acids at its N-terminus) with a calculated molecular mass of 82803 Da. An alignment of the primary structures (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/397/bj3970491add.htm) shows that ScTPase (GenBank®/GenPept accession no. ABC84380) is 76.7 and 73.0% identical with fungal trehalose phosphorylases from G. frondosa (GenBank®/GenPept accession no. BAA31350) [18] and Pleurotus sajor-caju (GenBank®/GenPept accession no. AAF22230) [22] respectively. Screening of the non-redundant protein sequence database with the amino acid sequence of ScTPase confirmed the proposed membership [10] of the enzyme to family GT-4 glycosyltransferases (results not shown). Interestingly, the C-terminal part of ScTPase is 20.2% identical with trehalose synthase, a 416-amino-acid GT-4 glycosyltransferase from the archaeon Pyrococcus horikoshii (GenBank®/GenPept accession no. BAA30133) [23]. The trehalose synthase appears to be a truncated homologue of ScTPase, lacking entirely the approx. 330-amino-acid-long N-terminal domain of the fungal enzyme.
The specific ScTPase activity of the induced E. coli cell extract was 0.7 unit/mg and can be compared with a value of 0.06 unit/mg found in S. commune [14]. Highly purified recombinant ScTPase shows a specific activity of 6.3 units/mg. It was obtained in a yield of 32% by using an optimized method, where an anion exchange and a hydyroxylapatite chromatographic step were employed sequentially. (A detailed summary of the purification is available in Supplementary Table 2 at http://www.BiochemJ.org/bj/397/bj3970491add.htm.) Stabilizers such as glycerol or α,α-trehalose that were required to prevent inactivation of the natural enzyme [14] were not needed during isolation and storage (at −21 °C) of the recombinant ScTPase.
Structural properties of recombinant ScTPase
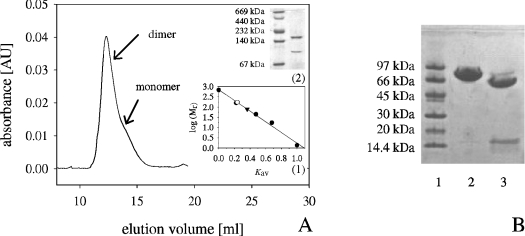
Recombinant ScTPase shows a molecular mass of approx. 80 kDa (Figure 4B), in good agreement with the predicted mass of the full-length protein. The purified enzyme was eluted from a Superdex size-exclusion column as shown in Figure 4(A), where the main protein peak corresponded to a 165 kDa dimer and contained approx. 60% of the total protein and 85% of the total activity. A minor fraction, eluted as a distinct shoulder of the main peak, accounted for the remaining protein and activity, and corresponded to a 81 kDa monomer. The specific activity of the 81 kDa fraction (1.6 units/mg) was only one-fourth that of the major form. Using non-denaturing anionic PAGE, the co-existence of dimeric and monomeric forms in the recombinant enzyme was confirmed (Figure 4A, inset). Analysis by inductively coupled plasma MS showed that recombinant ScTPase contained less than 0.01 g-atom of Mg2+ per protein monomer, in marked contrast with the natural enzyme isolated from S. commune in which each monomer had 1 g-atom equivalent of Mg2+ bound in non-dissociable manner [10].
Figure 4. Structural characterization of recombinant ScTPase by size-exclusion chromatography (A) and spontaneous truncation of the full-length enzyme during storage at 4 °C (B).
(A) The inset (1) shows the molecular mass determination for ScTPase, where the open circle and the closed triangle indicate the dimeric (165 kDa) and monomeric (81 kDa) enzyme forms respectively and closed circles are the molecular mass marker proteins. The inset (2) shows the detection of dimeric and monomeric ScTPase by using non-denaturing anionic PAGE. (B) An SDS/polyacrylamide gel of the purified recombinant ScTPase (2.5 mg/ml) is shown before (lane 2) and after (lane 3) incubation at 4 °C for approx. 340 h in 50 mM potassium phosphate buffer (pH 7.0). Lane 1 contained molecular-mass markers.
Upon storage at 4 °C (50 mM potassium phosphate buffer, pH 7.0), we observed a defined and highly reproducible conversion of the recombinant ScTPase into two fragments of approx. 65 kDa and approx. 16 kDa molecular mass (Figure 4B). After clear-cut separation of the two protein forms by gel filtration, the 65 kDa fragment (61005 Da by LC-ESI-Q-TOF MS) was found to have the same specific activity as the full-length precursor protein (6 units/mg), whereas the 16 kDa protein was completely inactive. The truncated ScTPase was eluted from the size-exclusion column in a single fraction corresponding to a 125 kDa dimer. Interestingly, natural ScTPase was isolated from the fungus as a shortened protein variant of a similar mass of approx. 61 kDa, but was monomeric [14]. Peptide mass ‘fingerprints’ of the tryptic digests of full-length ScTPase and the two fragments derived from it are reported in Table 1. It is not known whether polypeptide chain cleavage in ScTPase is autocatalytic, uncatalysed, or results from a minor contamination with protease not detectable at the protein level. Our attempts to produce in E. coli a truncated version of recombinant ScTPase (1644 bp; residues Asn189 to Asp737) containing or lacking an N-terminal His tag failed due to excessive formation of protein inclusion bodies, even at low growth temperatures of 15 °C. The proteolytic conversion of ScTPase was not further pursued, in particular because it does not affect the enzyme activity.
