Abstract
Over the last several decades, research on snake venom toxins has provided not only new tools to decipher molecular details of various physiological processes, but also inspiration to design and develop a number of therapeutic agents. Blood circulation, particularly thrombosis and haemostasis, is one of the major targets of several snake venom proteins. Among them, anticoagulant proteins have contributed to our understanding of molecular mechanisms of blood coagulation and have provided potential new leads for the development of drugs to treat or to prevent unwanted clot formation. Some of these anticoagulants exhibit various enzymatic activities whereas others do not. They interfere in normal blood coagulation by different mechanisms. Although significant progress has been made in understanding the structure–function relationships and the mechanisms of some of these anticoagulants, there are still a number of questions to be answered as more new anticoagulants are being discovered. Such studies contribute to our fight against unwanted clot formation, which leads to death and debilitation in cardiac arrest and stroke in patients with cardiovascular and cerebrovascular diseases, arteriosclerosis and hypertension. This review describes the details of the structure, mechanism and structure–function relationships of anticoagulant proteins from snake venoms.
Keywords: anticoagulant, C-type lectin, metalloproteinase, phospholipase A2, snake venom, three-finger toxin
Abbreviations: FVa etc., Factor Va, etc; Gla, γ-carboxyglutamic acid; PLA2, phospholipase A2; HsPLA2, human secretory PLA2; TF–FVIIa complex, tissue factor–Factor VIIa complex; TLE, thrombin-like enzyme
INTRODUCTION
Snake venoms are complex mixtures of pharmacologically active proteins and polypeptides. They play an important role in incapacitating and immobilizing, as well as in digesting, prey [1,2]. Thus toxins have evolved to specifically target various critical points in the physiological systems of prey animals. Neuromuscular and circulatory systems are the two main physiological systems that are targeted by a great many toxins, as interruption(s) in these systems make the prey succumb to the venom in a short time. Over the years, a number of toxins that affect blood circulation have been isolated and characterized from various snake venoms [3–6]. Some of them affect platelet aggregation (for recent reviews, see [7–9]), whereas others affect blood coagulation. Studies of these factors have contributed immensely to the deciphering of various molecular mechanisms involved in the physiological processes. In addition, these studies have helped us in the development of various new therapeutic agents for the treatment of cardiovascular and haematological disorders [10,11]. Venom proteins affecting blood coagulation can functionally be classified as pro-coagulant or anticoagulant proteins on the basis of their ability to shorten or prolong the blood-clotting process. Pro-coagulant proteins are either serine proteinases or metalloproteinases. Their sizes vary between 24 kDa and 300 kDa. They induce blood coagulation either by specifically activating zymogen, one of the blood coagulation factors, or by directly converting soluble fibrinogen into an insoluble fibrin clot. Structural and functional details of these pro-coagulant proteins from snake venoms have been recently reviewed [12–15].
Snake venom toxins that prolong blood coagulation are proteins or glycoproteins with molecular masses ranging from 6 kDa to 350 kDa. These factors inhibit blood coagulation by different mechanisms. Some of these anticoagulant proteins exhibit enzymatic activities, such as PLA2 (phospholipase A2) and proteinase, whereas others do not exhibit any enzymatic activity. The mechanism of anticoagulant activity of only a few of these proteins is well understood. Further research is required to delineate the structure–function relationships and mechanism of a number of new anticoagulant proteins. Studies on such anticoagulants contribute to our understanding of ‘vulnerable’ sites in the coagulation cascade. Thus these studies help us to design novel strategies to develop anticoagulant therapeutic agents. Earlier reviews, dealing with snake venom proteins affecting thrombosis and haemostasis, have only marginally dealt with anticoagulant proteins [3–8,12]. This review attempts to provide an overview of the current understanding of the structure, function and mechanism of anticoagulant proteins (Table 1).
Table 1. Anticoagulant proteins from snake venom.
| Protein | Mechanism of action | Remarks | |
|---|---|---|---|
| Enzymatic anticoagulant proteins | |||
| 1. Phospholipase A2 enzymes | Strongly anticoagulant | Inhibits activation of FX to FXa by extrinsic tenase complex | By both enzymatic and non-enzymatic mechanisms; target protein not known |
| Inhibits activation of prothrombin to thrombin by prothrombinase complex | By non-enzymatic mechanism; binds to FXa and interferes in the prothrombinase complex formation | ||
| Weakly anticoagulant | Inhibits activation of FX to FXa by extrinsic tenase complex | By non-enzymatic mechanism through hydrolysis of phospholipids | |
| 2. Metalloproteinases (α-fibrinogenase) | Weaker soft clot formation due to physical destruction of fibrinogen | By cleaving Aα-chain of fibrinogen | |
| 3. Serine proteinases | Protein C activators | Inactivation of cofactors FVa and FVIIIa degradation | Directly activate protein C |
| Thrombin-like enzymes | Deplete fibrinogen in the plasma | Releases either fibrinopeptide A or B; fibrin clots are removed leading to depletion of fibrinogen content | |
| Fibrinogenases | Physical destruction of fibrinogen | Mechanism not known | |
| 4. L-Amino acid oxidase | Inhibits FIX activity | Mechanism not known | |
| Non-enzymatic anticoagulant proteins | |||
| 1. C-type lectin related proteins | FX and FIX binding proteins | Inhibit the formation of coagulation complexes | Bind to the Gla domain of FIX and FX, and interfere in their binding to phospholipids |
| Bothrojaracin, bothroalternin | Inhibit the activity of thrombin | Bind to α-thrombin at both exosite-1 and exosite-2 | |
| 2. Three-finger toxin | Cardiotoxins from Naja nigricollis crawshawii venom | Act on the extrinsic pathway of the clotting cascade | Mechanism not known |
| Hemextin A and hemextin AB complex from Hemachatus haemachatus venom | Prevents clot initiation by inhibiting extrinsic tenase activity | Specifically binds to FVIIa |
ANTICOAGULANT PROTEINS WITH ENZYMATIC ACTIVITY
Several proteins with enzymatic activity, such as PLA2 and proteinases, inhibit blood coagulation. Some of them inhibit clot formation by the physical destruction of a factor that contributes directly to the coagulation. In these cases, the mechanisms appear to be simple and are directly dependent on the respective enzymatic activity. The study of such factors, in general, may not significantly contribute to our understanding of blood coagulation. However, at times, a careful examination of their mechanisms may be not only important, but also essential. For example, conventional wisdom suggests that PLA2 enzymes exert their anticoagulant effects by the hydrolysis and physical destruction of the membrane surface required for the formation of coagulation complexes. Interestingly, the anticoagulant activity of certain PLA2 enzymes is due to their interaction with blood coagulation proteins and not phospholipid hydrolysis (for details, see below). Thus non-enzymatic mechanisms of these enzymatic proteins cannot be ignored.
PLA2 enzymes
PLA2 enzymes are esterolytic enzymes which hydrolyse glycerophospholipids at the sn−2 position of the glycerol backbone releasing lysophospholipids and fatty acids. Snake venoms are rich sources of PLA2 enzymes. Several hundred snake venom PLA2 enzymes have been purified and characterized. Amino acid sequences of over 280 PLA2 enzymes have been determined [16,17]. (A database is available at http://sdmc.lit.org.sg/Templar/DB/snaketoxin_PLA2/index.html.) They are approx. 13 kDa proteins and contain 116–124 amino acid residues and six or seven disulphide bonds. They are rarely glycosylated. So far, three-dimensional structures of more than 30 PLA2 enzymes have been determined (for a comprehensive list, see [18]). The structural data indicate that snake venom PLA2 enzymes share strong structural similarity to mammalian pancreatic as well as secretory PLA2 enzymes. They have a core of three α-helices, a distinctive backbone loop that binds catalytically important calcium ions, and a β-wing that consists of a single loop of antiparallel β-sheet. The C-terminal segment forms a semicircular ‘banister’, particularly in viperid and crotalid PLA2 enzymes, around the Ca2+-binding loop. In addition, they have a similar catalytic function in hydrolysing phospholipids at the sn−2 position. However, in contrast with mammalian PLA2 enzymes, many snake venom PLA2 enzymes are toxic and induce a wide spectrum of pharmacological effects [19–21]. These include neurotoxic, cardiotoxic, myotoxic, haemolytic, convulsive, anticoagulant, antiplatelet, oedema-inducing and tissue-damaging effects. Thus PLA2 enzymes also form a family of snake venom toxins, which share a common structural fold but exhibit multiple functions. These factors make the structure–function relationships and the mechanisms of action intriguing, and pose exciting challenges to scientists.
Some snake venom PLA2 enzymes inhibit blood coagulation [22–25]. Boffa and colleagues [22,23] studied the anticoagulant properties of a number of PLA2 enzymes and classified them into strongly, weakly and non-anticoagulant enzymes. Strongly anticoagulant PLA2 enzymes inhibit blood coagulation at concentrations below 2 μg/ml. Weakly anticoagulant PLA2 enzymes show effects between 3 and 10 μg/ml. A number of venom PLA2 enzymes do not prolong the clotting times significantly even at 15 μg/ml. Thus the anticoagulant activity of different PLA2 enzymes varies significantly. Evans et al. [25] purified three anticoagulant proteins (CM-I, CM-II and CM-IV) from Naja nigricollis (black-necked spitting cobra) venom and showed their identity with PLA2 enzymes. CM-IV shows at least 100-fold more potent anticoagulant activity than CM-I and CM-II [26]. On the basis of their anticoagulant properties, they were classified as strongly (CM-IV) and weakly (CM-I, CMII) anticoagulant PLA2 enzymes respectively. Since phospholipids play a crucial role in the formation of several coagulation complexes, intuitively one might anticipate that the destruction of phospholipid surface would be the primary mechanism to account for anticoagulant effects of PLA2 enzymes. However, strongly anticoagulant PLA2 enzymes also affect blood coagulation by mechanisms that are independent of phospholipid hydrolysis (see below).
To explain the functional specificity and mechanism of induction of various pharmacological effects, the target model was proposed [21,27,28]. Accordingly, the susceptibility of a tissue to a particular PLA2 enzyme is due to the presence of specific ‘target sites’ on the surface of target cells or tissues. These target sites are recognized by specific ‘pharmacological sites’ on the PLA2 molecule that are complementary to ‘target sites’ in terms of charges, hydrophobicity and van der Waals contact surfaces [21,27,28]. Proteins (or glycoproteins) could act as specific target sites for PLA2 enzymes. The affinity between PLA2 and its target protein is in the low nanomolar range, whereas the binding between PLA2 and phospholipids is in the high micromolar range. Such a four to six orders of magnitude difference in affinity between the protein–protein interaction and the protein–phospholipid interaction explains why the interaction of PLA2 and its target protein governs the pharmacological specificity [27,28].
The target proteins such as membrane-bound receptors/acceptors are identified through studies using radiolabelled PLA2 enzymes and specific binding studies, as well as photoaffinity labelling techniques (for details, see [29]). Anticoagulant PLA2 enzymes, on the other hand, target one or more soluble proteins or their complexes in the coagulation cascade. Furthermore, the enzymes may interact with the active, but not the zymogen, form of the coagulation factor. Therefore different strategies have been used to identify the soluble target protein in order to understand the mechanism of anticoagulant effects of PLA2 enzymes.
