Abstract
The Candida albicans CDR1 and CDR2 genes code for highly homologous ATP-binding cassette (ABC) transporters which are overexpressed in azole-resistant clinical isolates and which confer resistance to multiple drugs by actively transporting their substrates out of the cells. These transporters are formed by two homologous halves, each with an intracellular domain containing an ATP-binding site followed by a membrane-associated domain. We have expressed Cdr1p and Cdr2p in Saccharomyces cerevisiae to investigate their functions. The two proteins were properly expressed and functional, as determined by Western blotting, drug susceptibility assays, and rhodamine efflux. Using total membrane proteins from these transformants, we showed that Cdr1p and Cdr2p bind to the photoreactive analogue of rhodamine 123, [125I]iodoaryl azido-rhodamine 123 (IAARh123). IAARh123 photoaffinity labeling of membranes prepared from cells expressing either the N half or the C half of Cdr2p, or both, demonstrated that both halves contribute to rhodamine binding and can bind to rhodamine independently. Interestingly, Cdr1p was found to confer hypersusceptibility to FK520, an immunosuppressant and antifungal agent, whereas Cdr2p conferred resistance to this compound, uncovering a major functional difference between the two transporters. Furthermore, when administered in combination with azoles, FK520 sensitized cells expressing CDR1 but not those expressing CDR2. Finally, we showed that Cdr2p confers hypersusceptibility to hydrogen peroxide and resistance to diamide, while Cdr1p has no effect against these oxidative agents. Taken together, our results demonstrate that, despite a high level of structural conservation, Cdr1p and Cdr2p exhibit major functional differences, suggesting distinct biological functions.
Multidrug resistance (MDR) or pleiotropic drug resistance (PDR) is characterized by cellular cross-resistance to a broad spectrum of structurally and functionally unrelated cytotoxic compounds. It operates in a wide variety of cell types and involves the overexpression of membrane-associated transporters functioning as drug efflux pumps. Many of these transporters belong to the ATP-binding cassette (ABC) superfamily and contain highly conserved consensus sequences for ATP binding and hydrolysis (34). Well-characterized ABC transporters involved in MDR in mammalian cells include P glycoprotein type 1 (P-gp1) and the MDR-associated protein type 1 (MRP1), whose overexpression causes resistance to several anticancer drugs (2, 35). In the yeast Saccharomyces cerevisiae, overexpression of the ABC transporters Pdr5p and Snq2p has been shown to confer resistance to several different compounds with antifungal activities (6). Pdr5p and Snq2p are homologous proteins formed by two similar halves, each with an N-terminal hydrophilic domain that contains the ABC motif followed by a C-terminal hydrophobic domain with six predicted transmembrane (TM) segments, a structure characteristic of the PDR subfamily of ABC transporters ([ABC-TM]2) (14, 67). Both proteins have nucleotide triphosphatase activities and can transport steroids in vivo, suggesting that steroids or related membrane lipids represent endogenous substrates for these transporters (45). Cells lacking the PDR5 gene but not the SNQ2 gene display an increase in phosphatidylethanolamine (PE) accumulation, and it has been proposed that Pdr5p functions as a PE translocator (15).
The yeast Candida albicans is an opportunistic human pathogen that causes severe infections in immunocompromised individuals (20). Azole derivatives such as fluconazole (FLC) are commonly used to treat Candida infections. However, resistant strains often emerge during long-term or prophylactic treatment (74). Two major mechanisms of FLC resistance have been identified so far in these strains: (i) alterations in the drug target (14-α-sterol demethylase, the product of the ERG11 gene), which results in an increased level of production of the enzyme or in its reduced binding affinity for FLC, and (ii) a reduced level of intracellular FLC accumulation, which correlates with the overexpression of the CDR1 and CDR2 (Candida drug resistance) genes encoding transporters of the ABC family and of the CaMDR1 gene coding for a major facilitator (for a review, see reference 74). These different mechanisms of azole resistance can coexist in different subpopulations of C. albicans cells within a given patient as well as within the same cell, contributing to the stepwise development of azole resistance in the clinical setting (1, 26, 43, 73).
CDR1 and CDR2 were cloned by functional complementation of an S. cerevisiae pdr5 mutant and were found to code for ABC transporters displaying extensive sequence homology with each other (84% identity, 92% similarity) and with S. cerevisiae Pdr5p and Snq2p (52, 60). Since clinical isolates overexpressing CDR1 and CDR2 display energy-dependent reductions in their levels of intracellular FLC accumulation compared to those of their azole-susceptible counterparts, it was suggested that Cdr1p and Cdr2p mediate azole resistance by causing active extrusion of the drug out of the cells (60, 61). Heterologous expression systems in S. cerevisiae have recently been used to confirm this hypothesis for Cdr1p and to demonstrate that Cdr1p and Cdr2p function as general phospholipid translocators and possess nucleotide triphosphatase activities (49, 66).
In the present study, we expressed the CDR1 and CDR2 genes in drug-hypersusceptible S. cerevisiae strain TY310 (68) and generated polyclonal antibodies against the Cdr1p and Cdr2p transporters. Using these tools, we show that Cdr1p and Cdr2p bind to a photoreactive analogue of rhodamine (Rh) 123, [125I]iodoaryl azido-rhodamine 123 (IAARh123) and that both halves of Cdr2p participate in IAARh123 binding. We also present experimental evidence demonstrating that, despite a high level of structural conservation, Cdr1p and Cdr2p exhibit major functional differences and probably possess distinct biological functions.
MATERIALS AND METHODS
Strain and culture conditions.
S. cerevisiae strain TY310 (MATα pdr1 pdr3::URA3 pdr5Δ::TRP1 ura3-52 ade2-101 trp-81 lys2-801 his3-Δ200 leu2::PET56) was used throughout this study and has been described elsewhere (68). Cells were grown in yeast peptone dextrose (YPD) medium or in synthetic dextrose (SD) medium lacking leucine (SD −leu) or lacking leucine and histidine (SD −leu −his) for plasmid selection (63). Cell transformation was performed by the lithium acetate procedure (36). Isolated clones were selected and used throughout the study. C. albicans clinical strains 5457 and 5674 were obtained from the Laboratoire de Santé Publique du Québec and will be described elsewhere (S. Saidane, S. Weber, X. De Deken, G. St-Germain, T. Parkinson, C. A. Hitchcock, and M. Raymond, unpublished data). Cultures were routinely grown at 30°C.
Plasmid construction.
A 4.5-kb DNA fragment comprising the entire CDR1 gene (positions −10 to +4506 with respect to the A of the initiation codon set at +1 [52]) was amplified by PCR with C. albicans 1006 genomic DNA as the template (27), high-fidelity Pfu DNA polymerase (Stratagene), and oligonucleotides 5′-GGACTAGTGAAAAAAATTATGTCAGATTCTAAG (forward) and 5′-GGACTAGTTTATTTCTTATTTTTTTTCTCTCTG (reverse), into both of which an SpeI site (underlined) was incorporated. A 4.6-kb fragment corresponding to positions −12 to +4668 in CDR2 (60) was amplified by PCR with C. albicans CAI4 genomic DNA as the template (25), Pfu DNA polymerase, and the oligonucleotides 5′-GGACTAGTCAATAAAAACATATGAGTACTGC (forward) and 5′-GGACTAGTCTACTACAACAACCAATACAGATC (reverse), into both of which an SpeI site (underlined) was incorporated. The CDR1 and CDR2 PCR fragments were gel purified and digested with SpeI for cloning into the SpeI-digested LEU2-based vector p425GPD (kindly provided by Martin Funk, Institut fur Molekularbiologie und Tumorforschung, Marburg, Germany) (47), yielding plasmids p425GPD-CDR1 and p425GPD-CDR2L, respectively (see below for an explanation of the plasmid nomenclature). Sequencing of the inserts was performed with a CEQ 2000 automated sequencer (Beckman Coulter, Fullerton, Calif.) and a series of internal primers. Finally, the insert of p425GPD-CDR1 was released by digestion with SpeI, gel purified, and cloned into the HIS3-based vector p423GPD at the SpeI site (47) for coexpression experiments.
The two CTG codons in CDR2 (positions +61 and +1894 with respect to the initiation codon) were mutated to TCT by the QuikChange PCR-based site-directed mutagenesis technique (Stratagene). The 0.8-kb NdeI and 2.8-kb PstI fragments (overlapping the first and second CTG codons, respectively) were isolated from p425GPD-CDR2L and cloned into pGEM-5Zf (Promega), yielding plasmids pGEM/NdeI-0.8 and pGEM/PstI-2.8, respectively. These two plasmids were submitted to separate PCRs with Pfu Turbo DNA polymerase (Stratagene) and a mutagenic pair of oligonucleotides, 5′-GCCATGGGTGGATGCATCTGACAATTCATCAGTTC and 5′-GAACTGATGAATTGTCAGATGCATCCACCCATGGC or 5′-GGTTAATGTGTGCATCTTGCACTTTGGTAATGTCCC and 5′-GGGACATTACCAAAGTGCAAGATGCACACATTAACC, which incorporate the mutations 61CTG to TCT and 1894CTG to TCT (underlined) in pGEM/NdeI-0.8 and pGEM/PstI-2.8, respectively. Sequencing of the resulting inserts confirmed the codon changes and the absence of additional mutations. The mutated 2.8-kb PstI fragment was excised from the pGEM backbone and used to replace the corresponding fragment in p425GPD-CDR2L cut with PstI, generating plasmid p425GPD-CDR2L632S. The mutated 0.8-kb NdeI fragment was then used to replace the corresponding fragment in p425GPD-CDR2L632S cut with NdeI, yielding plasmid p425GPD-CDR2L21S/L632S, abbreviated p425GPD-CDR2.