Table 1. Peptide masses obtained by tryptic digestion of intact recombinant ScTPase and the two fragmentation products derived from it by spontaneous cleavage.
Several peptides of the N-terminal protein part (approximately residues 1–200) were detectable in full-length ScTPase and the approx. 16 kDa fragment, but were missing in the truncated enzyme. #MC, number of missed tryptic cleavage sites; CS, detected charge state; calc. m/z, calculated m/z value for the peptides in the detected charge state; exp. m/z, experimentally acquired m/z value for the peptides in the detected charge state; n.d., not determined.
| LC-ESI-Q-TOF MS | exp. m/z | ||||||
|---|---|---|---|---|---|---|---|
| Position | Peptide sequence | #MC | CS | calc. m/z | Full-length ScTPase | Truncated ScTPase | ∼16 kDa Fraction |
| 23–33 | ASLTNRPTFTK | 0 | 2 | 618.34 | 618.39 | n.d. | n.d. |
| 97–103 | HVLDALR | 0 | 2 | 412.24 | 412.27 | n.d. | 412.26 |
| 113–124 | FLGAGITLALLR | 0 | 2 | 622.89 | 622.93 | n.d. | 622.89 |
| 163–203 | ISSTSGSYVPSGAETPTVYVEASHLGNNLSAGTASKLPIPR | 1 | 4 | 1029.78 | 1029.87 | n.d. | n.d. |
| 189–203 | NNLSAGTASKLPIPR | 2 | 769.94 | n.d. | 770.02 | n.d. | |
| 244–255 | IHLIDDIDEYRK | 1 | 2 | 765.40 | 765.47 | 765.39 | n.d. |
| 513–523 | GIPNVIDSYAR | 0 | 2 | 602.82 | 602.85 | 602.79 | n.d. |
| 731–737 | GGIKVQD | 1 | 1 | 716.39 | 716.46 | 716.39 | n.d. |
Kinetic properties and inhibition of recombinant ScTPase
Kinetic parameters of full-length ScTPase are summarized in Table 2 along with the corresponding parameters of the truncated enzyme isolated from S. commune, which have been reported [10]. Catalytic-centre activities (kcat) as well as binding constant (Ki) and Michaelis constant (Km) for the substrates were very similar for both phosphorylase forms. The results imply that the enzymatic reaction co-ordinate, at the points defined by Figure 2(A), is not significantly altered by the N-terminal truncation of the intact enzyme. Table 2 also shows inhibition constants (Kic) for the proposed transition-state analogue vanadate in combination with glucose (see Figure 2B) or α,α-trehalose. The Kic values were almost identical for the two enzymes.
Table 2. Kinetic parameters and inhibition constants of ScTPase.
Reported values for the recombinant enzyme are from nonlinear fits of initial rates to the appropriate equations for an ordered Bi Bi kinetic mechanism (see Figure 2A) and binding of a competitive inhibitor, as described elsewhere in detail [9,14,15]. Values for the native ScTPase are taken from the literature [10,14]. kcat is the catalytic-centre activity calculated by using a subunit molecular mass of 82.8 and 61 kDa for recombinant and natural ScTPase respectively; Km is the Michaelis constant; Ki is an apparent dissociation constant; and Kic is a competitive inhibitor binding constant. Data were recorded at pH 6.6 and 30 °C. The internal consistency of the kinetic parameters was checked with the Haldane relationship, and the calculated and experimentally determined values of the equilibrium constant Keq [9] are in reasonable agreement.
| Kinetic property | Recombinant ScTPase | Natural ScTPase |
|---|---|---|
| Phosphorolysis | ||
| Kmphosphate (mM) | 0.8±0.1 | 1.4±0.3 |
| Kiphosphate (mM) | 12±3 | 26±4 |
| Km α,α-trehalose (mM) | 53±4 | 71±5 |
| kcat (s−1) | 16.4±0.4 | 13.3±0.6 |
| Kicvanadate (μM) | 0.40±0.11 | 0.75±0.10 |
| Synthesis | ||
| Km α-D-Glc 1-P (mM) | 2.2±0.5 | 1.6±0.2 |
| Ki α-D-Glc 1-P (mM) | 5.9±0.8 | 5.8±0.9 |
| Kmglucose (mM) | 46±8 | 35±2 |
| kcat (s−1) | 14.1±0.6 | 9.5±0.7 |
| Kicvanadate (μM) | 1.3±0.3 | 1.1±0.1 |
| Calculated Keq | 0.482 | 0.154 |
The pseudo-disaccharide validoxylamine A (Figure 3) is a potent inhibitor of ScTPase, acting competitively against α,α-trehalose with a Ki value of 1.7 (±0.2) μM. It binds approx. 350-fold more tightly to the enzyme–phosphate complex than α,α-thio-trehalose [10], a close structural analogue of α,α-trehalose (see Figure 3) that is not phosphorolysed by the enzyme and can probably mimic disaccharide substrate binding in the ground state.