Mechanism of anticoagulant effects
A simple ‘dissection approach’ was used to identify the specific stage of the coagulation cascade that is inhibited by anticoagulant PLA2 enzymes (for details, see [30,31]). In this approach, the effects of an anticoagulant on three commonly used clotting time assays, namely prothrombin time, Stypven (Russell viper venom) time and thrombin time, were studied to identify the stage in the extrinsic coagulation cascade. The anticoagulant will prolong clotting times when the cascade is initiated ‘upstream’ of the inhibited step, whereas it will not affect the clotting times when the cascade is initiated ‘downstream’ of the inhibited step. Since the above clotting assays specifically initiate the coagulation cascade at three different stages, it is easier to pinpoint the specific step(s) that is (are) inhibited by the anticoagulant (for details, see [18,30,31]). Using this strategy as well as the inhibition studies of specific reconstituted complexes, it was shown that the extrinsic tenase [TF–FVIIa (tissue factor–Factor VIIa)] complex is inhibited by all three anticoagulant PLA2 enzymes from N. nigricollis venom (i.e. CM-I, CM-II and CM-IV), whereas the prothrombinase complex is inhibited only by CM-IV. Thus the strongly anticoagulant enzyme CM-IV inhibits both the extrinsic tenase and prothrombinase complexes, creating two ‘bottlenecks’ in the coagulation cascade, whereas the weakly anticoagulant enzymes CM-I and CM-II only inhibit the extrinsic tenase complex, and create only a single bottleneck [30].
The prothrombinase complex is inhibited by the strongly anticoagulant PLA2 enzyme CM-IV via a non-enzymatic mechanism [32,33]. The inhibition of this complex does not increase with an increase in incubation time with CM-IV. In contrast, weakly anticoagulant CM-I and CM-II fail to inhibit the prothrombinase complex even after 30 min incubation [32,33]. Despite the complete hydrolysis of phospholipids by CM-I and CM-II, thrombin formation is not significantly reduced. Thus the inhibition of the prothrombinase complex is independent of phospholipid hydrolysis. Alkylation of the active-site residue His48 in CM-IV results in the loss of enzymatic activity, but it retains more than 60% of the inhibition of the prothrombinase complex. Furthermore, CM-IV inhibits thrombin formation more strongly in the absence of phospholipids than in their presence (for details, see [33]). Studies of inhibition kinetics indicate that CM-IV is a non-competitive inhibitor [33]. Thus CM-IV neither competes with prothrombin to bind to the active site of the prothrombinase complex nor binds to prothrombin. However, CM-IV competes with FVa (Factor Va) and interferes with complex formation [32,34]. The addition of increasing amounts of FVa to the mixture reverses the inhibition, suggesting that CM-IV may compete with FVa for binding to FXa (Factor Xa) [34]. Direct binding studies using isothermal calorimetry showed that CM-IV forms a 1:1 complex with FXa (Kd 500 nM) and blocks the formation of the prothrombinase complex [34]. Thus the strongly anticoagulant PLA2 enzyme CM-IV inhibits the key step in blood coagulation by a novel non-enzymatic mechanism, and FXa is the target protein for the anticoagulant activity of this enzyme [32,34].
All three PLA2 enzymes from N. nigricollis venom inhibit the extrinsic tenase (TF–FVIIa) complex [35]. Interestingly, the strongly anticoagulant enzyme CM-IV inhibits the tenase by both enzymatic and non-enzymatic mechanisms. On the other hand, weakly anticoagulant CM-I and CM-II inhibit the complex mostly by their enzymatic activity (for details, see [35]). Further studies are needed to clarify the non-enzymatic mechanism of inhibition by CM-IV.
Structure–function relationships
PLA2 enzymes share similar protein folds and three-dimensional structures, but exhibit diverse biological properties. Thus understanding their structure–function relationships and identifying their functional sites is a subtle, complicated and challenging task. Using a combination of theoretical and experimental approaches, we and others have successfully identified some of the functional sites in PLA2 enzymes (for references and details, see [21,27,28]).
The anticoagulant region was identified by a systematic and direct comparison of the amino acid sequences of strongly, weakly and non-anticoagulant enzymes [26]. To minimize phylogenetic and structural parameters, we initially restricted the sequence comparison to seven PLA2 enzymes isolated from venoms of the genus Naja with different anticoagulant potencies, and then extended this to others. The region between residues 54 and 77 is positively charged in strongly anticoagulant PLA2 enzymes, but negatively charged in weakly and non-anticoagulant enzymes. At both ends of the region is a pair of lysine residues that are replaced by neutral or negatively charged amino acid residues [26]. This region is located on the surface and is accessible for interaction (Figure 1A). The modification of lysine residues by carbamoylation [36], ethoxyformylation or guanidination [37] affects the anticoagulant properties of the basic PLA2 of N. nigricollis venom. Neutralization of the positive charges of lysines by carbamoylation resulted in almost complete loss of anticoagulant activity, whereas the enzymatic activity was not affected significantly [36]. In contrast, guanidination of lysine residues, which leads to a retention of the positive charges, reduced the anticoagulant potency of the enzyme by only 50% [37]. This supports the essential nature of the positive charges in the proposed anticoagulant site.
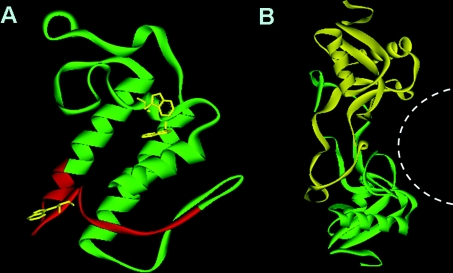
Figure 1. Structure–function relationships of anticoagulant proteins.
(A) The predicted anticoagulant region of anticoagulant PLA2 enzymes [26]. The ribbon model of N. naja PLA2 was generated from the PDB file (#1POA) [171]. The predicted anticoagulant region (highlighted in red) is fully exposed on the surface and easily accessible for interaction. Tryptophan residues are shown in yellow. (B) The binding site of FIX/FX-binding protein. The ribbon model was generated from the PDB file (#1IXX) [99]. The concave central region formed through domain swapping between the two subunits is the binding site for the Gla domain [108,109], and is indicated by the white broken line).
Strongly anticoagulant PLA2 enzymes exhibit high penetrating ability, whereas non-anticoagulant ones show weak penetrating ability [23,24]. Furthermore, strongly anticoagulant PLA2 enzymes bind to phospholipid vesicles with a significant increase in their intrinsic fluorescence, whereas poor anticoagulants show little or no effect [26,38]. The changes in the fluorescence were correlated to the microenvironment of Trp68 in the anticoagulant region (for details, see [26]). On the basis of a comparison of three-dimensional structures of class II PLA2 enzymes, three independent groups of researchers supported the predicted anticoagulant site [39–41]. This region shows conformational similarity, and the presence of a positively charged residue free for intermolecular interactions at the corner of the molecule corresponding to the stretch of residues at positions 55 to 67 seems to be a common feature of most of the anticoagulant PLA2 enzymes.
As mentioned above, the strongly anticoagulant PLA2 enzyme interferes with the activities of prothrombinase and extrinsic tenase complexes [33,35]. Therefore the predicted anticoagulant region of CM-IV was compared with the amino acid sequences of bovine FX, FV, prothrombin and TF. No homology was detected between the anticoagulant region of CM-IV and that of either prothrombin or FX. However, the anticoagulant region showed partial homology with a region of the light chain of FVa and with a section of TF [28,32]. This site in the light chain of FVa is closer to the site that binds to activated protein C [42]. The fact that binding of FXa to the light chain of FVa protects FVa from subsequent cleavage of the heavy chain by activated protein C [43] could be explained by the potential steric hindrance that arises when they compete for binding to two separate sites that are close to each other. The second half of the predicted anticoagulant region shares similarity with TF [28,32,44]. Thus it appears that there are two separate sites in the predicted anticoagulant region, each of the sites sharing similarity with FVa and TF respectively. We hypothesized that these sites are responsible for targeting to the prothrombinase and extrinsic tenase complexes respectively [44].
The predicted anticoagulant region is strongly supported by site-directed mutagenesis studies [45] as well as using synthetic peptides [46]. Insertion of a positive charge into porcine PLA2 (a D59R/S60G mutant) increased its ability to inhibit the prothrombinase complex, whereas a K56Q mutant of HsPLA2 (human secretory PLA2) lost 3-fold activity [45]. However, the affinity of HsPLA2 for FXa upon mutation of the residues Lys53, Arg54, Lys57 and Arg58 to negatively charged residues did not change significantly [47]. In contrast, mutations in the clusters of basic residues located on the interface binding site led to the loss of anti-prothrombinase activity of HsPLA2. Thus the authors proposed that several clusters of basic residues probably play an important role in the electrostatic interaction of HsPLA2 with FXa [47]. Recent studies using site-directed mutagenesis have also shown that the basic residues in the C-terminal tail and the β-wing of ammodytoxin A are responsible for its binding to FXa and the inhibition of the prothrombinase complex [48].
Metalloproteinases
Snake venom metalloproteinases are endoproteolytic enzymes. Their catalytic activity is dependent on Zn2+ ions. On the basis of size and domain structure characteristics, they are classified into P-I, P-II, P-III and P-IV classes [49,50]. P-I proteinases contain only a metalloproteinase domain, P-II proteinases contain metalloproteinase and disintegrin domains, P-III proteinases contain metalloproteinase, disintegrin-like and cysteine-rich domains, and P-IV proteinases contain the P-III domain structure plus lectin-like domains connected by disulphide bonds. To date, the sequences of over 40 metalloproteinases from snake venoms have been determined [50]. Six crystal structures of snake venom metalloproteinases are available, but all of them are from the P-I class. They are structurally similar to elastases and matrix metalloproteinases. They have a central core of a five-stranded β-sheet mixed with α-helices. There is a characteristic methionine-turn structure between the αD and αE helices. The structure is organized as an upper and lower domain with the substrate-binding cleft running between them. In addition to their role in the digestion of prey, they exhibit several biological effects, including haemorrhagic, pro-coagulant, anticoagulant and antiplatelet effects [50].
Some of the snake venom metalloproteinases inhibit blood coagulation. Most metalloproteinases are fibrinogenases and they release peptides from the C-terminal of fibrinogen. They are classified into α- and β-fibrinogenases on the basis of their specificity for the Aα or Bβ chain of fibrinogen [51]. α-Fibrinogenases inhibit blood coagulation, because truncated fibrinogen does not form as strong a fibrin clot as the native fibrinogen. Thus the subtle physical destruction leads to the anticoagulant action of metalloproteinases. The structure–function relationships of these metalloproteinases with respect to their anticoagulant effects have not been studied yet.