The following cloning strategy was used to express Cdr2p as two separate halves. An SpeI restriction site was inserted in the linker region of CDR2 (between nucleotide positions +2565 and +2566), along with proper stop and start codons, by PCR with Pfu polymerase, p425GPD-CDR2L as the template, and primer pair 5′-CGGTAGGTATTGATTGTAATTC (forward) and 5′-GACTAGTCTTATTCACGGTTTTCTGGG (reverse) or primer pair5′-GACTAGTCATGATATTTTTCTGGAGAG (forward) and 5′-CAGGTTGTCTAACTCCTTCC (reverse) to amplify the N- and C-terminal halves, respectively (the SpeI sites are underlined, and the stop and start codons are shown in boldface). The resulting PCR products were gel purified, digested with SpeI, and cloned into p425GPD and p423GPD cut with SpeI, yielding plasmids p425GPD-CDR2L(1-855)and p423GPD-CDR2(856-1499), respectively. The inserts were entirely sequenced, ruling out the presence of mutations introduced by PCR. These plasmids were transformed in different combinations in TY310 cells by using SD −leu −his selective medium.
Preparation of antisera.
The generic anti-Cdrp polyclonal antibody was raised against a multiple-antigen peptide (P13-MAP) consisting of a 13-amino-acid (aa) synthetic peptide (DSSFQRSIGYVQQ) linked to a polylysine core (Sheldon Biotechnology Center, McGill University, Montreal, Quebec, Canada). To generate the anti-Cdr2p antibody, a CDR2 PCR fragment overlapping nucleotide positions +9 to +302 of the CDR2 gene and cloned blunt into the SmaI site of pUC19 was excised from this plasmid by an EcoRI-SalI double digestion, blunt ended with T4 DNA polymerase, and cloned into the SmaI site of plasmid pGEX-4T-2 (Pharmacia), yielding plasmid pGEX-CDR2. The resulting Gst-Cdr2p fusion protein contains 97 aa from the NH2 terminus of Cdr2p (amino acid positions 4 to 101). Escherichia coli DH5α cells transformed with pGEX-CDR2 were treated with isopropyl-β-d-thiogalactopyranoside (0.1 mM) for 4 h at 30°C to induce expression of Gst-Cdr2p. The resulting fusion protein was soluble in aqueous solution and was thus purified from a crude bacterial lysate by affinity chromatography on immobilized glutathione (64). The synthetic P13-MAP peptide and the purified Gst-Cdr2p fusion protein were used to raise polyclonal antisera in New Zealand White rabbits by standard immunization protocols (30). One anti-P13mer (RX-B) and two anti-Cdr2p (R2a and R2b) antisera of high titers were obtained and used without any further purification.
Protein preparation and Western blot analysis.
Total membrane proteins were prepared as described previously (54) with the following modifications. Concentrated yeast cells were passed twice through a French press (SLM Instruments Inc., Rochester, N.Y.) at 20,000 lb/in2. After solubilization of the crude membranes in cold TNE buffer (10 mM Tris [pH 7.0], 150 mM NaCl, 1 mM EDTA) followed by a quick spin in a microcentrifuge to remove insoluble material, the membranes were centrifuged in a TLA100.3 ultramicrocentrifuge rotor (Beckman) at 50,000 rpm for 45 min at 4°C. The membrane pellets were resuspended in cold TS buffer (5 mM Tris [pH 7.4], 250 mM sucrose) by using 1-ml syringes and 21G11/2-gauge needles (Becton Dickinson). The membranes were aliquoted and stored at −80°C. All experimental steps for protein preparation were carried out in the presence of the protease inhibitors phenylmethylsulfonyl fluoride (Sigma) at 1 mM and leupeptin, pepstatin, and aprotinin (Roche Diagnostics), each at 5 μg/ml. The protein concentration was determined by the method described by Bradford (9). Proteins from total membrane extracts (25 to 50 μg) were solubilized for 15 min at room temperature in Laemmli buffer and were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) on 7.5% polyacrylamide gels (42). The proteins were transferred to nitrocellulose membranes with a Trans Blot SD Semi-Dry transfer apparatus (Bio-Rad). The anti-Cdr2p (R2a) polyclonal antibody was used at a 1:4,000 dilution, while the generic polyclonal anti-Cdrp (RX-B) antibody was used at a 1:1,000 dilution. Immune complexes were revealed by incubation with goat anti-rabbit immunoglobulin G antibodies coupled to alkaline phosphatase (Bio-Rad) and developed with 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt and nitroblue tetrazolium chroride substrates, as recommended by the manufacturer (Bio-Rad).
Antifungal compounds and susceptibility testing.
Stock solutions of FLC (a gift from Pfizer Canada Inc.) were prepared at a concentration of 5 mg/ml in water. Itraconazole (ITC; Janssen Pharmaceuticals) and ketoconazole (KTC; Medisca, Montreal, Canada) were solubilized in dimethyl sulfoxide (DMSO) at concentrations of 10 and 5 mg/ml, respectively. Rh 6G (Sigma) was dissolved at 20 mM in ethanol. FK520 (a gift from Merck Sharp & Dohme Research Laboratories, Rahway, N.J.) was prepared at a concentration of 10 mg/ml in methanol. Stock solutions of 1 M diamide (Sigma) were prepared in DMSO. Hydrogen peroxide (30%) was purchased from Aldrich Chemical Company Inc. For drug resistance assays in liquid medium, cells were grown overnight on selective plates (SD −leu or SD −leu −his) and diluted in selective liquid medium to an optical density at 600 nm (OD600) of 0.1, followed by a second 1:100 dilution in the same medium. Fifty microliters of this dilution was added to 50 μl of a drug solution concentrated two times or drug-free medium in 96-well microtiter plates. The ranges of drug concentrations tested were 0.1 to 250 μg/ml for FLC, 0.005 to 10 μg/ml for ITC, 0.05 to 50 μg/ml for KTC, and 0.5 to 200 μg/ml for Rh 6G. Growth was measured spectrophotometrically at an OD595 after 2 days of incubation at 30°C in a humid chamber. The relative growth was calculated as the percentage of growth in drug-containing medium relative to the control growth in drug-free medium containing the solvent that was used to dissolve the drug. For the FK520-azole synergy experiments, 10 μg of FK520 per ml (a nontoxic concentration for the p425GPD, p425GPD-CDR1, and p425GPD-CDR2 transformants) was added to FLC or KTC under the conditions described above. The concentrations of azoles selected for use in the experiments whose results are presented in Fig. 5 were 10 μg/ml for FLC and 0.5 μg/ml for KTC, which correspond to the respective MICs of these azoles for control TY310 cells transformed with p425GPD. For spot assays, cells were grown overnight on plates with selective (TY310 transformants) or YPD (C. albicans clinical isolates) medium and suspended in sterile water to an OD600 of 0.1. Three microliters of fivefold serial dilutions of each strain were spotted onto YPD medium plates supplemented with the indicated concentrations of the antifungal compounds or supplemented with the solvent alone. The ranges of concentrations tested were 20 to 80 μg/ml for FK520, 0.2 to 1.2 mM (TY310 transformants) or 1 to 5 mM (C. albicans isolates) for diamide, and 0.5 to 3 mM (TY310 transformants) or 1 to 5 mM (C. albicans isolates) for H2O2. The plates were incubated for 2 days at 30°C.
FIG. 5.
Effects of the combination of azoles with FK520 on the viability of S. cerevisiae TY310 cells expressing CDR1 or CDR2. The TY310 transformants were grown in SD −leu medium in the presence (+) or in the absence (−) of 10 μg of FK520 per ml in combination with 10 μg of FLC per ml (A) or 0.5 μg of KTC per ml (B). CTL, control. Values represent the means ± standard deviations of three independent experiments performed in duplicate.
Rh efflux.