ScTPase, intact or truncated, displays a very weak but significant hydrolytic activity towards α,α-trehalose and α-D-Glc 1-P in the absence of phosphate and glucose respectively. The disaccharide (400 mM) and α-D-Glc 1-P (25 mM) were converted at approx. 0.002% (±0.0003%) of the corresponding phosphorolysis and synthesis rate recorded under otherwise identical conditions in the presence of 50 mM phosphate and 400 mM glucose respectively. The limited availability of purified natural enzyme precluded detection of this low activity in our previous studies of ScTPase [10,14]. The result is interesting because it shows that occupancy of the phosphate site and sugar-binding subsite +1 is not an absolute requirement for glucosyl transfer to occur, even though the minute ‘error’ reaction with water is not a relevant component of the normal catalytic pathway.
Incubation of ScTPase with UDP-Glc (25 mM) in the presence or absence of glucose (400 mM) did not yield UDP within limits of detection by the enzymatic assay (∼0.001% of kcat for synthesis). UDP-Glc, however, acts as competitive inhibitor against phosphate and α-D-Glc 1-P as well as against α,α-trehalose when the phosphate concentration is non-saturating. A Kic of approx. 12 mM has been determined using initial rates measured in phosphorolysis direction at a constant concentration of α,α-trehalose (100 mM) and a varied concentration of phosphate. Phosphorylase inhibition by UDP-Glc, recorded at constant and non-saturating substrate levels of 5 mM phosphate and 100 mM α,α-trehalose, increased only marginally (<1.5-fold) in response to an increase in the glucose concentration from Km (50 mM) to a saturating level (400 mM), indicating that formation of an abortive ternary complex does not occur.
Sequence analysis and site-directed mutagenesis
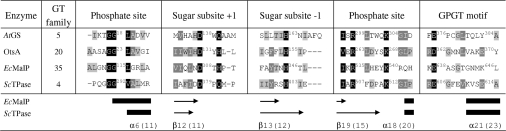
A pairwise alignment of the primary structures of ScTPase and EcMalP (maltodextrin phosphorylase from E. coli), a well-characterized α-retaining glucosyltransferase of family GT-35 [12,24–26], revealed that in spite of the low overall sequence identity of 11.6%, the catalytic residues of EcMalP appear to be highly conserved in ScTPase. Recent X-ray structures of enzyme members of families GT-5 (glycogen synthase) [27,28], GT-20 (trehalose 6-phosphate synthase) [29,30] and GT-72 [AGT (α-glucosyltransferase)] [31] support a distinct pattern of active site conservation among clan IV glycosyltransferases of fold family GT-B. Figure 5 shows a partial multiple sequence alignment in which the known catalytic centre residues were used to identify their likely functional homologues in ScTPase and thus select candidates for site-directed mutagenesis. (The full alignment is found in Supplementary Figure 2 at http://www.BiochemJ.org/bj/397/bj3970491add.htm.) Glu606 and Glu614 of ScTPase comprise the so-called GPGT (glycogen phosphorylase glycosyltransferase) superfamily motif (EXXXXXXXE, where X is any amino acid), originally identified in the sequence work of Wrabl and Grishin [32], whereby Glu606 may be replaced by an aspartate residue (family GT-20 [29]) and Glu614 by a histidine, tyrosine or phenylalanine residue (family GT-5 [27,33]). Furthermore, Glu614 is replaced by the pyridoxal 5′-phosphate-binding lysine in family GT-35 [24]. Results of mutagenesis experiments with different glycosyltransferases [34,35], among them the α-mannosyltransferase AceA of family GT-4 [36], suggest that GPGT motif residues are essential, and the available crystal structures reveal their conserved role in positioning the glycosyl donor for catalysis in subsite −1 [12,27,30]. Arg507 and Lys512 of ScTPase are having a common evolutionary origin in the sequence with key phosphate-binding residues of EcMalP [12,24] as well as of OtsA (trehalose 6-phosphate synthase from E. coli) [29,30]. Their exact function in enzymes of family GT-4 is not well defined, except for the reported complete loss of activity in lysine-into-alanine variant of AceA [36]. The motif Gly291-Gly-Val293 of ScTPase corresponds to similar motifs in EcMalP (Gly114-Gly-Leu116) and OtsA (Gly22-Gly-Leu24), whereby the main chain NH of the second glycine residue interacts with an oxygen of the substrate phosphate in EcMalP and the β-phosphate group of the UDP leaving group in OtsA. Comparison of the known secondary structure compositions of EcMalP and OtsA with that predicted for ScTPase suggests that Asp379 and His403 are having a common evolutionary origin with Asp308 and His348 of EcMalP (Figure 5). Both residues are important for the activity of EcMalP. Asp308 seems to position the substrate for catalysis through strong hydrogen-bonding interactions with the leaving group at sugar-binding subsite +1 ([12] and references therein). His348 is thought to stabilize the glucosyl oxocarbenium ion-like transition at the catalytic subsite −1 [12,37].