Serine proteinases
Snake venom serine proteinases, in addition to their contribution to the digestion of prey, affect various physiological functions. They affect platelet aggregation, blood coagulation, fibrinolysis, the complement system, blood pressure and the nervous system [6–9,12–15,52]. Among the serine proteinases, only protein C activators exhibit direct anticoagulant effects. Physiologically, the zymogen of protein C circulating in the blood is activated by thrombin. This activated protein C degrades FV/FVa and FVIII/FVIIIa, and releases a tissue-type plasminogen activator. It also stimulates fibrinolysis through its interaction with plasminogen activator inhibitor [53–55]. Venoms from snake species belonging to the genus Agkistrodon [copperhead snakes: A. contortrix contortrix (southern copperhead), A. contortrix mokasen (northern copperhead), A. contortrix pictigaster (Trans-Pecos copperhead), A. piscivorus (cottonmouth), A. piscivorus leucostoma (western cottonmouth), A. halys halys (Siberian moccasin), A. blomhoffi ussuriensis (Ussurian mamushi) and A. bilineatus (cantil)] contain protein C activators. These are glycoproteins with a molecular mass of approx. 36–40 kDa. They activate protein C at low salt concentrations in the absence of Ca2+ ions. High salt concentrations and the presence of Ca2+ ions inhibit their ability to activate protein C [56–58]. So far, the amino acid sequence of only the protein C activator from A.c. contortrix venom has been determined [59]. They prolong clotting times [60,61] and thrombus formation in the arteriovenous shunt [62] in vivo. So far, no significant data are available on the structure–function relationships of this class of proteinases.
Another group of serine proteinases, namely TLEs (thrombin-like enzymes), deplete the fibrinogen and makes the plasma unclottable. They are widely distributed within several pit viper genera (Agkistrodon, Bothrops, Crotalus, Lachesis and Trimeresurus), as well as some true vipers (Bitis and Cerastes) and the colubrid, Dispholidus typus (for an inventory and reviews, see [63–65]). They are single-chain proteins or glycoproteins (for example, see [66]) with a molecular mass of 26–33 kDa. They share a high degree of sequence similarity among themselves (≈67%). However, they show less than 40% similarity to human thrombin. They preferentially release either fibrinopeptide A or B, although rarely both with equal efficiency, unlike thrombin [64,67]. Classical low-molecular-mass serine proteinase inhibitors inhibit them, but most are not inhibited by thrombin inhibitors like antithrombin III and hirudin [4,64,67]. They act on blood plasma and induce friable and translucent clots, presumably due to lack of cross-linking of fibrin by FXIIIa. They often also act on the coagulation factor FXIII, but appear to degrade rather than activate it [4]. Unlike thrombin, they do not activate other coagulation factors [67]. Thus, although TLEs ‘resemble’ thrombin to an extent, they are structurally and functionally dissimilar to the coagulation factor [4,14,15,64]. Furthermore, flavoxobin, a TLE from Trimeresurus flavoviridis (Habu snake) venom, activates complement C3 protein and acts as a heterologous C3 convertase [68]. These unique properties enable their clinical use as defibrinogenating agents; for example, ancrod [Arvin®; from Calloselasma rhodostoma (the Malayan pit viper)] and batroxobin [Defibrase®; from Bothrops moojeni (the Brazilian lancehead snake)] (reviewed in [69,70]). Since the fibrin formed is not cross-linked, it is readily degraded by the fibrinolytic system.
Two anticoagulant serine fibrinogenases from Vipera lebetina (blunt-nosed viper) venom have been characterized [71]. One is a basic (pI>10) α-fibrinogenase, whereas the other is an acidic (pI<3) β-fibrinogenase [72–74]. Both enzymes are structurally similar to other snake venom serine proteinases [71]. They have the catalytic triad, and, in both enzymes, Asp189, which is located in the bottom of the primary specificity pocket, is replaced by Gly189. (For more details on proteinases affecting thrombosis and haemostasis, their structure and properties, see [52,74–76].)
L-Amino acid oxidases
L-Amino acid oxidases catalyse the oxidative deamination of a number of L-amino acids and generate hydrogen peroxide (H2O2). It is widely known that these enzymes affect haemostasis by modulating platelet function [77–80]. Recently, Sakurai et al. [81] showed that L-amino acid oxidase purified from Agkistrodon halys blomhoffii exhibits anticoagulant activity. This enzyme affects only the intrinsic pathway, having little effect on the extrinsic pathway. Furthermore, they showed that it selectively inhibits FIX activity. H2O2 production does not appear to be involved in the inactivation. Interestingly, L-amino acid oxidase does not bind or interact directly with FIX, as shown by surface plasmon resonance [81]. Further studies are needed to clarify the mechanism of inactivation.
NON-ENZYMATIC ANTICOAGULANT PROTEINS
Several snake venom proteins with no ‘detectable’ (known or tested) enzymatic activity inhibit blood coagulation. A number of non-enzymatic anticoagulant proteins have been purified and characterized. These proteins inhibit the coagulation process through their direct interaction with a specific coagulation factor. The mechanisms appear to be simple, and these proteins interfere in either complex formation or inhibit the activity of one of the proteinases. The study of such factors significantly contributes to our understanding of blood coagulation. Furthermore, the structure–function relationships of these proteins and identification of the functional sites may be useful in the development of new anticoagulant agents.
C-type lectin-related proteins
C-type lectins are homodimers and possess the ability to agglutinate red blood cells through their interaction with carbohydrate moieties. C-type lectin-related proteins, on the other hand, are heterodimers or oligomeric complexes of heterodimers and do not possess lectin-like activity [82–84]. At times, they are also found in the snake venom as a complex with metalloproteinases. C-type lectin-related proteins form the integral part of pro-coagulant proteins, such as FX activator from Daboia russelli (Russell's viper; formerly Vipera russelli) venom and prothrombin activators from Echis carinatus (saw-scaled viper) and Echis multisquamatus (Central Asian sand viper) venoms [85–88]. In all these cases, C-type lectin-related subunits act as regulatory subunits and are involved in determining the substrate specificity in the presence of Ca2+ ions (for details, see [89]).
FX and FIX-binding proteins
Anticoagulant C-type lectin-related proteins were among the first non-enzymatic proteins to be isolated and characterized from snake venoms. They were first isolated and purified from Deinagkistrodon acutus (hundred-pace pit viper; formerly Agkistrodon acutus) and Trimeresurus stejneri (Stejneger's bamboo viper; formerly, Trimeresurus gramineus) venoms [90,91]. They showed that these anticoagulant proteins inhibit prothrombin activation by non-enzymatic mechanisms [92,93]. However, these studies were not followed by detailed studies on their structure and mechanism of action. Atoda and Morita [94] purified an anticoagulant protein from T. flavoviridis venom using a FX-affinity column. This protein binds to FX/FXa as well as to FIX/FIXa. This anticoagulant was shown to be the first C-type lectin-related protein on the basis of its amino acid sequence and disulphide linkages [95,96]. Subsequently, they also purified and characterized a specific FIX-binding protein and FX-binding protein from T. flavoviridis and D. acutus venoms respectively [97,98]. These are heterodimeric proteins with α- and β-chains. Both chains share the common structural scaffold of C-type lectin [99,100]. The core structure is similar to the carbohydrate-recognition domain of mannose-binding protein, a C-type lectin [101,102]. A distinctive structural feature among C-type lectin-related proteins [99,100,103–106] is that the central loop of the individual subunit extends away from the core structure and forms a large open loop. This central loop forms the dimeric interface through domain swapping; a domain from the α subunit replaces essentially an identical domain in the β subunit. At the same time, this domain from the β subunit is swapped for the same domain in the α subunit. This is the first demonstrated example of three-dimensional swapping in the central region, whereas all other domain swapping occurs at the N- or C-terminus [107]. Furthermore, domain swapping is found mostly in the formation of homodimers or homo-oligomers, but not in the formation of heterodimers, as in the case of C-type lectin-related proteins [99,100,103–106]. This swapped dimeric interface, along with core structures of the α and β subunits, forms the concave ligand-binding site (see below).
The snake venom anticoagulant C-type lectin-related proteins inhibit the activity of the coagulation factors FIX and FX [96–98]. They bind to these coagulation factors with nanomolar and subnanomolar affinities. The Gla (γ-carboxyglutamic acid) domain peptides of FX (comprising residues 1–44 and 1–41) bind to FX-binding protein in the presence of Ca2+ with apparent dissociation constants of 1.0 and 100 nM respectively [108]. Thus most of the interaction occurs through the Gla domain. Interestingly, although FIX/FX-binding protein interacts with both FIX and FX, it has a low affinity for FX Gla domain peptides but binds to the Gla peptide of FIX-(1–46) [109]. The three-dimensional structures of the complexes [108,109] show that the Gla domains bind to the concave ligand-binding site between the two subunits (Figure 1B). The FX Gla domain has eight bound Ca2+ ions [108]. One of the Ca2+ ions participates in the binding interface between the Gla domain and the FX-binding protein. There are nine salt-bridges between the negatively charged Gla domain and the positively charged FX-binding protein, and 21 water molecules form an extensive network of hydrogen-bonds between the α-chain and the Gla domain [108]. Phe4, Leu5 and Val8 in the N-terminal loop of the Gla domain interact with Arg112, Met113 and Ile114 of the β-chain. Thus salt-bridges along with hydrophobic interactions and hydrogen bonds stabilize the complex between the Gla domain and the FX-binding protein (for details, see [108]). This binding interferes in the Ca2+-dependent binding of FIX and FX to phospholipid membranes, and hence exhibits potent anticoagulant effects.
Bothrojaracin
A second group of snake venom anticoagulants belonging to C-type lectin-related proteins interact specifically with thrombin/prothrombin. They include bothrojaracin [110,111] and bothroalternin [112]. Bothrojaracin was purified from Bothrops jararaca venom as a component that inhibited thrombin-induced platelet aggregation [110], with IC50 values of 1–20 nM; it also inhibited binding of α-thrombin to fibrinogen with a Ki of 15 nM [110]. It, however, did not inhibit platelet aggregation induced by other agonists, such as collagen, platelet activation factor, arachidonic acid, ADP and cerestocytin, a TLE from Cerastes vipera (Sahara sand viper) venom. Bothrojaracin inhibited the binding of 125I-labelled α-thrombin to platelets [110]. To test whether this inhibition is due to either the direct interaction with thrombin receptor(s) on the platelets or thrombin itself, Zingali et al. [110] performed an elegant experiment. They incubated 10 nM bothrojaracin (sufficient to completely inhibit thrombin-induced aggregation) with platelets and removed the supernatant. The platelets that were resuspended in a bothrojaracin-free medium aggregate when stimulated by thrombin. These results indicated that bothrojaracin interacted with thrombin, and not thrombin receptor(s) on platelets. Furthermore, they showed that 125I-labelled bothrojaracin did not bind to platelets [110]. Bothrojaracin–thrombin complexes were identified on non-denaturing gel electrophoresis. Bothrojaracin inhibited thrombin effects on macromolecular substrates, but not on the small chromogenic substrate S-2238 [110]. It also neutralized the inhibition of amidolytic activity of thrombin by hirudin. From these results, Zingali et al. [110] concluded that it is an exosite inhibitor. The high-affinity binding of α-thrombin to immobilized bothrojaracin (Kd 0.6 nM) is inhibited by a C-terminal peptide (comprising residues 54–65) of hirudin, indicating that bothrojaracin binds to the exosite 1. On the other hand, γ-thrombin, in which exosite 1 is disrupted, binds only with a Kd of 0.3 μM [111]. As γ-thrombin still binds to bothrojaracin, Arocas et al. [111] examined its interaction at exosite 2 (the heparin-binding site). Bothrojaracin does not modify the rate of inhibition of α-thrombin by antithrombin in the absence of heparin. In contrast, it significantly lowers the rate of inhibition by antithrombin in the presence of heparin. Further, bothrojaracin does not bind to a heparin column, indicating that it interacts with thrombin at exosite 2 [111]. Data from solid-phase binding studies showed that γ-thrombin clearly supported the binding of bothrojaracin to exosite 2. Thus the high affinity of interaction is due to its ability to bind to both exosites 1 and 2 [111]. Structurally, it is a heterodimeric protein held together by an interchain disulphide bond. The A and B chains are similar to C-type lectin-related proteins [113]. More than one isoform of bothrojaracin has been identified in individual Bothrops jararaca venom [114–116]. It has been found in six Bothrops species [B. atrox (Barba Amarilla snake), B. cotiara (cotiara), B. jararacussu (jararacussu), B. moojeni and B. neuwiedi (jararaca pintada)] and in small amounts in Lachesis muta (bushmaster snake) venom, but not in Crotalus durissus terrificus (South American rattlesnake) venom [117]. In addition to its ability to inhibit thrombin directly, bothrojaracin exhibits its anticoagulant effects by inhibiting the feedback activation of coagulation factor FV [118] and activation of prothrombin to thrombin [119,120]. The inhibition of FV activation by thrombin is due to its ability to bind to exosite 1, as exosite 2 does not appear to play a direct role in FV recognition by thrombin [118]. In prothrombin, bothrojaracin binds to partially exposed anion-binding exosite (pro-exosite 1) [121]. By studying the effects of dithiothreitol and urea on subunit dissociation, unfolding and inactivation of bothrojaracin, Monteiro et al. [122] proposed a denaturation model for C-type lectin-related proteins. Overall, bothrojaracin is an excellent inhibitor of thrombin through its interaction with both the exosites, but not with the active site. Furthermore, it exhibits allosteric effects on the thrombin active site and provides a tool to study allosteric changes in thrombin [123].