Cells were grown in SD −leu medium and harvested in the early logarithmic phase (OD600 = 0.7 to 1.0). Two OD600 U of cells were collected by centrifugation in 1.5-ml microcentrifuge tubes (4,700 × g for 1 min at room temperature). The cell pellets were washed four times with 1 ml of phosphate-buffered saline (PBS), resuspended in 1 ml HEPES-buffered saline (pH 7.0; without dextrose)-10 mM 2-deoxy-d-glucose (Sigma)-5 μM Rh 6G, and incubated for 3 h at room temperature in the dark. The cells were washed once with 1 ml of PBS, spun again, and resuspended in 1 ml of prewarmed PBS containing 1% glucose (Sigma). After 14 min at 30°C in the dark, the tubes were spun for 1 min at room temperature (for a total incubation period of 15 min) and placed in an ice-water bath (0°C) to stop the efflux activity (a 15-min time point was chosen since it was the optimal condition for discrimination of the different transformants; a 10-min incubation was too short to measure significant Cdr1p activity above that for the control values, whereas longer incubation times were in the plateau phase for Cdr2p). Six aliquots (100 μl each) of sample supernatant and of PBS with 1% glucose (blank) each were placed in a Dynex Microfluor microplate, and Rh fluorescence was measured by spectrofluorometry (LS-50B; Perkin-Elmer). Excitation and emission wavelengths were set at 529 nm (slit 2.5 nm) and 553 nm (slit 2.5 nm), respectively. A 515-nm band-pass filter was applied, the photomultiplier tube voltage was automatically set, and the acquisition time was 0.1 s. To normalize the Rh efflux activity with respect to the number of cells present in each sample, the remaining supernatant was removed, the cells were resuspended in 1 ml of cold PBS, and the OD600 was measured. The efflux activity was calculated as follows: (sample mean fluorescence units − blank mean fluorescence units)/OD600.
Photoaffinity labeling and immunoprecipitation.
Total membrane proteins in TS buffer (50 μg for direct labeling or 300 μg for immunoprecipitation, in a final volume of 20 μl) were incubated with 0.5 to 1.0 μM IAARh123 (50) for 30 min at room temperature under subdued light conditions. The samples were further incubated for 10 min on ice, followed by UV irradiation at 254 nm (1800 UV cross-linker; Stratagene, La Jolla, Calif.) for 10 min on ice. All the photoaffinity labeling and immunoprecipitation steps were performed in the presence of the four protease inhibitors described above in the membrane protein extraction procedure. Samples that were not subjected to immunoprecipitation were solubilized for 15 min at room temperature in Laemmli buffer and separated by SDS-PAGE on a 7.5% polyacrylamide gel (42). For immunoprecipitation, the samples were incubated for 15 min on ice in lysis buffer A (1% SDS, 50 mM Tris [pH 7.4]) and then diluted to 420 μl with lysis buffer B (1.25% Triton X-100, 190 mM NaCl, 50 mM Tris [pH 7.4]). After incubation for 1 h on ice, the polyclonal antibody (anti-Cdrp or anti-Cdr2p) was added at a 1:100 dilution and the tubes were placed on a rotating wheel at 4°C overnight. A protein A-Sepharose CL-4B (Amersham Pharmacia) suspension (100 μl) was added to each sample, followed by another incubation on the rotating wheel at 4°C for 3 h. The beads were washed four times with wash A buffer (0.1% Triton X-100, 0.05% SDS, 150 mM NaCl, 50 mM Tris [pH 7.5], 5 mM EDTA) and two times with detergent-free wash B buffer (150 mM NaCl, 50 mM Tris [pH 7.5], 5 mM EDTA). The immune complexes were solubilized in Laemmli buffer for 20 min at room temperature, followed by a 2-min incubation at 37°C, and were resolved by SDS-PAGE on 7.5% polyacrylamide gels. The gels were fixed, dried, and exposed to Kodak BioMax MS films with two BioMax MS intensifying screens at −80°C.
RESULTS
Expression of CDR1 and CDR2 in S. cerevisiae.
CDR1 and CDR2 expression plasmids were constructed by isolating the coding region of each CDR gene by PCR amplification and cloning them under the control of the strong constitutive glyceraldehyde-3-phosphate dehydrogenase (GPD) promoter in the multicopy vector p425GPD (47). DNA sequencing indicated that there was no difference between the nucleotide sequence of the amplified CDR1 gene and the published sequence of CDR1 (52). The sequence of the amplified CDR2 gene had three nucleotide differences (C946G, G2325C, and C3263A; the PCR-amplified CDR2 sequence is underlined) that caused three amino acid changes (N15K, G475A, and L788M) compared to the published CDR2 sequence (60). However, our sequence was identical to that in the Stanford C. albicans genome sequence database and probably corresponds to the second allele of CDR2. Since C. albicans uses an alternative genetic code, in which the CTG codon is decoded as a serine instead of a leucine, the product of a C. albicans gene containing such codons contains leucine rather than serine residues at the corresponding positions when it is expressed in S. cerevisiae (62). Sequence analysis indicated that CDR1 had no CTG codons, while our cloned allele of CDR2 had two (at amino acid positions 21 and 632) and was designated CDR2L. By using site-directed mutagenesis, the two CTG codons in CDR2L were replaced by TCT codons specifying serines. The resulting plasmids, designated p425GPD-CDR1, p425GPD-CDR2L, and p425GPD-CDR2L21S/L632S (abbreviated p425GPD-CDR2), as well as p425GPD as a control, were transformed into S. cerevisiae strain TY310, which harbors a triple pdr1 pdr3 pdr5 mutation that renders the cells hypersusceptible to many drugs (68). TY310 cells transformed with p425GPD-CDR2 or p425GPD-CDR2L displayed similar drug resistance profiles (data not shown), indicating that the presence of leucines rather than serines at positions 21 and 632 does not significantly alter the activity of Cdr2p. Plasmid p425GPD-CDR2 was used throughout this study except where mentioned otherwise.
To examine the expression of the Cdr1p and Cdr2p proteins in the TY310 transformants, total membranes were prepared and analyzed by Western blotting with two different polyclonal antibodies (Fig. 1). First, we used an anti-Cdrp antibody raised against a 13-aa synthetic peptide whose sequence, located downstream of the C-terminal Walker A motif (Fig. 1A), is perfectly conserved between the two proteins (DSSFQRSIGYVQQ; amino acid positions 930 to 942 in Cdr1p and 928 to 940 in Cdr2p) (52, 60). This antibody detected a protein of high molecular weight in membranes from the CDR1 or the CDR2 transformants but not in membranes from the control cells, indicating that these two proteins correspond to Cdr1p and Cdr2p, respectively (Fig. 1B, left panel). This antibody also detects an endogenous S. cerevisiae protein of high molecular weight which can be used as an internal control for protein loading (Fig. 1B, left panel). This experiment demonstrated that Cdr1p and Cdr2p are properly expressed in the TY310 transformants, with about 1.5- to 2.0-fold more Cdr2p than Cdr1p, but the reason for this difference is unclear (Fig. 1B, left panel). We also used an anti-Cdr2p antibody raised against the NH2-terminal domain of Cdr2p (Fig. 1A), a region where the level of amino acid sequence homology between Cdr1p and Cdr2p is the lowest. This antibody recognized a protein of high molecular weight only in membranes prepared from the CDR2 transformants, confirming that this protein corresponds to Cdr2p and demonstrating the isoform specificity of the antibody (Fig. 1B, right panel). As determined by indirect immunofluorescence, cells expressing CDR1 and CDR2 exhibited predominantly intracellular staining which was not detected in the control transformants (data not shown), indicating that the two proteins are similarly located in internal membranes of the TY310 transformants, probably as a consequence of the high level of expression achieved with the p425GPD vector.
FIG. 1.
Expression of Cdr1p and Cdr2p in S. cerevisiae TY310. (A) Schematic representation of the Cdrp proteins. Shaded boxes represent the nucleotide-binding domains (NBD1 and NBD2), and striped boxes correspond to the predicted TM domains (TM 1 to 6 and TM 7 to 12). The protein segments used as antigens to generate the generic anti-Cdrp (P13) and anti-Cdr2p polyclonal antibodies are indicated by the filled boxes. (B) Immunodetection of Cdr1p and Cdr2p. Total membrane proteins (25 μg) prepared from TY310 cells transformed with p425GPD (control [CTL]), p425GPD-CDR1 (CDR1), or p425GPD-CDR2 (CDR2) were separated by SDS-PAGE, transferred to nitrocellulose membranes, and analyzed with the indicated antibodies. The positions of the molecular mass standards (in kilodaltons) are shown on the left. (C) Azole resistance profiles of the CDR1 and CDR2 transformants. TY310 cells transformed with plasmid p425GPD-CDR1 (squares), p425GPD-CDR2 (circles), or p425GPD (triangles) were analyzed by liquid microdilution assay for their ability to grow in medium containing the indicated azoles. Cell growth is presented as the percentage of growth in azole-containing medium relative to the growth in azole-free (control) medium containing the solvent alone. Values represent the means ± standard deviations of three independent experiments performed in duplicate.
Analysis of the azole resistance profiles of these transformants by a microtiter plate assay showed that CDR1- and CDR2-expressing cells were more resistant than control cells to the three azoles tested: FLC, ITC, and KTC (Fig. 1C). The p425GPD-CDR1 transformants were able to withstand higher concentrations of FLC and ITC than the p425GPD-CDR2 transformants, suggesting that Cdr1p is more active than Cdr2p at effluxing FLC and ITC (Fig. 1C). Conversely, the p425GPD-CDR2 transformants were able to grow in the presence of higher concentrations of KTC than the p425GPD-CDR1 transformants, although this difference could be attributed to the higher levels of Cdr2p expression compared to those of Cdr1p expression (Fig. 1B, left panel). Taken together, these results demonstrate that Cdr1p and Cdr2p are functionally expressed in S. cerevisiae TY310.
Photoaffinity labeling of Cdr1p and Cdr2p with IAARh123.