Figure 5. Partial multiple alignment of primary structures of ScTPase and retaining glycosyltransferases of fold family GT-B.
The regions surrounding catalytic centre residues in EcMalP [12] (GenBank®/GenPept accession no. AAC76442), OtsA [30] (GenBank®/GenPept accession no. BAA15717) and AtGS [27] (glycogen synthase from Agrobacterium tumefaciens; GenBank®/GenPept accession no. AAD03474) are shown and compared with the counterpart regions in ScTPase (GenBank®/GenPept accession no. ABC84380). Also displayed are the secondary structural elements (black bars for α-helices and arrows for β-sheets) for EcMalP, whereby the numbering corresponds to that of Watson et al. [24], aligned to the predicted secondary structural elements for ScTPase, numbers indicated in parentheses. Secondary structure prediction was made with the public PSIPRED server (http://bioinf.cs.ucl.ac.uk/psipred/) using mGenTHREADER method.
By site-directed mutagenesis, ScTPase residues Asp379, His403, Arg507 and Lys512 were individually replaced with an alanine residue. Because the D379A mutant could not be produced as a soluble recombinant protein in E. coli, Asp379 was alternatively replaced by an asparagine residue. Each ScTPase mutant was prepared by using procedures exactly as described for the wild-type. An SDS/polyacrylamide gel displaying the purified point mutants along with the wild-type is shown in Figure 6. Initial-rate measurements performed in the direction of phosphorolysis of α,α-trehalose under conditions of apparent substrate saturation of the wild-type (400 mM α,α-trehalose; 50 mM phosphate) revealed that each site-directed replacement had introduced a large, between 103.2 and 105.6-fold, catalytic defect in the corresponding ScTPase variant. The R507A mutant was also assayed for its ability to catalyse the synthesis of α,α-trehalose (50 mM α-D-Glc 1-P; 400 mM glucose), and the activity loss in the mutant in comparison with the wild-type was comparable in each direction of the reaction. The hydrolase activity of each mutant was determined in the absence of phosphate using 400 mM α,α-trehalose as the substrate, and in marked contrast with the phosphorylase activity, it was similar or even increased in the mutants, compared with the wild-type activity. The ratio of apparent catalytic rates of phosphorolysis and hydrolysis of α,α-trehalose thus decreased by a factor of up to 106 from a value of 4.6×104 in the wild-type to 7.8×10−2 in R507A. Three ScTPase mutants (D379N, R507A and K512A) were ‘better’ hydrolases than phosphorylases under the conditions used. The results are summarized in Table 3.
Figure 6. Analysis by SDS/PAGE of purified recombinant ScTPase and site-directed mutants thereof.
Lanes 1 and 7, low-molecular-mass standard; lane 2, recombinant ScTPase; lane 3, D379N; lane 4, H403A; lane 5, R507A; lane 6, K512A.
Table 3. Kinetic comparison of recombinant ScTPase and site-directed mutants thereof.
kcatP and kcatH are apparent catalytic-centre activities for, respectively, phosphorolysis (P) and hydrolysis (H) of 400 mM α,α-trehalose in the presence (P) and absence (H) of 50 mM phosphate recorded at pH 6.6 and 30 °C. They were calculated with a value of 82.8 kDa for the molecular mass of the enzyme subunit.
| Parameter | Recombinant ScTPase | D379N | H403A | R507A | K512A |
|---|---|---|---|---|---|
| kcatP (10−4 s−1) | 160000 | 0.88 | 110 | 0.39 | 1.4 |
| kcatH for α,α-trehalose (10−4 s−1) | 3.5 | 5.6 | 5.2 | 5.0 | 15 |
| Ratio (P/H) | 46000 | 0.16 | 21 | 0.08 | 0.09 |
DISCUSSION
Structure–function relationships for ScTPase and related family GT-4 glycosyltransferases
Family GT-4 is the currently second largest glycosyltransferase family [1,3]. Its members are from all three domains of life and represent a wide range of donor and acceptor substrate specificities. However, little is known about structure–function relationships and the mechanism of family GT-4 enzymes. The reaction catalysed by trehalose phosphorylase is unique among those reported for family GT-4 glycosyltransferases and appears to be particularly conducive to mechanistic studies. It is freely reversible in vitro, and reactants and products have relatively simple chemical structures for which site-specifically modified analogues are readily accessible [10,25,32]. Point mutants of recombinant ScTPase can now also be generated as particular probes of mechanism.