Three-finger toxins
This is a family of non-enzymatic polypeptides containing 60–74 amino acid residues [124,125]. This family of proteins is found commonly in the venoms of elapids (cobras, kraits and mambas) and hydrophids (sea snakes). Recently, they have been found in colubrid venoms [126–130], but not those of vipers and crotalids (rattlesnakes) [131]. They contain four or five disulphide bridges, of which four are conserved in all the members [125]. Consequently, all proteins of this family show a similar pattern of protein folding: three β-stranded loops extending from a central core containing the four conserved disulphide bridges [132,133]. Because of this appearance, this family of proteins is called the three-finger toxin family. Despite the overall similarity in structure, at times they differ from each other in their biological activities. Members of this family include α-neurotoxins [133–135], κ-bungarotoxins [136], muscarinic toxins [137], fasciculins [138], calciseptine [139,140], cardiotoxins (cytotoxins) [124,141], dendroaspins [142] and anticoagulant proteins [143–145]. They exhibit such varied activities through interaction with different target protein receptors/acceptors, ion channels or phospholipids (for details, see [146]). Interestingly, several other non-venom proteins and polypeptides also belong to this superfamily of proteins [147–151]. Structure–function relationships of a number of these polypeptides have been well elucidated, and their functional sites are located on distinct surfaces (for details, see [146]).
Anticoagulant three-finger toxins
The anticoagulant and antiplatelet effects of three-finger toxins were first identified in cardiotoxins isolated from Naja nigricollis crawshawii (spitting cobra) venom [143,144]. The mechanism of antiplatelet action [152] and structure–function relationships of these cardiotoxins [153,154] have been well elucidated. We have recently initiated studies to characterize a number of three-finger toxins with anticoagulant effects (see below).
Hemextin AB complex
Recently, a novel anticoagulant complex was characterized from Hemachatus haemachatus venom [145]. It has two three-finger toxins, hemextin A and hemextin B, as subunits. Individually, hemextin A prolongs blood coagulation, but hemextin B does not show any effect on blood clotting. However, hemextin B forms a 1:1 complex and synergistically enhances the anticoagulant effects of hemextin A. The dissection approach [30,31] was used to identify the coagulation step that is (are) inhibited by hemextin AB complex. Hemextin A and hemextin AB complex prolong the prothrombin time, but not the Stypven or the thrombin time, and hence we proposed that they inhibit the extrinsic tenase complex [145]. Hemextin A inhibits the reconstituted extrinsic tenase (TF–FVIIa) complex. As expected, hemextin B by itself does not inhibit the complex, but through complex formation enhances the inhibitory effects of hemextin A. Hemextin AB complex non-competitively inhibits the TF–FVIIa complex with a Ki value of 50 nM [145]. Of the 12 serine proteinases tested, hemextin A and hemextin AB complex specifically inhibit FVIIa and its complexes. In addition, they mildly inhibit plasma kallikrein activity. Thus hemextin AB complex is a highly specific natural inhibitor of the initiation of blood coagulation. It is also the first anticoagulant complex isolated from snake venom [145].
Proteinase inhibitors
Several snake venoms contain a number of isoforms of serine proteinase inhibitors [155–158]. They contain 57–60 amino acid residues and three disulphide bridges, and belong to the Kunitz pancreatic trypsin-inhibitor family [156,157]. Some of the closely related polypeptides from snake venoms block potassium and calcium channels [159–162]. Overall three-dimensional structures of proteinase inhibitors and their isoforms are similar [163–166]. As all the proteinases in blood coagulation and fibrinolysis are serine proteinases, these group polypeptides were thought to be potential anticoagulants [155]. Textilinins from Pseudonaja textilis textilis (Australian common brown snake) venom are being investigated for their plasmin inhibitive and antifibrinolytic activities [167,168]. However, no specific inhibitors of proteinases in blood coagulation and fibrinolysis have been identified yet.
Recently, a new family of snake venom proteins, waprins, was isolated [169]. Members of this family contain 50–52 amino acid residues with four disulphide bridges. They show significant similarity to elafin (elastase inhibitor), and other whey acidic proteins. Nawaprin, the first member of this family, shows a structural fold similar to that of elafin [169]. Although cysteine residues are conserved, waprins differ from each other in their intercysteine segments. It would be interesting to study their ability to inhibit various proteinases in blood coagulation.
FUTURE PROSPECTS
Aberrations in normal blood coagulation functions can result in thrombotic disorders or haemorrhage. In thrombosis, largely unknown conditions promote the apparently spontaneous formation of clots large enough to block circulation. Formation of such blocks in the arteries supplying vital organs, such as the heart or brain, can cause myocardial infarction or stroke respectively. Thus a life-saving mechanism of blood coagulation becomes a potentially life-threatening disease mechanism. Several conditions, such as atherosclerosis, contribute significantly to promote the spontaneous initiation of clotting. Anticoagulants are pivotal for the prevention and treatment of thromboembolic disorders, and approx. 0.7% of the Western population receives oral anticoagulant treatment [170]. With the increasingly aging population throughout the world, more people will require antithrombotic therapies in the future. Thus various new anticoagulant and antiplatelet agents are being sought after. Proteins from snake venom affecting blood coagulation and platelet aggregation can provide us with new lead compounds to design novel therapeutic agents, providing new paradigms in the treatment of thromboembolic disorders. So far, tremendous progress has been made in understanding the structure–function relationships and mechanisms of a number of anticoagulant proteins from snake venoms. In recent years, several new anticoagulant proteins have been isolated from snake venoms. Further studies are needed to decipher the structure–function relationships and mechanisms of newly isolated anticoagulant proteins. Studies on these anticoagulant proteins have potential in identifying new drug leads.
Acknowledgments
I thank Dr Prakash Kumar for his constructive comments and Mr Yajnavalka Banerjee for his help in the preparation of this manuscript. This work was supported in part by the Biomedical Research Council, Agency for Science and Technology, Singapore.
References
- 1.Tu A. T. New York: Marcel Decker; 1991. Reptile Venoms and Toxins. [Google Scholar]
- 2.Harvey A. L. New York: Pergamon Press; 1991. Snake Toxins. [Google Scholar]
- 3.Pirkle H., Markland F. S., Jr . New York: Marcel Decker; 1987. Hemostasis and Animal Venoms. [Google Scholar]
- 4.Hutton R. A., Warrell D. A. Action of snake venom components on the haemostatic system. Blood Rev. 1993;7:176–189. doi: 10.1016/0268-960x(93)90004-n. [DOI] [PubMed] [Google Scholar]
- 5.Lu Q., Clemetson J. M., Clemetson K. J. Snake venoms and hemostasis. J. Thromb. Haemostasis. 2005;3:1791–1799. doi: 10.1111/j.1538-7836.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- 6.Markland F. S. Snake venoms and the hemostatic system. Toxicon. 1998;36:1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 7.Meier J., Stocker K. Effects of snake venoms on hemostasis. Crit. Rev. Toxicol. 1991;21:171–182. doi: 10.3109/10408449109089878. [DOI] [PubMed] [Google Scholar]
- 8.Braud S., Bon C., Wisner A. Snake venom proteins acting on hemostasis. Biochimie. 2000;82:851–859. doi: 10.1016/s0300-9084(00)01178-0. [DOI] [PubMed] [Google Scholar]
- 9.Kini R. M. Platelet aggregation and exogenous factors from animal sources. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004;4:301–325. doi: 10.2174/1568006043335835. [DOI] [PubMed] [Google Scholar]
- 10.Scarborough R. M., Naughton M. A., Teng W., Rose J. W., Phillips D. R., Nannizzi L., Arfsten A., Campbell A. M., Charo I. F. Design of potent and specific integrin antagonists. Peptide antagonists with high specificity for glycoprotein IIb-IIIa. J. Biol. Chem. 1993;268:1066–1073. [PubMed] [Google Scholar]
- 11.Phillips D. R., Scarborough R. M. Clinical pharmacology of eptifibatide. Am. J. Cardiol. 1997;80(suppl. 4A):11B–20B. doi: 10.1016/s0002-9149(97)00572-9. [DOI] [PubMed] [Google Scholar]
- 12.Kornalik F. The influence of snake venom proteins on blood coagulation. In: Harvey A. L., editor. Snake Toxins. New York: Pergamon Press; 1991. pp. 323–383. [Google Scholar]
- 13.Kini R. M., Rao V. S., Joseph J. S. Procoagulant proteins from snake venoms. Haemostasis. 2002;31:218–224. doi: 10.1159/000048066. [DOI] [PubMed] [Google Scholar]
- 14.Kini R. M., Joseph J. S., Rao V. S. Prothrombin activators from snake venoms. In: Mènez A., editor. Perspectives in Molecular Toxinology. Chichester: John Wiley; 2002. pp. 341–355. [Google Scholar]
- 15.Joseph J. S., Kini R. M. Snake venom prothrombin activators similar to blood coagulation factor Xa. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004;4:397–416. doi: 10.2174/1568006043335781. [DOI] [PubMed] [Google Scholar]
- 16.Danse J. M., Gasparini S., Ménez A. Molecular biology of snake venom phospholipases A2. In: Kini R. M., editor. Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism. Chichester: John Wiley; 1997. pp. 29–71. [Google Scholar]