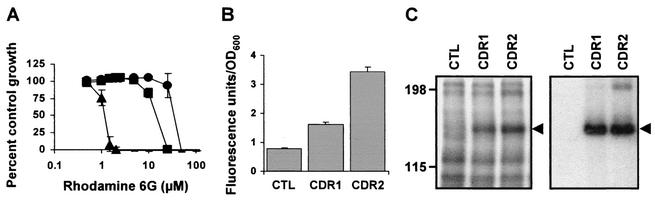
Rh, a cationic fluorescent dye with antifungal activity, is a substrate for several ABC transporters, including Cdr1p and Cdr2p (49, 66). As anticipated, TY310 cells transformed with p425GPD-CDR1 or p425GPD-CDR2 were more resistant to Rh 6G and displayed increased levels of efflux of this compound compared to control cells (Fig. 2A and B). Cells expressing Cdr2p grew in the presence of higher concentrations of Rh 6G (Fig. 2A) and effluxed more Rh 6G than those expressing Cdr1p (Fig. 2B), although these differences were not significant when the results were normalized for protein content (Fig. 1B). Given the abilities of Cdr1p and Cdr2p to confer Rh resistance and to mediate its efflux, it was of interest to investigate the abilities of the two proteins to bind to this compound. To this end, we performed cross-linking experiments with the photoreactive-iodinated analogue of Rh 123, IAARh123, which was previously used to demonstrate binding of Rh to P-gp1 and MRP1 (13, 50). Membranes from the TY310 transformants were incubated with IAARh123, UV cross-linked, and resolved by SDS-PAGE (Fig. 2C, left panel). A high-molecular-weight protein was specifically photolabeled with IAARh123 in total membranes isolated from the p425GPD-CDR1 or the p425GPD-CDR2 transformants but not from the p425GPD transformants (control), suggesting that the labeled proteins correspond to Cdr1p and Cdr2p. This was confirmed by immunoprecipitation of the photolabeled membranes with the generic anti-Cdrp polyclonal antibody (Fig. 2C, right panel). When normalized for the amount of Cdr1p and Cdr2p proteins present in the membrane samples (Fig. 1B), the levels of photolabeling of Cdr1p and Cdr2p were roughly equivalent. Taken together, our results demonstrate that Cdr1p and Cdr2p can be photoaffinity labeled with IAARh123 and that this compound may be used to characterize the Rh 123 binding site(s) in the Cdrp transporters.
FIG. 2.
Rh transport and binding by Cdr1p and Cdr2p. (A) Rh 6G resistance profile of TY310 cells transformed with p425GPD-CDR1 (squares), p425GPD-CDR2 (circles), or p425GPD (triangles) as determined by liquid microdilution assay. Values represent the means ± standard deviations of three independent experiments performed in duplicate. (B) Rh 6G efflux activities of the TY310 transformants mentioned in panel A. Extracellular Rh 6G fluorescence was measured by spectrofluorometry and normalized for the amount of cells present in each sample, as determined by measurement of the OD600. Values represent the means ± standard deviations of three independent experiments. (C) Photoaffinity labeling of Cdr1p and Cdr2p with IAARh123. Left panel, total membrane proteins (50 μg) from the transformants described above were incubated with 0.5 μM IAARh123, UV cross-linked, and separated by SDS-PAGE; right panel, total membrane proteins (300 μg) were incubated with 1 μM IAARh123, UV cross-linked, and immunoprecipitated with the anti-Cdrp antibody. The immune complexes were resolved by SDS-PAGE. The gels were dried and exposed to Kodak BioMax MS films with two intensifying screens at −80°C for 4 h (left panel) or 5 days (right panel). The results shown are representative of those of at least three independent experiments. The positions of the molecular mass standards (in kilodaltons) are indicated on the left. CTL, control.
As a first step in that direction, we used a coexpression system to determine whether both halves of Cdr2p bind to Rh 123. Plasmids expressing the N- or the C-terminal half of Cdr2p (p425GPD-CDR2L(1-855) and p423GPD-CDR2(856-1499),respectively; Fig. 3A) were transformed independently or simultaneously into TY310 cells, along with the appropriate control vectors. Analysis of the drug resistance profiles of the resulting transformants showed that cells coexpressing the two halves of Cdr2p were significantly resistant to FLC and Rh 6G, with about 50% resistance compared to the level of resistance of cells expressing full-length Cdr2p, while cells expressing each half alone were as susceptible to FLC and Rh 6G as the control cells transformed with the empty vectors (Fig. 3B). These results demonstrate that each half of Cdr2p is unable to confer resistance by itself and that coexpression of the two halves is necessary to reconstitute a functional transporter. Western blot analysis of total membrane extracts by use of the N-terminal-specific anti-Cdr2p antibody or the C-terminal-specific anti-Cdrp antibody (Fig. 3A) confirmed that the two halves are properly expressed in the TY310 transformants. The N-terminal half migrates at a position corresponding to its predicted molecular mass of 96 kDa (Fig. 3C, upper panel) and is detected as a doublet, possibly due to posttranslational modifications such as glycosylation, ubiquitination, or phosphorylation (16, 22). The C-terminal half appears as a single band migrating approximately 10 kDa lower than its calculated molecular mass of 73 kDa (Fig. 3C, lower panel). The reason for this difference is unclear, but expression of the C-terminal half of the yeast ABC transporter Ste6p also gives rise to a similar discrepancy between its calculated and apparent molecular masses (8). Taken together, these results indicate that both halves of Cdr2p are properly expressed in S. cerevisiae, thus providing a system that can be used to evaluate the ability of each half to bind to Rh within the context of a functionally reconstituted transporter. Photoaffinity labeling with IAARh123 was performed by using total membrane extracts from the different transformants, followed by immunoprecipitation with either the anti-Cdr2p antibody (Fig. 3D, upper panel) or the anti-Cdrp antibody (Fig. 3D, lower panel). Figure 3 shows that each half of Cdr2p can be photolabeled with IAARh123 when it is expressed either alone or in combination with the other half (Fig. 3D, lanes N, C, and N + C). These results demonstrate the presence of at least one Rh-binding site in each half of Cdr2p and suggest that each half is involved in Rh recognition. They also show that the inability of individual halves to confer Rh resistance is not due to impaired Rh binding. Finally, they indicate that the presence of leucines rather than serines at positions 21 and 632 in Cdr2p does not affect the binding of Rh to the transporter (compare Fig. 3D, lane FL, and Fig. 2C, lane CDR2).
FIG. 3.
Photoaffinity labeling of the N- and C-terminal halves of Cdr2p by IAARh123. (A) Schematic representation of Cdr2p showing the positions of the cleavage site and of the epitopes recognized by the anti-Cdr2p and anti-Cdrp polyclonal antibodies. (B) Drug resistance profiles of TY310 cells cotransformed with p425GPD-CDR2L and p423GPD (squares), p425GPD-CDR2L(1-855) and p423GPD-CDR2(856-1499) (circles), p425GPD-CDR2L(1-855) and p423GPD (triangles), p425GPD and p423GPD-CDR2(856-1499) (diamonds), as well as p425GPD and p423GPD (×), as determined by liquid microdilution assay. (C) Immunodetection of the halves of Cdr2p expressed in S. cerevisiae TY310. Total membrane proteins (50 μg) from TY310 cells cotransformed with p425GPD-CDR2L and p423GPD (lane FL, for full-length CDR2), p425GPD-CDR2L(1-855) and p423GPD (lane N), p425GPD and p423GPD-CDR2(856-1499) (lane C), p425GPD-CDR2L(1-855) and p423GPD-CDR2(856-1499) (lane N + C), as well as p425GPD and p423GPD (control [CTL]) were separated by SDS-PAGE, transferred to nitrocellulose membranes, and analyzed with the anti-Cdr2p (upper panel) or the anti-Cdrp (lower panel) polyclonal antibody. The positions of the N- and C-terminal halves are indicated by arrowheads. (D) Photoaffinity labeling of the two halves of Cdr2p by IAARh123. Total membrane proteins (300 μg) from the cotransformants for which the results are shown in panel C were incubated with 1 μM IAARh123, UV cross-linked, and immunoprecipitated (IP) with either the anti-Cdr2p (upper panel) or the anti-Cdrp (lower panel) antibody. The immune complexes were separated by SDS-PAGE. The gels were dried and exposed to Kodak BioMax MS films with two intensifying screens at −80°C for 5 days. The positions of the molecular mass standards (in kilodaltons) are indicated on the left.
Differential activity of Cdr1p or Cdr2p against FK520.