Lacking a three-dimensional structure of a GT-4 glycosyltransferase, identification of candidate residues of ScTPase for site-directed replacement has relied on comparison with template structures, using retaining glycosyltransferases of fold family GT-B. Results summarized in Figure 5 suggest close similarity in active-site topology between ScTPase and enzymes of families GT-5, GT-20 and GT-35. Analysis of kinetic consequences in ScTPase mutants revealed that residues pinpointed by a clear conservation pattern (Asp379, His403, Arg507 and Lys512 in addition to GPGT motif residues) are clearly important for the activity of ScTPase.
The results also advance, on a more global level than Figure 5, the structure–function relationships of ScTPase. The molecular sizes of family GT-4 proteins vary in the approximate range of approx. 300 to approx. 1100 amino acids; hence ScTPase with its 737 residues is among the large members. The highly purified enzyme is readily converted into a 65 kDa short-form variant, which probably explains why only the truncated ScTPase could be isolated from S. commune [14]. Cleavage of the full-length phosphorylase appears to occur predominantly between residues Asn189 and Gly188 (Table 1) and does not impact significantly on activity and stability. The results do not support an immediate correlation between N-terminal truncation and protein monomerization and Mg2+ binding, which are properties of the natural approx. 61 kDa enzyme [10,14] that are lacking in the recombinant counterpart. However, the kinetic parameters described imply that the crucial elements of catalytic function of ScTPase reside completely in the C-terminal protein part.
Family GT-4 comprises glycosyltransferases with different specificities for the leaving group of their respective glycosyl donor substrate. It is interesting therefore that ScTPase binds UDP-Glc, although it is unable to utilize the nucleotide sugar as an alternative substrate for the enzymatic reaction with glucose or water respectively. Binding studies reveal that sugar-binding subsite −1 and the phosphate site are both occupied in the enzyme–UDP–Glc complex. The binding affinity of UDP-Glc (Kic≈12 mM) is comparable with that of α-D-Glc 1-P (Ki=6 mM; Table 2) but three to four orders of magnitude smaller than the typical affinities of nucleotide sugar-dependent glycosyltransferases for their donor substrates [34,38]. The absence of detectable transferase activity with UDP-Glc is most simply explained by its non-productive binding to ScTPase. Another possibility suggested by the results is, however, that bound UDP-Glc does not promote glucose binding at subsite +1, perhaps because steric conflicts occur in a ternary complex or the required binding recognition of glucose is not induced. One of closest enzyme homologues of ScTPase that has been characterized is the UDP-Glc-dependent trehalose synthase from the archaeon P. horikoshii, which is not active as a phosphorylase [23]. We therefore performed a structure-based sequence comparison of ScTPase and P. horikoshii trehalose synthase using EcMalP and OtsA as templates of phospho-sugar and nucleotide-sugar-dependent glycosyltransferases respectively. The analysis did not pinpoint candidate residues determining the ‘phosphorylase to synthase’ switch in α,α-trehalose-metabolizing enzymes of family GT-4.
Implications for the catalytic mechanism
Figure 5 suggests that ScTPase probably shares salient features of the catalytic mechanism with other retaining glycosyltransferases of fold family GT-B. Residues contributed to the phosphate site (Arg507 and Lys512) and sugar subsite +1 (Asp379) of ScTPase are of particular interest, as they seem to determine the exquisite selectivity of the wild-type enzyme for the phosphorolysis compared with the hydrolysis of α,α-trehalose. Individual substitutions of Asp379, Arg507 and Lys512 nearly reversed the original selectivity of the enzyme such that hydrolysis of the glucosyl donor substrate was now preferred, up to 13-fold, in the respective site-directed mutant. Considering Figures 2(B) and 5, the three conserved residues are also relevant with respect to the transition-state-like inhibition of ScTPase by vanadate in combination with glucose [15], and a systematic dissection of this two-component inhibitor could now be possible by using mutagenesis. If ScTPase utilized the catalytic mechanism shown in Figure 1, Asp379, Arg507 and Lys512 might also be crucial to promote the initial attack of phosphate on the glycosidic bond of α,α-trehalose.