- 17.Tan P. T. J., Khan M. A., Brusic V. Bioinformatics for venom and toxin sciences. Brief. Bioinform. 2003;4:53–62. doi: 10.1093/bib/4.1.53. [DOI] [PubMed] [Google Scholar]
- 18.Kini R. M. Structure–function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon. 2005;45:1147–1161. doi: 10.1016/j.toxicon.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Harris J. B. Phospholipases in snake venoms and their effects on nerve and muscle. Pharmacol. Ther. 1985;31:79–102. doi: 10.1016/0163-7258(85)90038-5. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg P. Phospholipases. In: Shier W. T., Mebs D., editors. Handbook of Toxinology. New York: Marcel Dekker; 1990. pp. 667–677. [Google Scholar]
- 21.Kini R. M. Phospholipase A2 – a complex multifunctional protein puzzle. In: Kini R. M., editor. Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism. Chichester: John Wiley; 1997. pp. 1–28. [Google Scholar]
- 22.Boffa M. C., Boffa G. A. A phospholipase A2 with anticoagulant activity. II. Inhibition of the phospholipid activity in coagulation. Biochim. Biophys. Acta. 1976;429:839–852. doi: 10.1016/0005-2744(76)90330-2. [DOI] [PubMed] [Google Scholar]
- 23.Verheij H. M., Boffa M. C., Rothen C., Bryckert M. C., Verger R., de Haas G. H. Correlation of enzymatic activity and anticoagulant properties of phospholipase A2. Eur. J. Biochem. 1980;112:25–32. doi: 10.1111/j.1432-1033.1980.tb04982.x. [DOI] [PubMed] [Google Scholar]
- 24.Boffa M. C., Rothen C., Verheij H. M., Verger R., de Haas G. H. Correlation of enzymatic activity and anticoagulant properties of phospholipase A2. In: Eaker D., Walstrom T., editors. Natural Toxins. Oxford: Pergamon Press; 1980. pp. 131–138. [DOI] [PubMed] [Google Scholar]
- 25.Evans H. J., Franson R., Qureshi G. D., Moo-Penn W. F. Isolation of anticoagulant proteins from cobra venom (Naja nigricollis). Identity with phospholipases A2. J. Biol. Chem. 1980;255:3793–3797. [PubMed] [Google Scholar]
- 26.Kini R. M., Evans H. J. Structure–function relationships of phospholipases: the anticoagulant region of phospholipases A2. J. Biol. Chem. 1987;262:14402–14407. [PubMed] [Google Scholar]
- 27.Kini R. M., Evans H. J. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon. 1989;27:613–635. doi: 10.1016/0041-0101(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 28.Kini R. M. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42:827–840. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Lambeau G., Cupillard L., Lazdunski M. Membrane receptors for venom phospholipases A2. In: Kini R. M., editor. Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism. Chichester: John Wiley; 1997. pp. 389–412. [Google Scholar]
- 30.Stefansson S., Kini R. M., Evans H. J. The inhibition of clotting complexes of the extrinsic coagulation cascade by the phospholipase A2 isoenzymes from Naja nigricollis venom. Thromb. Res. 1989;55:481–491. doi: 10.1016/0049-3848(89)90056-x. [DOI] [PubMed] [Google Scholar]
- 31.Kini R. M., Banerjee Y. Dissection approach: a simple strategy for identification of the step of action of anticoagulant agents in the blood coagulation cascade. J. Thromb. Haemostas. 2005;3:170–171. doi: 10.1111/j.1538-7836.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 32.Stefansson S. Ph.D. Thesis. Richmond, VA, U.S.A.: Virginia Commonwealth University; 1990. The mechanism of the anticoagulant effect of the basic phospholipase A2 from Naja nigricollis venom. [Google Scholar]
- 33.Stefansson S., Kini R. M., Evans H. J. The basic phospholipase A2 from Naja nigricollis venom inhibits the prothrombinase complex by a novel nonenzymatic mechanism. Biochemistry. 1990;29:7742–7746. doi: 10.1021/bi00485a024. [DOI] [PubMed] [Google Scholar]
- 34.Kerns R. T., Kini R. M., Stefansson S., Evans H. J. Targeting of venom phospholipases: the strongly anticoagulant phospholipase A2 from Naja nigricollis venom binds to coagulation factor Xa to inhibit the prothrombinase complex. Arch. Biochem. Biophys. 1999;369:107–113. doi: 10.1006/abbi.1999.1345. [DOI] [PubMed] [Google Scholar]
- 35.Kini R. M., Evans H. J. The role of enzymatic activity in inhibition of the extrinsic tenase complex by phospholipase A2 isoenzymes from Naja nigricollis venom. Toxicon. 1995;33:1585–1590. doi: 10.1016/0041-0101(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 36.Condrea E., Fletcher J. E., Rapuano B. E., Yang C.-C., Rosenberg P. Dissociation of enzymatic activity from lethality and pharmacological properties by carbamoylation of lysines in Naja nigricollis and Naja naja atra snake venom phospholipases A2. Toxicon. 1981;19:705–720. doi: 10.1016/0041-0101(81)90108-2. [DOI] [PubMed] [Google Scholar]
- 37.Condrea E., Rapuano B. E., Fletcher J. E., Yang C.-C., Rosenberg P. Ethoxyformylation and guanidination of snake venom phospholipases A2: effects on enzymatic activity, lethality and some pharmacological properties. Toxicon. 1983;21:209–218. doi: 10.1016/0041-0101(83)90005-3. [DOI] [PubMed] [Google Scholar]
- 38.Prigent-Dachary J., Boffa M. C., Boisseau M. R., Dufourcq J. Snake venom phospholipases A2. A fluorescent study of their binding to phospholipid vesicles. Correlation with their anticoagulant activities. J. Biol. Chem. 1980;255:7734–7739. [PubMed] [Google Scholar]
- 39.Carredano E., Westerlund B., Persson B., Saarinen M., Ramaswamy S., Eaker D., Eklund H. The three-dimensional structures of two toxins from snake venom throw light on the anticoagulant and neurotoxic sites of phospholipase A2. Toxicon. 1998;36:75–92. doi: 10.1016/s0041-0101(97)00051-2. [DOI] [PubMed] [Google Scholar]
- 40.Zhao K., Zhou Y., Lin Z. Structure of basic phospholipase A2 from Agkistrodon halys Pallas: implications for its association, hemolytic and anticoagulant activities. Toxicon. 2000;38:901–916. doi: 10.1016/s0041-0101(99)00193-2. [DOI] [PubMed] [Google Scholar]
- 41.Singh G., Gourinath S., Sharma S., Paramasivam M., Srinivasan A., Singh T. P. Sequence and crystal structure determination of a basic phospholipase A2 from common krait (Bungarus caeruleus) at 2.4 Å resolution: identification and characterization of its pharmacological sites. J. Mol. Biol. 2001;307:1049–1059. doi: 10.1006/jmbi.2001.4550. [DOI] [PubMed] [Google Scholar]
- 42.Walker F. J., Scandella D., Fay P. J. Identification of the binding site for activated protein C on the light chain of factors V and VIII. J. Biol. Chem. 1990;265:1484–1489. [PubMed] [Google Scholar]
- 43.Nesheim M. E., Canfield W. M., Kisiel W., Mann K. G. Studies of the capacity of factor Xa to protect factor Va from inactivation by activated protein C. J. Biol. Chem. 1982;257:1443–1447. [PubMed] [Google Scholar]
- 44.Evans H. J., Kini R. M. Anticoagulant effects of phospholipases. In: Kini R. M., editor. Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism. Chichester: John Wiley; 1997. pp. 353–368. [Google Scholar]
- 45.Inada M., Crowl R. M., Bekkers A. C., Verheij H., Weiss J. Determinants of the inhibitory action of purified 14-kDa phospholipases A2 on cell-free prothrombinase complex. J. Biol. Chem. 1994;269:26338–26343. [PubMed] [Google Scholar]
- 46.Mounier C. M., Hackeng T. M., Schaeffer F., Faure G., Bon C., Griffin J. H. Inhibition of prothrombinase by human secretory phospholipase A2 involves binding to factor Xa. J. Biol. Chem. 1998;273:23764–23772. doi: 10.1074/jbc.273.37.23764. [DOI] [PubMed] [Google Scholar]
- 47.Mounier C. M., Luchetta P., Lecut C., Koduri R. S., Faure G., Lambeau G., Valentin E., Singer A., Ghomashchi F., Beguin S., et al. Basic residues of human group IIA phospholipase A2 are important for binding to factor Xa and prothrombinase inhibition comparison with other mammalian secreted phospholipases A2. Eur. J. Biochem. 2000;267:4960–4969. doi: 10.1046/j.1432-1327.2000.01523.x. [DOI] [PubMed] [Google Scholar]
- 48.Prijatelj P., Charnay M., Ivanovski G., Jenko Z., Pungercar J., Krizaj I, Faure G. The C-terminal and β-wing regions of ammodytoxin A, a neurotoxic phospholipase A2 from Vipera ammodytes ammodytes, are critical for binding to factor Xa and for anticoagulant effect. Biochimie. 2006;88:69–76. doi: 10.1016/j.biochi.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Bjarnason J. B., Fox J. W. Snake venom metalloproteinases: reprolysins. Methods Enzymol. 1995;248:345–368. doi: 10.1016/0076-6879(95)48023-4. [DOI] [PubMed] [Google Scholar]
- 50.Fox J. W., Serrano S. M. T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005;45:969–985. doi: 10.1016/j.toxicon.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Ouyang C., Teng C. M. Fibrinogenolytic enzymes of Trimeresurus venom. Biochim. Biophys. Acta. 1976;420:298–308. doi: 10.1016/0005-2795(76)90321-4. [DOI] [PubMed] [Google Scholar]
- 52.Kini R. M. Serine proteases affecting blood coagulation and fibrinolysis from snake venoms. Pathophysiol. Haemostasis Thromb. 2005;34:200–204. doi: 10.1159/000092424. [DOI] [PubMed] [Google Scholar]
- 53.Fay W. P., Owen W. G. Platelet plasminogen activator inhibitor: purification and characterization of interaction with plasminogen activators and activated protein C. Biochemistry. 1989;28:5773–5778. doi: 10.1021/bi00440a011. [DOI] [PubMed] [Google Scholar]
- 54.Moniwa N. Relationship of urokinase type plasminogen activator, plasminogen activator inhibitor type 1 and activated protein C in fibrinolysis of human placenta. Pol. J. Pharmacol. 1996;48:215–220. [PubMed] [Google Scholar]
- 55.Sakamoto T., Ogawa H., Takazoe K., Yoshimura M., Shimomura H., Moriyama Y., Arai H., Okajima K. Effect of activated protein C on plasma plasminogen activator inhibitor activity in patients with acute myocardial infarction treated with alteplase: comparison with unfractionated heparin. J. Am. Coll. Cardiol. 2003;42:1389–1394. doi: 10.1016/s0735-1097(03)01059-3. [DOI] [PubMed] [Google Scholar]