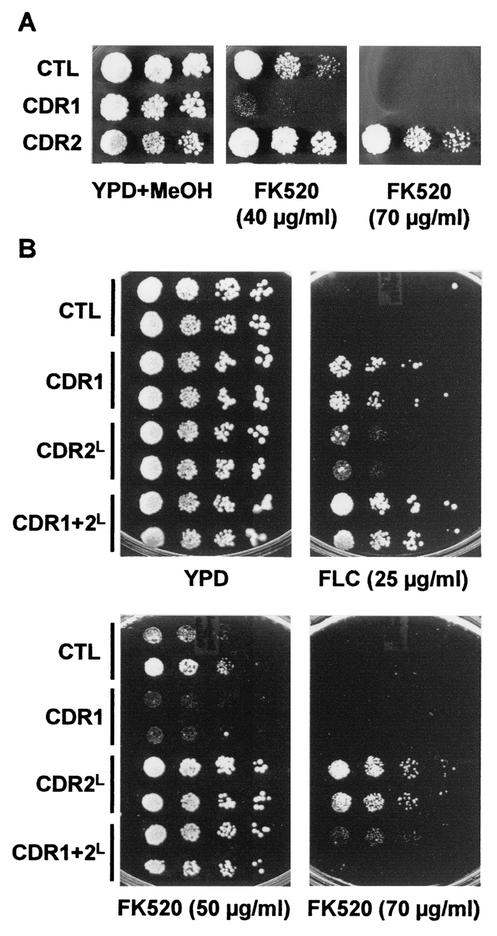
FK506 (tacrolimus) and FK520 (ascomycin; an FK506 derivative in which the C-21 allyl group is replaced by an ethyl group [31]) are macrolide compounds with potent immunosuppressive and antimicrobial activities (4, 21). They exert their action by forming a complex with FKBP12, a peptidyl-prolyl isomerase, and inhibiting the calmodulin-dependent protein phosphatase calcineurin, which is involved in signal transduction and regulation of gene expression (10, 32). In addition, they function as modulators of P-gp1-mediated MDR in mammalian cells: FK506 has been shown to compete with anticancer drugs for binding and transport by P-gp1 and both compounds stimulate P-gp1 ATPase activity with high efficiencies (3, 53, 57). Also, P-gp encoded by the mouse mdr3 gene confers resistance to both compounds when it is expressed in S. cerevisiae (55, 71). Finally, FK506 has been shown to inhibit the transport of drugs and steroids by Pdr5p, suggesting a potential clinical role for this compound as an agent that can be used to reverse azole resistance (23, 40). It was therefore of interest to determine whether Cdr1p and Cdr2p expression in S. cerevisiae affects the tolerance of cells to this class of compounds. Using a spot assay, we compared the ability of TY310 cells transformed with p425GPD, p425GPD-CDR1, or p425GPD-CDR2 to grow in the presence of increasing concentrations of FK520. Interestingly, we found that CDR1 expression and CDR2 expression have opposite effects: Cdr1p conferred hypersusceptibility to FK520, whereas Cdr2p conferred resistance to this compound (Fig. 4A), uncovering a major functional difference between the two transporters.
FIG. 4.
Differential susceptibilities of TY310 cells expressing Cdr1p and Cdr2p in the presence of FK520. (A) TY310 cells transformed with plasmid p425GPD (control), p425GPD-CDR1 (CDR1), or p425GPD-CDR2 (CDR2) were analyzed by spot assay on YPD plates containing the solvent alone (0.7% methanol [YPD + MeOH]) or FK520 at the indicated concentrations. (B) Coexpression of CDR1 and CDR2. Two different TY310 transformants harboring plasmids p425GPD and p423GPD (control), p425GPD and p423GPD-CDR1 (CDR1), p425GPD-CDR2L and p423GPD (CDR2L), or p423GPD-CDR1 and p425GPD-CDR2L (CDR1 + 2L) were analyzed by spot assay on YPD plates in the absence of FLC and FK520 (YPD) or in the presence of FLC and FK520 at the indicated concentrations. The plates were incubated at 30°C for 2 days. CTL, control.
Since azole-resistant C. albicans strains overexpress both CDR1 and CDR2, we investigated the functional consequences of CDR1 and CDR2 coexpression on cellular resistance to FLC and FK520. To this end, we cloned CDR1 into p423GPD and generated the appropriate sets of double transformants by transforming TY310/p425GPD-CDR2L cells with p423GPD-CDR1 for CDR1 and CDR2 coexpression, TY310/p423GPD-CDR1 with p425GPD and TY310/p425GPD-CDR2L with p423GPD for the individual expression of CDR1 and CDR2, respectively, and TY310/p425GPD cells with p423GPD as a control. Western blot analysis of the resulting Leu+ His+ clones was performed, confirming that Cdr1p and Cdr2p are properly expressed in the single-gene transformants as well as in the double-gene transformants (data not shown). We first analyzed these clones for their resistance to FLC by spot assay. At a concentration of 25 μg/ml, CDR1- and CDR2-expressing cells displayed moderate and low levels of FCZ resistance, respectively, compared to the level of resistance of control cells (Fig. 4B). Cells coexpressing CDR1 and CDR2 were more resistant to FLC than each single-gene transformant. These results demonstrate that Cdr1p and Cdr2p have additive activities against FLC, suggesting that the concomitant overexpression of CDR1 and CDR2 observed in many azole-resistant C. albicans strains helps to confer high levels of resistance in these cells. We then analyzed these transformants for their levels of resistance to FK520 (Fig. 4C). As expected, CDR1- and CDR2-overexpressing cells were hypersusceptible and resistant to FK520, respectively, compared to the susceptibilities of control cells. However, cells coexpressing CDR1 and CDR2 were more susceptible to FK520 than CDR2-expressing cells but more resistant than CDR1-expressing cells, demonstrating that Cdr1p and Cdr2p additivity also applies to susceptibilities to compounds against which the two transporters have opposite activities. These results further substantiate that CDR1 and CDR2 have similar but also distinct cellular functions.
Previous studies have shown that FK506 has synergistic activity with FLC against C. albicans and other pathogenic fungi (11, 17, 44). It has been proposed that this synergy could be due to the ability of FK506 to inhibit multidrug transporters, resulting in increased levels of intracellular FLC accumulation and thus increased FLC susceptibility (17, 44). We therefore used our expression system to examine possible functional interactions between this class of compounds and the azole resistance activities of the Cdr1p and Cdr2p transporters. TY310 cells transformed with p425GPD, p425GPD-CDR1, or p425GPD-CDR2 were grown in the presence or in the absence of a nontoxic concentration of FK520 (10 μg/ml) in combination with FLC (Fig. 5A) or KTC (Fig. 5B) in microtiter plate assays. Again, an important difference was observed between cells expressing Cdr1p or Cdr2p: while the addition of FK520 had no effect on the growth of the p425GPD (control) or the p425GPD-CDR2 transformants in the presence of azoles, it strongly inhibited the growth of the p425GPD-CDR1 transformants in the presence of the two azoles tested (Fig. 5A and B). This finding highlights yet another functional difference between Cdr1p and Cdr2p which has important clinical implications (see Discussion).
Functional consequences of CDR1 and CDR2 expression on cell response to oxidants.
We have observed that the acquisition of azole resistance in C. albicans clinical isolates is accompanied by an altered susceptibility of the cells to oxidative agents. For example, an azole-resistant C. albicans isolate (isolate 5674) overexpressing CDR1 and CDR2 but not CaMDR1 is resistant to diamide and hypersusceptible to hydrogen peroxide compared to the susceptibility of its matched parental azole-susceptible isolate (isolate 5457) (Fig. 6A), suggesting that CDR1 and/or CDR2 overexpression could be responsible for the phenotypes observed. However, molecular changes other than transporter overexpression may have taken place in these cells during the acquisition of azole resistance. To directly investigate this question, we analyzed the p425GPD-CDR1 and p425GPD-CDR2 transformants for their abilities to grow in the presence of increasing concentrations of these oxidants by spot assay. This experiment showed that Cdr2p expression causes hypersusceptibility to H2O2 and resistance to diamide, while Cdr1p expression has no effect on the susceptibilities to these two compounds (Fig. 6B). The inability of Cdr1p to generate these phenotypes confirms that they specifically result from the expression of CDR2 and not simply from high levels of expression of a polytopic membrane protein in a heterologous host. These results further demonstrate that Cdr1p and Cdr2p possess distinct activities. They also indicate that the altered susceptibilities to oxidative agents observed in azole-resistant C. albicans clinical isolates overexpressing CDR1 and CDR2 are likely to be a consequence of CDR2 overexpression and that ABC transporters like Cdr2p can influence cell response to oxidative stress.
FIG. 6.
Functional consequences of CDR1 and CDR2 expression on cellular responses to oxidants. (A) C. albicans isolates 5457 and 5674 were spotted on YPD plates containing diamide (1 mM) or H2O2 (3 mM). (B) TY310 cells transformed with p425GPD (control), p425GPD-CDR1 (CDR1), or p425GPD-CDR2 (CDR2) were spotted on YPD plates containing diamide (0.75 mM) or H2O2 (2 mM). Control growth in the absence of oxidants is also shown (YPD and YPD + DMSO). The plates were incubated at 30°C for 2 days. CTL, control.