Validoxylamine A is the most powerful inhibitor of ScTPase currently known. We see its particular prowess as a mechanistic probe for the enzyme from the fact that (i) it provides partial analogy to the proposed glucosyl oxocarbenium ion-like transition state of the reaction, particularly with regard to the build-up of positive charge at the substituted anomeric carbon (for a discussion, see [14]), and (ii) upon binding to the enzyme–phosphate complex, it mimics the catalytically critical ternary complex, which the previously examined iminosugars 1-deoxynojirimycin and isofagomine (Figure 3; [15]) could not. It was shown in earlier studies of α-glucosyl hydrolases that the inhibitory properties of N-linked pseudo-disaccharides are dependent on the whole unit and not on the individual constituent parts [17,39,40]. The very high affinity of validoxylamine A for binding to pig kidney trehalase (Ki=0.52 nM) derives from the synergistic interaction of its two cyclitol units with enzyme subsites −1 and +1 [17,39]. Strong inhibition of amylolytic enzymes by the pseudo-tetrasaccharide acarbose requires that the acarviosine moiety, as shown in Figure 3, is intact [40]. There is overlap in binding recognition of subsites −1 and +1 in ScTPase [15,16] and therefore the orientation of validoxylamine A in the active centre is not unambiguously defined. The conformation of the hydroxymethylconduritol unit of the inhibitor is constrained by the double bond between C-5 and C-7 (which corresponds to the endocyclic O-5 in glucose) to a 2H3 half-chair in which atoms C-4, C-5, C-7 and C-1 are co-planar (Figure 3). Atoms C-2 and C-3 are above and below the plane respectively. This conformation is clearly not an ideal mimic of the glucosyl oxocarbenium ion-like transition state which is expected to be constrained to a 4H3 half-chair or a sofa conformation because of the partial double bond between C-1 and O-5 that leads to co-planarity of the atoms C-5, O-5, C-1 and C-2. In the endocyclic enitol D-glucal (Figure 3), the atoms O-5, C-1, C-2 and C-3 are co-planar, and this compound binds well to subsite +1 (Ki=0.32 mM [10]), in spite of the fact that the sp2-hybridized C-1 would be a clear feature of a good transition state mimic, which should bind selectively to subsite −1. Therefore reversal of the anticipated binding mode of the pseudo-disaccharide in which the hydroxymethylconduritol group would be positioned in the catalytic subsite −1 cannot be discounted. Nevertheless, despite the lack of good complementarity with the proposed transition state structure and the perhaps incomplete subsite selectivity of validoxylamine A, the observed high affinity is interesting because it suggests that the inhibitor may exploit electrostatic interactions between its (positively charged) amino group and enzyme-bound phosphate for binding. In the SNi-like mechanism, the electrostatic ‘front-side’ stabilization of the oxocarbenium ion-like transition state by the attacking anionic nucleophile is thought to be a crucial catalytic factor [4].
Online Data
Acknowledgments
Financial support from the Austrian Science Funds (FWF; P15118-B09; W901 DK ‘Molecular Enzymology’) is gratefully acknowledged. We thank Professor Friedrich Altmann and Dr Daniel Kolarich (Department of Chemistry, University of Natural Resources and Applied Life Sciences, Vienna, Austria) for MS analysis, and Professor Dr Naoki Asano for the gift of validoxylamine A.
References
- 1.Davies G. J., Gloster T. M., Henrissat B. Recent structural insights into the expanding world of carbohydrate-active enzymes. Curr. Opin. Struct. Biol. 2005;15:637–645. doi: 10.1016/j.sbi.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Breton C., Snajdrova L., Jeanneau C., Koca J., Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16:29–37. doi: 10.1093/glycob/cwj016. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho P. M., Deleury E., Davies G. J., Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 2003;328:307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 4.Davies G. J., Sinnott M. L., Withers S. G. Glycosyl transfer. In: Sinnott M. L., editor. Comprehensive Biological Catalysis. San Diego, CA: Academic Press; 1998. pp. 119–208. [Google Scholar]
- 5.Lairson L. L., Withers S. G. Mechanistic analogies amongst carbohydrate modifying enzymes. Chem. Commun. 2004;20:2243–2248. doi: 10.1039/b406490a. [DOI] [PubMed] [Google Scholar]
- 6.Zechel D. L., Withers S. G. Glycosidase mechanisms: anatomy of a finely tuned catalyst. Acc. Chem. Res. 2000;33:11–18. doi: 10.1021/ar970172+. [DOI] [PubMed] [Google Scholar]