- 56.Klein J. D., Walker F. J. Purification of a protein C activator from the venom of the southern copperhead snake (Agkistrodon contortrix contortrix) Biochemistry. 1986;25:4175–4179. doi: 10.1021/bi00363a001. [DOI] [PubMed] [Google Scholar]
- 57.Kisiel W., Kondo S., Smith K. J., McMullen B. A., Smith L. F. Characterization of a protein C activator from Agkistrodon contortrix contortrix venom. J. Biol. Chem. 1987;262:12607–12613. [PubMed] [Google Scholar]
- 58.Bakker H. M., Tans G., Yukelson L. Y., Janssen-Claessen T. W., Bertina R. M., Hemker H. C., Rosing J. Protein C activation by an activator purified from the venom of Agkistrodon halys halys. Blood Coagulation Fibrinolysis. 1993;4:605–614. doi: 10.1097/00001721-199308000-00012. [DOI] [PubMed] [Google Scholar]
- 59.McMullen B. A., Fujikawa K., Kisiel W. Primary structure of a protein C activator from Agkistrodon contortrix contortrix venom. Biochemistry. 1989;28:674–679. doi: 10.1021/bi00428a039. [DOI] [PubMed] [Google Scholar]
- 60.Stocker K., Fischer H., Meier J., Brogli M., Svendsen L. Protein C activators in snake venoms. Behring Inst. Mitt. 1986;79:37–47. [PubMed] [Google Scholar]
- 61.Stocker K., Fischer H., Meier J., Brogli M., Svendsen L. Characterization of the protein C activator Protac from the venom of the southern copperhead (Agkistrodon contortrix) snake. Toxicon. 1987;25:239–252. doi: 10.1016/0041-0101(87)90253-4. [DOI] [PubMed] [Google Scholar]
- 62.Kogan A. E., Bashkov G. V., Bobruskin I. D., Romanova E. P., Makarov V. A., Strukova S. M. Protein C activator from the venom of Agkistrodon blomhoffi ussuriensis retards thrombus formation in the arterio-venous shunt in rats. Thromb. Res. 1993;70:385–393. doi: 10.1016/0049-3848(93)90080-8. [DOI] [PubMed] [Google Scholar]
- 63.Pirkle H., Stocker K. Thrombin-like enzymes from snake venoms; an inventory. Thromb. Haemostasis. 1991;65:444–450. [PubMed] [Google Scholar]
- 64.Bell W. R., Jr Defibrinogenating enzymes. Drugs. 1997;54(suppl. 3):18–30. doi: 10.2165/00003495-199700543-00005. [DOI] [PubMed] [Google Scholar]
- 65.Pirkle H., Theodor I. Thrombin-like enzymes. In: Bailey G. S., editor. Enzymes from Snake Venom. Fort Collins, CO: Alaken Inc.; 1998. pp. 39–69. [Google Scholar]
- 66.Au L. C., Lin S. B., Chou J. S., Teh G. W., Chang K. J., Shih C. M. Molecular cloning and sequence analysis of the cDNA for ancrod, a thrombin-like enzyme from the venom of Calloselasma rhodostoma. Biochem. J. 1993;294:387–390. doi: 10.1042/bj2940387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aronson D. L. Comparison of the actions of thrombin and the thrombin-like venom enzymes ancrod and batroxobin. Thromb. Haemostasis. 1976;36:1–13. [PubMed] [Google Scholar]
- 68.Yamamoto C., Tsuru D., Oda-Ueda N., Ohno M., Hattori S., Kim S. T. Flavoxobin, a serine protease from Trimeresurus flavoviridis (habu snake) venom, independently cleaves Arg726–Ser727 of human C3 and acts as a novel, heterologous C3 convertase. Immunology. 2002;107:111–117. doi: 10.1046/j.1365-2567.2002.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stocker K., Barlow G. H. The coagulant enzyme from Bothrops atrox venom (batroxobin) Methods Enzymol. 1976;45:214–223. doi: 10.1016/s0076-6879(76)45021-8. [DOI] [PubMed] [Google Scholar]
- 70.Stocker K. Research, diagnostic and medicinal uses of snake venom enzymes. In: Bailey G. S., editor. Enzymes from Snake Venom. Fort Collins, CO: Alaken Inc.; 1998. pp. 705–736. [Google Scholar]
- 71.Siigur E., Aaspollu A., Siigur J. Anticoagulant serine fibrinogenases from Vipera lebetina venom: structure–function relationships. Thromb. Haemostasis. 2003;89:826–831. [PubMed] [Google Scholar]
- 72.Siigur E., Mahar A., Siigur J. β-Fibrinogenase from the venom of Vipera lebetina. Toxicon. 1991;29:107–118. doi: 10.1016/0041-0101(91)90043-q. [DOI] [PubMed] [Google Scholar]
- 73.Mahar A., Siigur E., Siigur J. Purification and properties of a proteinase from Vipera lebetina (snake) venom. Biochim. Biophys. Acta. 1987;925:272–281. doi: 10.1016/0304-4165(87)90192-9. [DOI] [PubMed] [Google Scholar]
- 74.Samuel M., Subbi J., Siigur E., Siigur J. Biochemical characterization of fibrinogenolytic serine proteinases from Vipera lebetina snake venom. Toxicon. 2002;40:51–54. doi: 10.1016/s0041-0101(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 75.Matsui T., Fujimura Y., Titani K. Snake venom proteases affecting hemostasis and thrombosis. Biochim. Biophys. Acta. 2000;1477:146–156. doi: 10.1016/s0167-4838(99)00268-x. [DOI] [PubMed] [Google Scholar]
- 76.Serrano S. M., Maroun R. C. Snake venom serine proteinases: sequence homology vs. substrate specificity, a paradox to be solved. Toxicon. 2005;45:1115–1132. doi: 10.1016/j.toxicon.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 77.Nathan I., Dvilansky A., Yirmiyahu T., Aharon M., Livne A. Impairment of platelet aggregation by Echis colorata venom mediated by L-amino acid oxidase or H2O2. Thromb. Haemostasis. 1982;48:277–282. [PubMed] [Google Scholar]
- 78.Li Z. Y., Yu T. F., Lian E. C. Purification and characterization of L-amino acid oxidase from king cobra (Ophiophagus hannah) venom and its effects on human platelet aggregation. Toxicon. 1994;32:1349–1358. doi: 10.1016/0041-0101(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 79.Ali S. A., Stoeva S., Abbasi A., Alam J. M., Kayed R., Faigle M., Neumeister B., Voelter W. Isolation, structural, and functional characterization of an apoptosis-inducing L-amino acid oxidase from leafnosed viper (Eristocophis macmahoni) snake venom. Arch. Biochem. Biophys. 2000;384:216–265. doi: 10.1006/abbi.2000.2130. [DOI] [PubMed] [Google Scholar]
- 80.Sakurai Y., Takatsuka H., Yoshioka A., Matsui T., Suzuki M., Titani K., Fujimura Y. Inhibition of human platelet aggregation by L-amino acid oxidase purified from Naja naja kaouthia venom. Toxicon. 2001;39:1827–1833. doi: 10.1016/s0041-0101(01)00133-7. [DOI] [PubMed] [Google Scholar]
- 81.Sakurai Y., Shima M., Matsumoto T., Takatsuka H., Nishiya K., Kasuda S., Fujimura Y., Yoshioka A. Anticoagulant activity of M-LAO, L-amino acid oxidase purified from Agkistrodon halys blomhoffii, through selective inhibition of factor IX. Biochim. Biophys. Acta. 2003;1649:51–57. doi: 10.1016/s1570-9639(03)00157-2. [DOI] [PubMed] [Google Scholar]
- 82.Drickamer K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999;9:585–590. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 83.Morita T. C-type lectin-related proteins from snake venoms. Curr. Drug Targets Cardiovasc. Hematol. Disord. 2004;4:357–373. doi: 10.2174/1568006043335916. [DOI] [PubMed] [Google Scholar]
- 84.Ogawa T., Chijiwa T., Oda-Ueda N., Ohno M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon. 2005;45:1–14. doi: 10.1016/j.toxicon.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 85.Takeya H., Nishida S., Miyata T., Kawada S., Saisaka Y., Morita T., Iwanaga S. Coagulation factor X activating enzyme from Russell's viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J. Biol. Chem. 1992;267:14109–14117. [PubMed] [Google Scholar]
- 86.Gowda D. C., Jackson C. M., Hensley P., Davidson E. A. Factor X-activating glycoprotein of Russell's viper venom. Polypeptide composition and characterization of the carbohydrate moieties. J. Biol. Chem. 1994;269:10644–10650. [PubMed] [Google Scholar]
- 87.Yamada D., Sekiya F., Morita T. Isolation and characterization of carinactivase, a novel prothrombin activator in Echis carinatus venom with a unique catalytic mechanism. J. Biol. Chem. 1996;271:5200–5207. doi: 10.1074/jbc.271.9.5200. [DOI] [PubMed] [Google Scholar]
- 88.Yamada D., Morita T. Purification and characterization of a Ca2+-dependent prothrombin activator, multactivase, from the venom of Echis multisquamatus. J. Biochem. (Tokyo) 1997;122:991–997. doi: 10.1093/oxfordjournals.jbchem.a021862. [DOI] [PubMed] [Google Scholar]
- 89.Morita T. Proteases which activate factor X. In: Bailey G. S., editor. Snake Venom Enzymes. Fort Collins, CO: Alaken Inc.; 1998. pp. 179–208. [Google Scholar]
- 90.Ouyang C., Teng C. M. Purification and properties of the anticoagulant principle of Agkistrodon acutus venom. Biochim. Biophys. Acta. 1972;278:155–162. doi: 10.1016/0005-2795(72)90117-1. [DOI] [PubMed] [Google Scholar]
- 91.Ouyang C., Yang F. Y. Purification and properties of the anticoagulant principle of Trimeresurus gramineus venom. Biochim. Biophys. Acta. 1975;386:479–492. doi: 10.1016/0005-2795(75)90291-3. [DOI] [PubMed] [Google Scholar]
- 92.Ouyang C., Teng C. M. The effect of the purified anticoagulant principle of Agkistrodon acutus venom on blood coagulation. Toxicon. 1973;11:287–292. doi: 10.1016/0041-0101(73)90057-3. [DOI] [PubMed] [Google Scholar]
- 93.Teng C. M., Seegers W. H. Agkistrodon acutus snake venom inhibits prothrombinase complex formation. Thromb. Res. 1981;23:255–263. doi: 10.1016/0049-3848(81)90015-3. [DOI] [PubMed] [Google Scholar]
- 94.Atoda H., Morita T. A novel blood coagulation factor IX/factor X-binding protein with anticoagulant activity from the venom of Trimeresurus flavoviridis (Habu snake): isolation and characterization. J. Biochem. (Tokyo) 1989;106:808–813. doi: 10.1093/oxfordjournals.jbchem.a122935. [DOI] [PubMed] [Google Scholar]
- 95.Atoda H., Hyuga M., Morita T. The primary structure of coagulation factor IX/factor X-binding protein isolated from the venom of Trimeresurus flavoviridis. Homology with asialoglycoprotein receptors, proteoglycan core protein, tetranectin, and lymphocyte Fc3 receptor for immunoglobulin. E. J. Biol. Chem. 1991;266:14903–14911. [PubMed] [Google Scholar]