DISCUSSION
Transporter-mediated azole resistance represents an important clinical problem for the treatment of C. albicans infections. The molecular characterization of several azole-resistant clinical isolates has shown that a large proportion of these strains overexpress the CDR1 and CDR2 genes (74). This overexpression is stable and probably the consequence of mutations in a trans-acting factor coregulating the expression of both genes. Indeed, several studies have shown that the transcription of CDR1 and CDR2 is similarly regulated; i.e., both genes can be induced upon treatment of cells with different steroids and antifungal agents through a drug response element present in their promoters (18, 33, 41). In addition, studies performed so far have shown that Cdr1p and Cdr2p perform similar functions: both transporters confer resistance to multiple antifungal compounds, including azoles, Rh, and other drugs (although to various degrees) and function as phospholipid translocators (60, 66). In the present study, we have used a heterologous expression system in S. cerevisiae to compare the activities of these two transporters. Our system uses multicopy vectors containing a strong constitutive promoter, a situation mimicking the stable overexpression observed in azole-resistant clinical isolates. We have also generated and characterized polyclonal antibodies recognizing different epitopes in these transporters. These antibodies are advantageous since they permit (i) normalization of the activities of the transporters with respect to their expression levels, (ii) specific detection of different domains of the transporters, and (iii) analysis of these transporters without the use of tags that may impair their function. Using this well-characterized system, we were able to identify different phenotypes specifically associated with the expression of Cdr1p or Cdr2p, unraveling fundamental functional differences between the two transporters. In particular, we show that CDR2 confers resistance to FK520 and diamide but hypersusceptibility to H2O2, whereas CDR1 confers hypersusceptibility to FK520 but does not affect the tolerance of cells to oxidative agents. Since Cdr1p and Cdr2p display very high levels of structural conservation, these functional differences are possibly encoded by a limited number of amino acid residues. Alternatively, different subcellular localizations, half-lives, and/or phosphorylation status could account for the differences between the two transporters. The availability of a functional expression system in S. cerevisiae together with well-characterized antibodies now allows us to dissect the molecular mechanisms underlying these functional dissimilarities.
Although Cdr1p, Cdr2p, P-gp1, and MRP1 all function as MDR pumps, Cdr1p and Cdr2p are structurally different from the mammalian P-gp1 and MRP1 transporters. They possess an inverted domain organization ([ABC-TM]2) compared to those of P-gp1 and MRP1 ([TM-ABC]2). They also display a low level of sequence identity with P-gp1 and MRP1 (<17%) outside of the conserved ABC domains. Despite these differences, the results of our photoaffinity labeling analyses demonstrate that Cdr1p and Cdr2p have a number of characteristics in common with P-gp1 and MRP1 with respect to substrate binding. First, we show that, like P-gp1 and MRP1, Cdr1p and Cdr2p bind to IAARh123 (Fig. 2C) (13, 50), indicating that the Cdrp transporters share sites for binding to this compound with the mammalian transporters. Second, we found that Cdr1p and Cdr2p also bind to the photoactive dihydropyridine analog [3H]azidopine (data not shown), a well-characterized MDR modulator which inhibits drug binding on P-gp1 and which is exported by this transporter (59, 69). These data further demonstrate that the proteins Cdrp and P-gp1 recognize similar substrates and suggest that dihydropyridine may function as an inhibitor of Cdrp activity and, thus, of Cdrp-mediated azole resistance. Finally, we show that each half of Cdr2p can be photolabeled by IAARh123 (Fig. 3D), indicating the presence of at least one substrate-binding site in each half of the transporter, as demonstrated for P-gp1 and MRP1 (12, 19, 29, 58). Interestingly, our results show that each half of Cdr2p, although unable by itself to confer Rh resistance (Fig. 3B), can be independently photolabeled by IAARh123 (Fig. 3D). To our knowledge, this is the first demonstration that each half of an ABC transporter, expressed alone, can bind to a substrate independently of the other half. These results demonstrate that each half of Cdr2p contains all the structural determinants necessary for Rh binding. They also suggest that the inability of individual halves of Cdr2p to confer Rh resistance is not due to impaired Rh binding but rather to impaired Rh translocation. This hypothesis is supported by the recent demonstration that the two ABC domains of an ABC transporter cooperate to form the catalytic pockets involved in ATP binding and hydrolysis and, thus, are required to provide the energy necessary for substrate translocation (24, 65). Our coexpression system will be useful for investigation not only of the substrate binding sites in Cdr2p but also of the functions of its ABC domains in substrate translocation.
One question that remains to be answered is whether the IAARh123-binding sites in Cdr1p and Cdr2p overlap with those for azoles or other known Cdrp substrates (e.g., steroids and phospholipids) and, thus, whether they are clinically and/or physiologically relevant. Experimental evidence suggests that that should be the case. On the one hand, IAARh123 binding by P-gp1 and MRP1 is efficiently competed by anticancer drugs, MDR modulators, and physiological substrates, demonstrating the relevance of IAARh123 in studying the drug-binding sites on the mammalian transporters (13, 50). On the other hand, azoles such as ITC and KTC have been shown to inhibit the P-gp1-mediated transport of Rh 123 and anticancer drugs (72), suggesting that Rh 123 and these azoles have the same or an overlapping binding site(s) in P-gp1. On the basis of this evidence, we predict that IAARh123 should prove useful in characterizing the Cdr1p and Cdr2p substrate-binding sites and in exploring the molecular basis of substrate specificity in the Cdrp subfamily of ABC transporters, a question that we are addressing through competition experiments.
Our study with FK520 revealed that CDR2 confers resistance to FK520, whereas CDR1 confers hypersusceptibility to this compound (Fig. 4A). It also showed that CDR1 expression together with CDR2 expression decreases the ability of the CDR2-expressing cells to grow in the presence of FK520, demonstrating that these opposite phenotypes are additive and can take place in the same cell (Fig. 4B). These results are important because (i) FK520 is the only compound identified so far against which Cdr1p and Cdr2p have opposite activities and (ii) they underscore the major functional differences between Cdr1p and Cdr2p, despite the very high level of structural conservation between the two transporters. What are the molecular mechanisms underlying these opposite phenotypes? It is possible that Cdr2p confers resistance to FK520 by directly exporting this compound out of the cells, as previously shown for P-gp1 (56, 57), although indirect mechanisms such as decreased membrane permeability or activation of another transporter are also conceivable. The mechanism by which Cdr1p confers hypersusceptibility is less clear but could well be related to that recently reported for the Cdr1p-mediated hypersusceptibility of S. cerevisiae cells to FMDP [N3-(4-methoxyfumaroyl)-l-2,3-diaminopropanoic acid] antifungal peptides (46). This study showed that a proton motive force generated by Cdr1p stimulates uptake of these peptides, giving rise to the observed hypersusceptibility (46). It is therefore possible that Cdr1p mediates hypersusceptibility to FK520 by promoting its cellular uptake directly or indirectly.
The mechanisms underlying the azole-FK506 synergy are not fully understood and appear to vary among different fungi. On the one hand, Del Poeta et al. (17) have shown that this synergy is independent of FKBP12 and calcineurin in Cryptococcus neoformans and suggested that direct inhibition of azole-exporting transporters by FK506 might cause increased levels of intracellular accumulation of azoles and, thus, increased azole susceptibility. On the other hand, Cruz et al. have shown that the azole-FK506 synergy depends upon FKBP12 and calcineurin in C. albicans and concluded that there was no need to consider an additional effect of FK506 on MDR transporters to explain the synergy in this fungus (11). Our results showing (i) that FK520 in combination with azoles strongly inhibits the growth of S. cerevisiae cells expressing CDR1 (Fig. 5) and (ii) that CDR1 confers hypersusceptibility to FK520 and CDR2 confers resistance to this compound (Fig. 4A) indicate that the Cdr1p and Cdr2p transporters can affect cell tolerance to FK520 and suggest a possible involvement of these transporters in the azole-FK506 synergy in C. albicans. Interestingly, it has been shown that exposure of C. albicans cells to FLC promotes the intracellular accumulation of FK506 by about 20-fold, potentially through an increase in levels of uptake or a decrease in levels of export (11). It is thus possible that Cdr1p could function as the (or one of the) determinant(s) mediating the FLC-triggered accumulation of FK506 in C. albicans, with FKBP12 and calcineurin being further required to mediate the toxic effect of the intracellular FK506. Since normal (azole-susceptible) cells express only CDR1 (60, 61), there would be no involvement of Cdr2p in the observed phenotype. In azole-resistant isolates overexpressing CDR1 and CDR2, the function of CDR2 would add to that of CDR1 in conferring azole resistance but would partly overcome the CDR1-mediated susceptibility of the cells to FK506, as suggested by the results of our CDR1-CDR2 coexpression experiment with S. cerevisiae (Fig. 4B). CDR2 expression would reduce, to some extent, the effect of a CDR1-mediated azole-FK506 synergy, a hypothesis consistent with the demonstration that clinical isolates overexpressing CDR1 and CDR2 are slightly more resistant to the FLC-FK506 combination than their parental isolates which express only CDR1 (11). Indeed, this model is still tentative and remains to be tested in C. albicans with CDR1- and CDR2-overexpressing strains and strains with CDR1 and CDR2 deletions. Understanding the molecular basis of the different behaviors of Cdr1p and Cdr2p toward FK520 is important, since it has clinical implications for the design of strategies aimed at overcoming azole resistance in C. albicans with inhibitors of both efflux pumps and drugs that act synergistically with azoles.