- 7.Vasella A., Davies G. J., Bohm M. Glycosidase mechanisms. Curr. Opin. Chem. Biol. 2002;6:619–629. doi: 10.1016/s1367-5931(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 8.Mosi R., Withers S. G. Synthesis and kinetic evaluation of 4-deoxymaltopentaose and 4-deoxymaltohexaose as inhibitors of muscle and potato α-glucan phosphorylases. Biochem. J. 1999;338:251–256. [PMC free article] [PubMed] [Google Scholar]
- 9.Ly H. D., Lougheed B., Wakarchuk W. W., Withers S. G. Mechanistic studies of a retaining α-galactosyltransferase from Neisseria meningitidis. Biochemistry. 2002;41:5075–5085. doi: 10.1021/bi012031s. [DOI] [PubMed] [Google Scholar]
- 10.Eis C., Watkins M., Prohaska T., Nidetzky B. Fungal trehalose phosphorylase: kinetic mechanism, pH-dependence of the reaction and some structural properties of the enzyme from Schizophyllum commune. Biochem. J. 2001;356:757–767. doi: 10.1042/0264-6021:3560757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persson K., Ly H. D., Dieckelmann M., Wakarchuk W. W., Withers S. G., Strynadka N. C. Crystal structure of the retaining galactosyltransferase LgtC from Neisseria meningitidis in complex with donor and acceptor sugar analogs. Nat. Struct. Biol. 2001;8:166–175. doi: 10.1038/84168. [DOI] [PubMed] [Google Scholar]
- 12.Watson K. A., McCleverty C., Geremia S., Cottaz S., Driguez H., Johnson L. N. Phosphorylase recognition and phosphorolysis of its oligosaccharide substrate: answers to a long outstanding question. EMBO J. 1999;18:4619–4632. doi: 10.1093/emboj/18.17.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinnott M. L., Jencks W. P. Solvolysis of D-glucopyranosyl derivatives in mixtures of ethanol and 2,2,2-trifluoroethanol. J. Am. Chem. Soc. 1980;102:2026–2032. [Google Scholar]
- 14.Eis C., Nidetzky B. Characterization of trehalose phosphorylase from Schizophyllum commune. Biochem. J. 1999;341:385–393. [PMC free article] [PubMed] [Google Scholar]
- 15.Nidetzky B., Eis C. α-Retaining glucosyl transfer catalysed by trehalose phosphorylase from Schizophyllum commune: mechanistic evidence obtained from steady-state kinetic studies with substrate analogues and inhibitors. Biochem. J. 2001;360:727–736. doi: 10.1042/0264-6021:3600727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eis C., Nidetzky B. Substrate-binding recognition and specificity of trehalose phosphorylase from Schizophyllum commune examined in steady-state kinetic studies with deoxy and deoxyfluoro substrate analogues and inhibitors. Biochem. J. 2002;363:335–340. doi: 10.1042/0264-6021:3630335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asano N. Glycosidase inhibitors: update and perspectives on practical use. Glycobiology. 2003;13:93–104. doi: 10.1093/glycob/cwg090. [DOI] [PubMed] [Google Scholar]
- 18.Saito K., Yamazaki H., Ohnishi Y., Fujimoto S., Takahashi E., Horinouchi S. Production of trehalose synthase from a basidiomycete, Grifola frondosa, in Escherichia coli. Appl. Microbiol. Biotechnol. 1998;50:193–198. doi: 10.1007/s002530051276. [DOI] [PubMed] [Google Scholar]
- 19.Fujii H., Miwa S. Pyruvate kinase. In: Bergmeyer H. U., editor. Methods of Enzymatic Analysis. Weinheim: Verlag Chemie; 1983. pp. 496–507. [Google Scholar]
- 20.Kolarich D., Altmann F. N-Glycan analysis by matrix-assisted laser desorption/ionization mass spectrometry of electrophoretically separated nonmammalian proteins: application to peanut allergen Ara h 1 and olive pollen allergen Ole e 1. Anal. Biochem. 2000;285:64–75. doi: 10.1006/abio.2000.4737. [DOI] [PubMed] [Google Scholar]
- 21.Wang W., Malcolm B. A. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. BioTechniques. 1999;26:680–682. doi: 10.2144/99264st03. [DOI] [PubMed] [Google Scholar]
- 22.Han S. E., Kwon H. B., Lee S. B., Yi B. Y., Murayama I., Kitamoto Y., Byun M. O. Cloning and characterization of a gene encoding trehalose phosphorylase (TP) from Pleurotus sajor-caju. Protein Expr. Purif. 2003;30:194–202. doi: 10.1016/s1046-5928(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 23.Ryu S. I., Park C. S., Cha J., Woo E. J., Lee S. B. A novel trehalose-synthesizing glycosyltransferase from Pyrococcus horikoshii: molecular cloning and characterization. Biochem. Biophys. Res. Commun. 2005;329:429–436. doi: 10.1016/j.bbrc.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 24.Watson K. A., Schinzel R., Palm D., Johnson L. N. The crystal structure of Escherichia coli maltodextrin phosphorylase provides an explanation for the activity without control in this basic archetype of a phosphorylase. EMBO J. 1997;16:1–14. doi: 10.1093/emboj/16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Reilly M., Watson K. A., Johnson L. N. The crystal structure of the Escherichia coli maltodextrin phosphorylase–acarbose complex. Biochemistry. 1999;38:5337–5345. doi: 10.1021/bi9828573. [DOI] [PubMed] [Google Scholar]