- 96.Atoda H., Morita T. Arrangement of the disulfide bridges in a blood coagulation factor IX/factor X-binding protein from the venom of Trimeresurus flavoviridis. J. Biochem. (Tokyo) 1993;113:159–163. doi: 10.1093/oxfordjournals.jbchem.a124020. [DOI] [PubMed] [Google Scholar]
- 97.Atoda H., Ishikawa M., Yoshihara E., Sekiya F., Morita T. Blood coagulation factor IX-binding protein from the venom of Trimeresurus flavoviridis: purification and characterization. J. Biochem. (Tokyo) 1995;118:965–973. doi: 10.1093/jb/118.5.965. [DOI] [PubMed] [Google Scholar]
- 98.Atoda H., Ishikawa M., Mizuno H., Morita T. Coagulation factor X-binding protein from Deinagkistrodon acutus venom is a Gla domain-binding protein. Biochemistry. 1998;37:17361–17370. doi: 10.1021/bi981177x. [DOI] [PubMed] [Google Scholar]
- 99.Mizuno H., Fujimoto Z., Koizumi M., Kano H., Atoda H., Morita T. Structure of coagulation factors IX/X-binding protein, a heterodimer of C-type lectin domains. Nat. Struct. Biol. 1997;4:438–441. doi: 10.1038/nsb0697-438. [DOI] [PubMed] [Google Scholar]
- 100.Mizuno H., Fujimoto Z., Koizumi M., Kano H., Atoda H., Morita T. Crystal structure of coagulation factor IX-binding protein from habu snake venom at 2.6 Å: implication of central loop swapping based on deletion in the linker region. J. Mol. Biol. 1999;289:103–112. doi: 10.1006/jmbi.1999.2756. [DOI] [PubMed] [Google Scholar]
- 101.Weis W. I., Kahn R., Fourme R., Drickamer K., Hendrickson W. A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991;254:1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- 102.Weis W. I., Drickamer K., Hendrickson W. A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature (London) 1992;360:127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 103.Fukuda K., Mizuno H., Atoda H., Morita T. Crystal structure of flavocetin-A, a platelet glycoprotein Ib-binding protein, reveals a novel cyclic tetramer of C-type lectin-like heterodimers. Biochemistry. 2000;39:1915–1923. doi: 10.1021/bi992134z. [DOI] [PubMed] [Google Scholar]
- 104.Sen U., Vasudevan S., Subbarao G., McClintock R. A., Celikel R., Ruggeri Z. M., Varughese K. I. Crystal structure of the von Willebrand factor modulator botrocetin. Biochemistry. 2001;40:345–352. doi: 10.1021/bi0021737. [DOI] [PubMed] [Google Scholar]
- 105.Hirotsu S., Mizuno H., Fukuda K., Qi M. C., Matsui T., Hamako J., Morita T., Titani K. Crystal structure of bitiscetin, a von Willebrand factor-dependent platelet aggregation inducer. Biochemistry. 2001;40:13592–13597. doi: 10.1021/bi0114933. [DOI] [PubMed] [Google Scholar]
- 106.Batuwangala T., Leduc M., Gibbins J. M., Bon C., Jones E. Y. Structure of the snake-venom toxin convulxin. Acta Crystallogr. Sect. D: Biol. Crystallogr. 2004;60:46–53. doi: 10.1107/s0907444903021620. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y., Eisenberg D. 3D domain swapping: as domains continue to swap. Protein Sci. 2002;11:1285–1299. doi: 10.1110/ps.0201402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mizuno H., Fujimoto Z., Atoda H., Morita T. Crystal structure of an anticoagulant protein in complex with the Gla domain of factor X. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7230–7234. doi: 10.1073/pnas.131179698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shikamoto Y., Morita T., Fujimoto Z., Mizuno H. Crystal structure of Mg2+- and Ca2+-bound Gla domain of factor IX complexed with binding protein. J. Biol. Chem. 2003;278:24090–24094. doi: 10.1074/jbc.M300650200. [DOI] [PubMed] [Google Scholar]
- 110.Zingali R. B., Jandrot-Perrus M., Guillin M. C., Bon C. Bothrojaracin, a new thrombin inhibitor isolated from Bothrops jararaca venom: characterization and mechanism of thrombin inhibition. Biochemistry. 1993;32:10794–10802. doi: 10.1021/bi00091a034. [DOI] [PubMed] [Google Scholar]
- 111.Arocas V., Zingali R. B., Guillin M. C., Bon C., Jandrot-Perrus M. Bothrojaracin: a potent two-site-directed thrombin inhibitor. Biochemistry. 1996;35:9083–9089. doi: 10.1021/bi960043l. [DOI] [PubMed] [Google Scholar]
- 112.Castro H. C., Dutra D. L., Oliveira-Carvalho A. L., Zingali R. B. Bothroalternin, a thrombin inhibitor from the venom of Bothrops alternatus. Toxicon. 1998;36:1903–1912. doi: 10.1016/s0041-0101(98)00111-1. [DOI] [PubMed] [Google Scholar]
- 113.Arocas V., Castro H. C., Zingali R. B., Guillin M. C., Jandrot-Perrus M., Bon C., Wisner A. Molecular cloning and expression of bothrojaracin, a potent thrombin inhibitor from snake venom. Eur. J. Biochem. 1997;248:550–557. doi: 10.1111/j.1432-1033.1997.00550.x. [DOI] [PubMed] [Google Scholar]
- 114.Monteiro R. Q., Carlini C. R., Guimaraes J. A., Bon C., Zingali R. B. Distinct bothrojaracin isoforms produced by individual jararaca (Bothrops jararaca) snakes. Toxicon. 1997;35:649–657. doi: 10.1016/s0041-0101(96)00176-6. [DOI] [PubMed] [Google Scholar]
- 115.Monteiro R. Q., Yamanouye N., Carlini C. R., Guimaraes J. A., Bon C., Zingali R. B. Variability of bothrojaracin isoforms and other venom principles in individual jararaca (Bothrops jararaca) snakes maintained under seasonally invariant conditions. Toxicon. 1998;36:153–163. doi: 10.1016/s0041-0101(97)00061-5. [DOI] [PubMed] [Google Scholar]
- 116.Monteiro R. Q., Dutra D. L., Machado O. L., Carlini C. R., Guimaraes J. A., Bon C., Zingali R. B. Bothrops jararaca snakes produce several bothrojaracin isoforms following an individual pattern. Comp. Biochem. Physiol. Part B. Biochem. Mol. Biol. 1998;120:791–798. doi: 10.1016/s0305-0491(98)10070-6. [DOI] [PubMed] [Google Scholar]
- 117.Castro H. C., Fernandes M., Zingali R. B. Identification of bothrojaracin-like proteins in snake venoms from Bothrops species and Lachesis muta. Toxicon. 1999;37:1403–1416. doi: 10.1016/s0041-0101(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 118.Arocas V., Lemaire C., Bouton M. C., Bezeaud A., Bon C., Guillin M. C., Jandrot-Perrus M. Inhibition of thrombin-catalyzed factor V activation by bothrojaracin. Thromb. Haemostasis. 1998;79:1157–1161. [PubMed] [Google Scholar]
- 119.Monteiro R. Q., Zingali R. B. Inhibition of prothrombin activation by bothrojaracin, a C-type lectin from Bothrops jararaca venom. Arch. Biochem. Biophys. 2000;382:123–128. doi: 10.1006/abbi.2000.2006. [DOI] [PubMed] [Google Scholar]
- 120.Monteiro R. Q., Zingali R. B. Bothrojaracin, a proexosite I ligand, inhibits factor Va-accelerated prothrombin activation. Thromb. Haemostasis. 2002;87:288–293. [PubMed] [Google Scholar]
- 121.Monteiro R. Q., Bock P. E., Bianconi M. L., Zingali R. B. Characterization of bothrojaracin interaction with human prothrombin. Protein Sci. 2001;10:1897–1904. doi: 10.1110/ps.09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Monteiro R. Q., Foguel D., Castro H. C., Zingali R. B. Subunit dissociation, unfolding, and inactivation of bothrojaracin, a C-type lectin-like protein from snake venom. Biochemistry. 2003;42:509–515. doi: 10.1021/bi020571z. [DOI] [PubMed] [Google Scholar]
- 123.Monteiro R. Q., Raposo J. G., Wisner A., Guimaraes J. A., Bon C., Zingali R. B. Allosteric changes of thrombin catalytic site induced by interaction of bothrojaracin with anion-binding exosites I and II. Biochem. Biophys. Res. Commun. 1999;262:819–822. doi: 10.1006/bbrc.1999.1297. [DOI] [PubMed] [Google Scholar]
- 124.Dufton M. T., Hider R. C. Structure and pharmacology of elapid cytotoxins. Pharmacol. Ther. 1988;36:1–40. doi: 10.1016/0163-7258(88)90111-8. [DOI] [PubMed] [Google Scholar]
- 125.Endo T., Tamiya N. Structure–function relationships of postsynaptic neurotoxins from snake venoms. In: Harvey A. L., editor. Snake Toxins. New York: Pergamon Press; 1991. pp. 165–222. [Google Scholar]
- 126.Fry B. G., Lumsden N. G., Wüster W., Wickramaratna J. C., Hodgson W. C., Kini R. M. Isolation of a neurotoxin (α-colubritoxin) from a ‘non-venomous’ colubrid: evidence for early origin of venom in snakes. J. Mol. Evol. 2003;57:446–452. doi: 10.1007/s00239-003-2497-3. [DOI] [PubMed] [Google Scholar]
- 127.Fry B. G., Wuster W., Ramjan R. S. F., Jackson T., Martelli P., Kini R. M. Analysis of Colubroidea snake venoms by liquid chromatography with mass spectrometry: evolutionary and toxinological implications. Rapid Commun. Mass Spectrom. 2003;17:2047–2062. doi: 10.1002/rcm.1148. [DOI] [PubMed] [Google Scholar]
- 128.Lumsden N. G., Fry B. G., Kini R. M., Hodgson W. C. In vitro neuromuscular activity of ‘colubrid’ venoms: clinical and evolutionary implications. Toxicon. 2004;43:819–827. doi: 10.1016/j.toxicon.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 129.Mackessy S. P. Biochemistry and pharmacology of colubrid snake venoms. J. Toxicol. Toxin Rev. 2002;21:43–83. [Google Scholar]
- 130.Lumsden N. G., Fry B. G., Ventura S., Kini R. M., Hodgson W. C. Pharmacological characterisation of a neurotoxin from the venom of Boiga dendrophila (Mangrove snake) Toxicon. 2005;45:329–334. doi: 10.1016/j.toxicon.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 131.Fry B. G., Wüster W., Kini R. M., Brusic V., Khan A., Venkataraman D., Rooney A. P. Molecular evolution and phylogeny of elapid snake venom three finger toxins. J. Mol. Evol. 2003;57:110–129. doi: 10.1007/s00239-003-2461-2. [DOI] [PubMed] [Google Scholar]
- 132.Menez A. Functional architectures of animal toxins: a clue to drug design? Toxicon. 1998;36:1557–1572. doi: 10.1016/s0041-0101(98)00148-2. [DOI] [PubMed] [Google Scholar]
- 133.Tsetlin V. Snake venom α-neurotoxins and other ‘three-finger’ proteins. Eur. J. Biochem. 1999;264:281–286. doi: 10.1046/j.1432-1327.1999.00623.x. [DOI] [PubMed] [Google Scholar]
- 134.Chang C. C. The action of snake venoms on nerve and muscle. Handb. Exp. Pharmacol. 1979;52:309–376. [Google Scholar]