We observed that an azole-resistant C. albicans clinical isolate overexpressing CDR1 and CDR2 (but not CaMDR1) displays altered sensitivities to compounds which induce oxidative stress, namely, resistance to diamide and hypersusceptibility to H2O2 (Fig. 6A). Likewise, we have found that FR2, an azole-resistant strain that was derived from azole-susceptible strain SGY243 by stepwise selection with FLC and that overexpresses CDR1, CDR2, and CaMDR1 (1), is also resistant to diamide and hypersusceptible to H2O2 compared to the susceptibility of SGY243 (unpublished data), demonstrating that our observation also applies to other azole-resistant strains. Our results showing that the heterologous expression of CDR2 in S. cerevisiae sensitizes the cells to hydrogen peroxide and confers resistance to diamide (Fig. 6B) suggest that the altered susceptibilities of these azole-resistant C. albicans strains to oxidative agents may result from the overexpression of CDR2. On the one hand, Cdr2p may confer resistance to diamide by directly exporting this compound, either in an unmodified form or with diamide coupled to glutathione (39), or by an indirect mechanism, as postulated for FK520. On the other hand, the increased susceptibilities to H2O2 of cells expressing Cdr2p could be the consequence of changes in the plasma membrane composition induced by the activity of this transporter with respect to phospholipid translocation and/or sterol transport (38, 66). These changes would alter the fluidity of the membrane and therefore the diffusion of the different oxidative agents. Indeed, it has been shown that the acquisition of CDR1- and CDR2-mediated azole resistance in C. albicans is accompanied by changes in membrane fluidity and asymmetry (37). Interestingly, such phenotypes are not restricted to CDR2. We have shown that overexpression of the S. cerevisiae FLR1 gene, which codes for a major facilitator homologous to C. albicans CaMdr1p, also confers diamide resistance and H2O2 hypersusceptibility (51). Taken together, our findings suggest that the acquisition of azole resistance in C. albicans through the upregulation of transporter-encoding genes such as CDR2 may be accompanied by an altered susceptibility of the cells to oxidative stress.
Since the production of reactive oxygen species by human phagocytic neutrophils is a major line of defense against fungal infections (48), the ability of Cdr2p to elicit H2O2 hypersusceptibility suggests that the acquisition of azole resistance through CDR2 upregulation in C. albicans could be achieved at the expense of the capacity of the cells to counteract the host immune response. Studies of the virulence of C. albicans strains have been performed in a murine model of systemic candidiasis and agree to some extent with this prediction. Graybill and colleagues (28) have shown that azole-resistant strains isolated from two different patients and overexpressing CDR1 and CDR2 displayed reduced virulence compared to the virulence of their matched azole-susceptible isolates and suggested that the observed link between FLC resistance and decreased virulence may relate to the overexpression of a pump of the CDR family in these strains. However, those investigators also reported that an azole-resistant strain that was isolated from a third patient and that showed no overexpression of the CDR genes also displayed reduced virulence, demonstrating that the interaction between C. albicans and its host is complex and probably involves many different factors besides transporter overexpression (28). Also, the interaction between the immune system and MDR transporters in C. albicans may also extend to transporters of the major facilitator superfamily, since deletion of the CaMDR1 gene in C. albicans has been shown to result in reduced virulence (7). Finally, other studies have shown that the virulence of C. albicans strains does not appear to be related to their azole resistance status (5, 70), although the molecular mechanism of azole resistance in these strains was not identified. It will be important to determine the capacity of multidrug transporters to modulate C. albicans virulence in animal models by using well-characterized strains engineered to overexpress such transporters individually or in combination in otherwise identical genetic backgrounds.
In conclusion, the heterologous expression system for Cdr1p and Cdr2p presented here has allowed us to make novel and important observations pertaining to the functions of these transporters. This system will be useful for the identification of the drug-binding sites in Cdr1p and Cdr2p and to address the molecular basis of their substrate specificities. It will also permit the dissection of the molecular determinants underlying the functional similarities and the differences between these two clinically important multidrug transporters.
Acknowledgments
We are grateful to Martin Funk for providing the p423GPD and p425GPD vectors.
This work was supported by research grants from the Canadian Institutes of Health Research (CIHR; grant MT-15679) to M.R. and from the Natural Sciences and Engineering Research Council of Canada (grant OGP0121558) to E.G. C.G. is supported by a studentship from CIHR, and M.R. is a senior scientist from le Fonds de la Recherche en Santé du Québec.
REFERENCES
- 1.Albertson, G. D., M. Niimi, R. D. Cannon, and H. F. Jenkinson. 1996. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 40:2835-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambudkar, S. V., S. Dey, C. A. Hrycyna, M. Ramachandra, I. Pastan, and M. M. Gottesman. 1999. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 39:361-398. [DOI] [PubMed] [Google Scholar]
- 3.Arceci, R. J., K. Stieglitz, and B. E. Bierer. 1992. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood 80:1528-1536. [PubMed] [Google Scholar]
- 4.Arndt, C., M. C. Cruz, M. E. Cardenas, and J. Heitman. 1999. Secretion of FK506/FK520 and rapamycin by Streptomyces inhibits the growth of competing Saccharomyces cerevisiae and Cryptococcus neoformans. Microbiology 145:1989-2000. [DOI] [PubMed] [Google Scholar]
- 5.Barchiesi, F., L. K. Najvar, M. F. Luther, G. Scalise, M. G. Rinaldi, and J. R. Graybill. 1996. Variation in fluconazole efficacy for Candida albicans strains sequentially isolated from oral cavities of patients with AIDS in an experimental murine candidiasis model. Antimicrob. Agents Chemother. 40:1317-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer, B. E., H. Wolfger, and K. Kuchler. 1999. Inventory and function of yeast ABC proteins: about sex, stress, pleiotropic drug and heavy metal resistance. Biochim. Biophys. Acta 1461:217-236. [DOI] [PubMed] [Google Scholar]
- 7.Becker, J. M., L. K. Henry, W. Jiang, and Y. Koltin. 1995. Reduced virulence of Candida albicans mutants affected in multidrug resistance. Infect. Immun. 63:4515-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkower, C., and S. Michaelis. 1991. Mutational analysis of the yeast a-factor transporter STE6, a member of the ATP binding cassette (ABC) protein superfamily. EMBO J. 10:3777-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 10.Carreras, C. W., H. Fu, and D. V. Santi. 2001. An FKBP12 binding assay based upon biotinylated FKBP12. Anal. Biochem. 298:57-61. [DOI] [PubMed] [Google Scholar]
- 11.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daoud, R., M. Julien, P. Gros, and E. Georges. 2001. Major photoaffinity drug binding sites in multidrug resistance protein 1 (MRP1) are within transmembrane domains 10-11 and 16-17. J. Biol. Chem. 276:12324-12330. [DOI] [PubMed] [Google Scholar]
- 13.Daoud, R., C. Kast, P. Gros, and E. Georges. 2000. Rhodamine 123 binds to multiple sites in the multidrug resistance protein (MRP1). Biochemistry 39:15344-15352. [DOI] [PubMed] [Google Scholar]
- 14.Decottignies, A., and A. Goffeau. 1997. Complete inventory of the yeast ABC proteins. Nat. Genet. 15:137-145. [DOI] [PubMed] [Google Scholar]
- 15.Decottignies, A., A. M. Grant, J. W. Nichols, H. de Wet, D. B. McIntosh, and A. Goffeau. 1998. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 273:12612-12622. [DOI] [PubMed] [Google Scholar]
- 16.Decottignies, A., G. Owsianik, and M. Ghislain. 1999. Casein kinase I-dependent phosphorylation and stability of the yeast multidrug transporter pdr5p. J. Biol. Chem. 274:37139-37146. [DOI] [PubMed] [Google Scholar]
- 17.Del Poeta, M., M. C. Cruz, M. E. Cardenas, J. R. Perfect, and J. Heitman. 2000. Synergistic antifungal activities of bafilomycin A(1), fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Micheli, M., J. Bille, C. Schueller, and D. Sanglard. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43:1197-1214. [DOI] [PubMed] [Google Scholar]
- 19.Dey, S., M. Ramachandra, I. Pastan, M. M. Gottesman, and S. V. Ambudkar. 1998. Photoaffinity labeling of human P-glycoprotein: effect of modulator interaction and ATP hydrolysis on substrate binding. Methods Enzymol. 292:318-328. [DOI] [PubMed] [Google Scholar]
- 20.Dixon, D. M., M. M. McNeil, M. L. Cohen, B. G. Gellin, and J. R. La Montagne. 1996. Fungal infections: a growing threat. Public Health Rep. 111:226-235. [PMC free article] [PubMed] [Google Scholar]
- 21.Dumont, F. J. 2000. FK506, an immunosuppressant targeting calcineurin function. Curr. Med. Chem. 7:731-748. [DOI] [PubMed] [Google Scholar]