- 26.Geremia S., Campagnolo M., Schinzel R., Johnson L. N. Enzymatic catalysis in crystals of Escherichia coli maltodextrin phosphorylase. J. Mol. Biol. 2002;322:413–423. doi: 10.1016/s0022-2836(02)00771-4. [DOI] [PubMed] [Google Scholar]
- 27.Buschiazzo A., Ugalde J. E., Guerin M. E., Shepard W., Ugalde R. A., Alzari P. M. Crystal structure of glycogen synthase: homologous enzymes catalyze glycogen synthesis and degradation. EMBO J. 2004;23:3196–3205. doi: 10.1038/sj.emboj.7600324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horcajada C., Guinovart J. J., Fita I., Ferrer J. C. Crystal structure of an archaeal glycogen synthase: insights into oligomerization and substrate binding of eukaryotic glycogen synthases. J. Biol. Chem. 2006;281:2923–2931. doi: 10.1074/jbc.M507394200. [DOI] [PubMed] [Google Scholar]
- 29.Gibson R. P., Turkenburg J. P., Charnock S. J., Lloyd R., Davies G. J. Insights into trehalose synthesis provided by the structure of the retaining glucosyltransferase OtsA. Chem. Biol. 2002;9:1337–1346. doi: 10.1016/s1074-5521(02)00292-2. [DOI] [PubMed] [Google Scholar]
- 30.Gibson R. P., Tarling C. A., Roberts S., Withers S. G., Davies G. J. The donor subsite of trehalose-6-phosphate synthase: binary complexes with UDP-glucose and UDP-2-deoxy-2-fluoro-glucose at 2 A resolution. J. Biol. Chem. 2004;279:1950–1955. doi: 10.1074/jbc.M307643200. [DOI] [PubMed] [Google Scholar]
- 31.Lariviere L., Sommer N., Morera S. Structural evidence of a passive base-flipping mechanism for AGT, an unusual GT-B glycosyltransferase. J. Mol. Biol. 2005;352:139–150. doi: 10.1016/j.jmb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Wrabl J. O., Grishin N. V. Homology between O-linked GlcNAc transferases and proteins of the glycogen phosphorylase superfamily. J. Mol. Biol. 2001;314:365–374. doi: 10.1006/jmbi.2001.5151. [DOI] [PubMed] [Google Scholar]
- 33.Yep A., Ballicora M. A., Preiss J. The active site of the Escherichia coli glycogen synthase is similar to the active site of retaining GT-B glycosyltransferases. Biochem. Biophys. Res. Commun. 2004;316:960–966. doi: 10.1016/j.bbrc.2004.02.136. [DOI] [PubMed] [Google Scholar]
- 34.Yep A., Ballicora M. A., Sivak M. N., Preiss J. Identification and characterization of a critical region in the glycogen synthase from Escherichia coli. J. Biol. Chem. 2004;279:8359–8367. doi: 10.1074/jbc.M312686200. [DOI] [PubMed] [Google Scholar]
- 35.Schinzel R., Palm D. Escherichia coli maltodextrin phosphorylase: contribution of active site residues glutamate-637 and tyrosine-538 to the phosphorolytic cleavage of α-glucans. Biochemistry. 1990;29:9956–9962. doi: 10.1021/bi00494a028. [DOI] [PubMed] [Google Scholar]
- 36.Abdian P. L., Lellouch A. C., Gautier C., Ielpi L., Geremia R. A. Identification of essential amino acids in the bacterial α-mannosyltransferase AceA. J. Biol. Chem. 2000;275:40568–40575. doi: 10.1074/jbc.M007496200. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz A., Pierfederici F. M., Nidetzky B. Catalytic mechanism of α-retaining glucosyl transfer by Corynebacterium callunae starch phosphorylase: the role of histidine-334 examined through kinetic characterization of site-directed mutants. Biochem. J. 2005;387:437–445. doi: 10.1042/BJ20041593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flint J., Taylor E., Yang M., Bolam D. N., Tailford L. E., Martinez-Fleites C., Dodson E. J., Davis B. G., Gilbert H. J., Davies G. J. Structural dissection and high-throughput screening of mannosylglycerate synthase. Nat. Struct. Mol. Biol. 2005;12:608–614. doi: 10.1038/nsmb950. [DOI] [PubMed] [Google Scholar]
- 39.Asano N., Kato A., Matsui K. Two subsites on the active center of pig kidney trehalase. Eur. J. Biochem. 1996;240:692–698. doi: 10.1111/j.1432-1033.1996.0692h.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim M.-J., Lee S.-B., Lee H.-S., Lee S.-Y., Baek J.-S., Kim D., Moon T.-W., Robyt J. F., Park K.-H. Comparative study of the inhibition of α-glucosidase, α-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine-glucose. Arch. Biochem. Biophys. 1999;371:277–283. doi: 10.1006/abbi.1999.1423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.