- 135.Changeux J. P. The TiPS lecture. The nicotinic acetylcholine receptor: an allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol. Sci. 1990;11:485–492. doi: 10.1016/0165-6147(90)90049-e. [DOI] [PubMed] [Google Scholar]
- 136.Grant G. A., Chiappinelli V. A. κ-Bungarotoxin: complete amino acid sequence of a neuronal nicotinic receptor probe. Biochemistry. 1985;24:1532–1537. doi: 10.1021/bi00327a036. [DOI] [PubMed] [Google Scholar]
- 137.Jerusalinsky D., Harvey A. L. Toxins from mamba venoms: small proteins with selectivities for different subtypes of muscarinic acetylcholine receptors. Trends Pharmacol. Sci. 1994;15:424–430. doi: 10.1016/0165-6147(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 138.Cervenansky C., Dajas F., Harvey A. L., Karlsson E. Fasciculins, anticholinesterase toxins from mamba venoms: biochemistry and pharmacology. In: Harvey A. L., editor. Snake Toxins. New York: Pergamon Press; 1991. pp. 303–321. [Google Scholar]
- 139.De Weille J. R., Schweitz H., Maes P., Tartar A., Lazdunski M. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc. Natl Acad. Sci. U.S.A. 1991;88:2437–2440. doi: 10.1073/pnas.88.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Albrand J. P., Blackledge M. J., Pascaud F., Hollecker M., Marion D. NMR and restrained molecular dynamics study of the three-dimensional solution structure of toxin FS2, a specific blocker of the L-type calcium channel, isolated from black mamba venom. Biochemistry. 1995;34:5923–5937. doi: 10.1021/bi00017a022. [DOI] [PubMed] [Google Scholar]
- 141.Bilwes A., Rees B., Moras D., Menez R., Menez A. X-ray structure at 1.55 Å of toxin γ, a cardiotoxin from Naja nigricollis venom. Crystal packing reveals a model for insertion into membranes. J. Mol. Biol. 1994;239:122–136. doi: 10.1006/jmbi.1994.1357. [DOI] [PubMed] [Google Scholar]
- 142.McDowell R. S., Dennis M. S., Louie A., Shuster M., Mulkerrin M. G., Lazarus R. A. Mambin, a potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor structurally related to the short neurotoxins. Biochemistry. 1992;31:4766–4772. doi: 10.1021/bi00135a004. [DOI] [PubMed] [Google Scholar]
- 143.Kini R. M., Stefansson S., Evans H. J. Non-phospholipase anticoagulants from Naja nigricollis venom. In: Gopalakrishnakone P., Tan C. K., editors. Progress in Venom and Toxin Research. Singapore: National University of Singapore; 1987. pp. 175–185. [Google Scholar]
- 144.Kini R. M., Haar N. C., Evans H. J. Non-enzymatic inhibitors of coagulation and platelet aggregation from Naja nigricollis venom are cardiotoxins. Biochem. Biophys. Res. Commun. 1988;150:1012–1016. doi: 10.1016/0006-291x(88)90729-2. [DOI] [PubMed] [Google Scholar]
- 145.Banerjee Y., Mizuguchi J., Iwanaga S., Kini R. M. Hemextin AB complex, a unique anticoagulant protein complex from Hemachatus haemachatus (African Ringhals cobra) venom that inhibits clot initiation and factor VIIa activity. J. Biol. Chem. 2005;280:42601–42611. doi: 10.1074/jbc.M508987200. [DOI] [PubMed] [Google Scholar]
- 146.Kini R. M. Molecular molds with multiple missions: functional sites in three-finger toxins. Clin. Exp. Pharmacol. Physiol. 2002;29:815–822. doi: 10.1046/j.1440-1681.2002.03725.x. [DOI] [PubMed] [Google Scholar]
- 147.Fleming T. J., O'hUigin C., Malek T. R. Characterization of two novel Ly-6 genes. Protein sequence and potential structural similarity to α-bungarotoxin and other neurotoxins. J. Immunol. 1993;150:5379–5390. [PubMed] [Google Scholar]
- 148.Ploug M., Ellis V. Structure–function relationships in the receptor for urokinase-type plasminogen activator. Comparison to other members of the Ly-6 family and snake venom α-neurotoxins. FEBS Lett. 1994;349:163–168. doi: 10.1016/0014-5793(94)00674-1. [DOI] [PubMed] [Google Scholar]
- 149.Gumley T. P., McKenzie I. F. C., Sandrin M. S. Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunol. Cell Biol. 1995;73:277–296. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- 150.Miwa J. M., Ibanez-Tallon I., Crabtree G. W., Sanchez R., Sali A., Role L. W., Heintz N. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23:105–114. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- 151.Cordero-Erausquin M., Marubio L. M., Klink R., Changeux J. P. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol. Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- 152.Kini R. M., Evans H. J. Mechanism of platelet effects of cardiotoxins from Naja nigricollis crawshawii (spitting cobra) snake venom. Thromb. Res. 1988;52:185–195. doi: 10.1016/0049-3848(88)90078-3. [DOI] [PubMed] [Google Scholar]
- 153.Kini R. M., Evans H. J. A common cytolytic region in myotoxins, hemolysins, cardiotoxins and antibacterial peptides. Int. J. Pept. Protein Res. 1989;34:277–286. doi: 10.1111/j.1399-3011.1989.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 154.Kini R. M., Evans H. J. Role of cationic amino acid residues in cytolytic activity. Modifications of lysine residues in the cardiotoxin from Naja nigricollis venom and correlation between cytolytic and antiplatelet activities. Biochemistry. 1989;28:9209–9215. doi: 10.1021/bi00449a037. [DOI] [PubMed] [Google Scholar]
- 155.Takahashi H., Iwanaga S., Suzuki T. Distribution of proteinase inhibitors in snake venoms. Toxicon. 1974;12:193–197. doi: 10.1016/0041-0101(74)90245-1. [DOI] [PubMed] [Google Scholar]
- 156.Hokama Y., Iwanaga S., Tatsuki T., Suzuki T. Snake venom proteinase inhibitors. III. Isolation of five polypeptide inhibitors from the venoms of Hemachatus haemachatus (Ringhal's corbra) and Naja nivea (Cape cobra) and the complete amino acid sequences of two of them. J. Biochem. (Tokyo) 1976;79:559–578. doi: 10.1093/oxfordjournals.jbchem.a131100. [DOI] [PubMed] [Google Scholar]
- 157.Strydom D. J. Snake venom toxins. Purification and properties of low-molecular-weight polypeptides of Dendroaspis polylepis polylepis (black mamba) venom. Eur. J. Biochem. 1976;69:169–176. doi: 10.1111/j.1432-1033.1976.tb10870.x. [DOI] [PubMed] [Google Scholar]
- 158.Ritonja A., Turk V., Gubensek F. Serine proteinase inhibitors from Vipera ammodytes venom. Isolation and kinetic studies. Eur. J. Biochem. 1983;133:427–432. doi: 10.1111/j.1432-1033.1983.tb07481.x. [DOI] [PubMed] [Google Scholar]
- 159.Harvey A. L. Twenty years of dendrotoxins. Toxicon. 2001;39:15–26. doi: 10.1016/s0041-0101(00)00162-8. [DOI] [PubMed] [Google Scholar]
- 160.Harvey A. L., Robertson B. Dendrotoxins: structure–activity relationships and effects on potassium ion channels. Curr. Med. Chem. 2004;11:3065–3072. doi: 10.2174/0929867043363820. [DOI] [PubMed] [Google Scholar]
- 161.Schweitz H., Heurteaux C., Bois P., Moinier D., Romey G., Lazdunski M. Calcicludine, a venom peptide of the Kunitz-type protease inhibitor family, is a potent blocker of high-threshold Ca2+ channels with a high affinity for L-type channels in cerebellar granule neurons. Proc. Natl. Acad. Sci. U.S.A. 1994;91:878–882. doi: 10.1073/pnas.91.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stotz S. C., Spaetgens R. L., Zamponi G. W. Block of voltage-dependent calcium channel by the green mamba toxin calcicludine. J. Membr. Biol. 2000;174:157–165. doi: 10.1007/s002320001040. [DOI] [PubMed] [Google Scholar]
- 163.Arseniev A. S., Wider G., Joubert F. J., Wuthrich K. Assignment of the H nuclear magnetic resonance spectrum of the trypsin inhibitor E from Dendroaspis polylepis polylepis. Two-dimensional nuclear magnetic resonance at 500 MHz. J. Mol. Biol. 1982;159:323–351. doi: 10.1016/0022-2836(82)90498-3. [DOI] [PubMed] [Google Scholar]
- 164.Keller R. M., Baumann R., Hunziker-Kwik E. H., Joubert F. J., Wuthrich K. Assignment of the 1H nuclear magnetic resonance spectrum of the trypsin inhibitor homologue K from Dendroaspis polylepis polylepis. Two-dimensional nuclear magnetic resonance at 360 and 500 MHz. J. Mol. Biol. 1983;163:623–646. doi: 10.1016/0022-2836(83)90115-8. [DOI] [PubMed] [Google Scholar]
- 165.Chen C., Hsu C. H., Su N. Y., Lin Y. C., Chiou S. H., Wu S. H. Solution structure of a Kunitz-type chymotrypsin inhibitor isolated from the elapid snake Bungarus fasciatus. J. Biol. Chem. 2001;276:45079–45087. doi: 10.1074/jbc.M106182200. [DOI] [PubMed] [Google Scholar]
- 166.Gilquin B., Lecoq A., Desne F., Guenneugues M., Zinn-Justin S., Menez A. Conformational and functional variability supported by the BPTI fold: solution structure of the Ca2+ channel blocker calcicludine. Proteins. 1999;34:520–532. [PubMed] [Google Scholar]
- 167.Masci P. P., Whitaker A. N., Sparrow L. G., de Jersey J., Winzor D. J., Watters D. J., Lavin M. F., Gaffney P. J. Textilinins from Pseudonaja textilis textilis. Characterization of two plasmin inhibitors that reduce bleeding in an animal model. Blood Coagulation Fibrinolysis. 2000;11:385–393. doi: 10.1097/00001721-200006000-00011. [DOI] [PubMed] [Google Scholar]
- 168.Flight S., Johnson L., Trabi M., Gaffney P., Lavin M., de Jersey J., Masci P. Pathophysiol. Haemostasis Thromb. 2006;34:188–193. doi: 10.1159/000092421. [DOI] [PubMed] [Google Scholar]
- 169.Torres A. M., Wong H. Y., Desai M., Moochhala S., Kuchel P. W., Kini R. M. Identification of a novel family of proteins in snake venoms: purification and structural characterization of nawaprin from Naja nigricollis snake venom. J. Biol. Chem. 2003;278:40097–40104. doi: 10.1074/jbc.M305322200. [DOI] [PubMed] [Google Scholar]
- 170.Gustafsson D., Bylund R., Antonsson T., Nilsson I., Nystrom J. E., Eriksson U., Bredberg U., Teger-Nilsson A. C. A new oral anticoagulant: the 50-year challenge. Nat. Rev. Drug Discov. 2004;3:649–659. doi: 10.1038/nrd1466. [DOI] [PubMed] [Google Scholar]
- 171.White S. P., Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990;250:1560–1563. doi: 10.1126/science.2274787. [DOI] [PubMed] [Google Scholar]