- 22.Egner, R., and K. Kuchler. 1996. The yeast multidrug transporter Pdr5 of the plasma membrane is ubiquitinated prior to endocytosis and degradation in the vacuole. FEBS Lett. 378:177-181. [DOI] [PubMed] [Google Scholar]
- 23.Egner, R., F. E. Rosenthal, A. Kralli, D. Sanglard, and K. Kuchler. 1998. Genetic separation of FK506 susceptibility and drug transport in the yeast Pdr5 ATP-binding cassette multidrug resisitance transporter. Mol. Biol. Cell 9:523-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fetsch, E. E., and A. L. Davidson. 2002. From the cover: vanadate-catalyzed photocleavage of the signature motif of an ATP-binding cassette (ABC) transporter. Proc. Natl. Acad. Sci. USA 99:9685-9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franz, R., S. L. Kelly, D. C. Lamb, D. E. Kelly, M. Ruhnke, and J. Morschhauser. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goshorn, A. K., and S. Scherer. 1989. Genetic analysis of prototrophic natural variants of Candida albicans. Genetics 123:667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graybill, J. R., E. Montalbo, W. R. Kirkpatrick, M. F. Luther, S. G. Revankar, and T. F. Patterson. 1998. Fluconazole versus Candida albicans: a complex relationship. Antimicrob. Agents Chemother. 42:2938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberger, L. M. 1998. Identification of drug interaction sites in P-glycoprotein. Methods Enzymol. 292:307-317. [DOI] [PubMed] [Google Scholar]
- 30.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Hatanaka, H., T. Kino, S. Miyata, N. Inamura, A. Kuroda, T. Goto, H. Tanaka, and M. Okuhara. 1988. FR-900520 and FR-900523, novel immunosuppressants isolated from a Streptomyces. II. Fermentation, isolation and physico-chemical and biological characteristics. J. Antibiot. (Tokyo) 41:1592-1601. [DOI] [PubMed] [Google Scholar]
- 32.Hemenway, C. S., and J. Heitman. 1999. Calcineurin. Structure, function, and inhibition. Cell Biochem. Biophys. 30:115-151. [DOI] [PubMed] [Google Scholar]
- 33.Henry, K. W., M. C. Cruz, S. K. Katiyar, and T. D. Edlind. 1999. Antagonism of azole activity against Candida albicans following induction of multidrug resistance genes by selected antimicrobial agents. Antimicrob. Agents Chemother. 43:1968-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 35.Hipfner, D. R., R. G. Deeley, and S. P. Cole. 1999. Structural, mechanistic and clinical aspects of MRP1. Biochim. Biophys. Acta 1461:359-376. [DOI] [PubMed] [Google Scholar]
- 36.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohli, A., K. Smriti, A. Mukhopadhyay, R. Rattan, and R. Prasad. 2002. In vitro low-level resistance to azoles in Candida albicans is associated with changes in membrane lipid fluidity and asymmetry. Antimicrob. Agents Chemother. 46:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kontoyiannis, D. P. 2000. Efflux-mediated resistance to fluconazole could be modulated by sterol homeostasis in Saccharomyces cerevisiae. J. Antimicrob. Chemother. 46:199-203. [DOI] [PubMed] [Google Scholar]
- 39.Kosower, N. S., and E. M. Kosower. 1995. Diamide: an oxidant probe for thiols. Methods Enzymol. 251:123-133. [DOI] [PubMed] [Google Scholar]
- 40.Kralli, A., and K. R. Yamamoto. 1996. An FK506-sensitive transporter selectively decreases intracellular levels and potency of steroid hormones. J. Biol. Chem. 271:17152-17156. [DOI] [PubMed] [Google Scholar]
- 41.Krishnamurthy, S., V. Gupta, R. Prasad, and S. L. Panwar. 1998. Expression of CDR1, a multidrug resistance gene of Candida albicans: transcriptional activation by heat shock, drugs and human steroid hormones. FEMS Microbiol. Lett. 160:191-197. [DOI] [PubMed] [Google Scholar]
- 42.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Ribot, J. L., R. K. McAtee, S. Perea, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 1999. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:1621-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maesaki, S., P. Marichal, M. A. Hossain, D. Sanglard, H. Vanden Bossche, and S. Kohno. 1998. Synergic effects of tactolimus and azole antifungal agents against azole-resistant Candida albican strains. J. Antimicrob. Chemother. 42:747-753. [DOI] [PubMed] [Google Scholar]
- 45.Mahe, Y., Y. Lemoine, and K. Kuchler. 1996. The ATP binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisiae can mediate transport of steroids in vivo. J. Biol. Chem. 271:25167-25172. [DOI] [PubMed] [Google Scholar]
- 46.Milewski, S., F. Mignini, R. Prasad, and E. Borowski. 2001. Unusual susceptibility of a multidrug-resistant yeast strain to peptidic antifungals. Antimicrob. Agents Chemother. 45:223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mumberg, D., R. Muller, and M. Funk. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119-122. [DOI] [PubMed] [Google Scholar]
- 48.Murphy, J. W. 1991. Mechanisms of natural resistance to human pathogenic fungi. Annu. Rev. Microbiol. 45:509-538. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura, K., M. Niimi, K. Niimi, A. R. Holmes, J. E. Yates, A. Decottignies, B. C. Monk, A. Goffeau, and R. D. Cannon. 2001. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 45:3366-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nare, B., R. K. Prichard, and E. Georges. 1994. Characterization of rhodamine 123 binding to P-glycoprotein in human multidrug-resistant cells. Mol. Pharmacol. 45:1145-1152. [PubMed] [Google Scholar]
- 51.Nguyen, D. T., A. M. Alarco, and M. Raymond. 2000. Multiple Yap1p-binding sites mediate induction of the yeast major facilitator FLR1 gene in response to drugs, oxidants, and alkylating agents. J. Biol. Chem. 276:1138-1145. [DOI] [PubMed] [Google Scholar]
- 52.Prasad, R., P. Dewergifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320-329. [DOI] [PubMed] [Google Scholar]
- 53.Rao, U. S., and G. A. Scarborough. 1994. Direct demonstration of high affinity interactions of immunosuppressant drugs with the drug binding site of the human P-glycoprotein. Mol. Pharmacol. 45:773-776. [PubMed] [Google Scholar]
- 54.Raymond, M., P. Gros, M. Whiteway, and D. Y. Thomas. 1992. Functional complementation of yeast ste6 by a mammalian multidrug resistance mdr gene. Science 256:232-234. [DOI] [PubMed] [Google Scholar]
- 55.Raymond, M., S. Ruetz, D. Y. Thomas, and P. Gros. 1994. Functional expression of P-glycoprotein in Saccharomyces cerevisiae confers cellular resistance to the immunosuppressive and antifungal agent FK520. Mol. Cell. Biol. 14:277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruetz, S. 1998. Yeast secretory vesicle system for expression and functional characterization of P-glycoproteins. Methods Enzymol. 292:382-396. [DOI] [PubMed] [Google Scholar]
- 57.Saeki, T., K. Ueda, Y. Tanigawara, R. Hori, and T. Komano. 1993. Human P-glycoprotein transports cyclosporin A and FK506. J. Biol. Chem. 268:6077-6080. [PubMed] [Google Scholar]
- 58.Safa, A. R. 1998. Photoaffinity labels for characterizing drug interaction sites of P-glycoprotein. Methods Enzymol. 292:289-307. [DOI] [PubMed] [Google Scholar]
- 59.Safa, A. R., C. J. Glover, J. L. Sewell, M. B. Meyers, J. L. Biedler, and R. L. Felsted. 1987. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J. Biol. Chem. 262:7884-7888. [PubMed] [Google Scholar]
- 60.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 61.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos, M. A. S., and M. F. Tuite. 1995. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res. 23:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 64.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 65.Smith, P. C., N. Karpowich, L. Millen, J. E. Moody, J. Rosen, P. J. Thomas, and J. F. Hunt. 2002. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell 10:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smriti, S. Krishnamurthy, B. L. Dixit, C. M. Gupta, S. Milewski, and R. Prasad. 2002. ABC transporters Cdr1p, Cdr2p and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast 19:303-318. [DOI] [PubMed] [Google Scholar]
- 67.Taglicht, D., and S. Michaelis. 1997. A complete catalog of Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol. 292:130-162. [DOI] [PubMed] [Google Scholar]
- 68.Talibi, D., and M. Raymond. 1999. Isolation of a putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 mutation in Saccharomyces cerevisiae. J. Bacteriol. 181:231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamai, I., and A. R. Safa. 1991. Azidopine noncompetitively interacts with vinblastine and cyclosporin A binding to P-glycoprotein in multidrug resistant cells. J. Biol. Chem. 266:16796-16800. [PubMed] [Google Scholar]
- 70.Taylor, B. N., C. Fichtenbaum, M. Saavedra, I. J. Slavinsky, R. Swoboda, K. Wozniak, A. Arribas, W. Powderly, and P. L. Fidel, Jr. 2000. In vivo virulence of Candida albicans isolates causing mucosal infections in people infected with the human immunodeficiency virus. J. Infect. Dis. 182:955-959. [DOI] [PubMed] [Google Scholar]
- 71.Urbatsch, I. L., M. Julien, I. Carrier, M. E. Rousseau, R. Cayrol, and P. Gros. 2000. Mutational analysis of conserved carboxylate residues in the nucleotide binding sites of P-glycoprotein. Biochemistry 39:14138-14149. [DOI] [PubMed] [Google Scholar]
- 72.Wang, E. J., K. Lew, C. N. Casciano, R. P. Clement, and W. W. Johnson. 2002. Interaction of common azole antifungals with P glycoprotein. Antimicrob. Agents Chemother. 46:160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]