Abstract
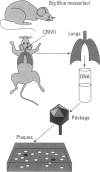
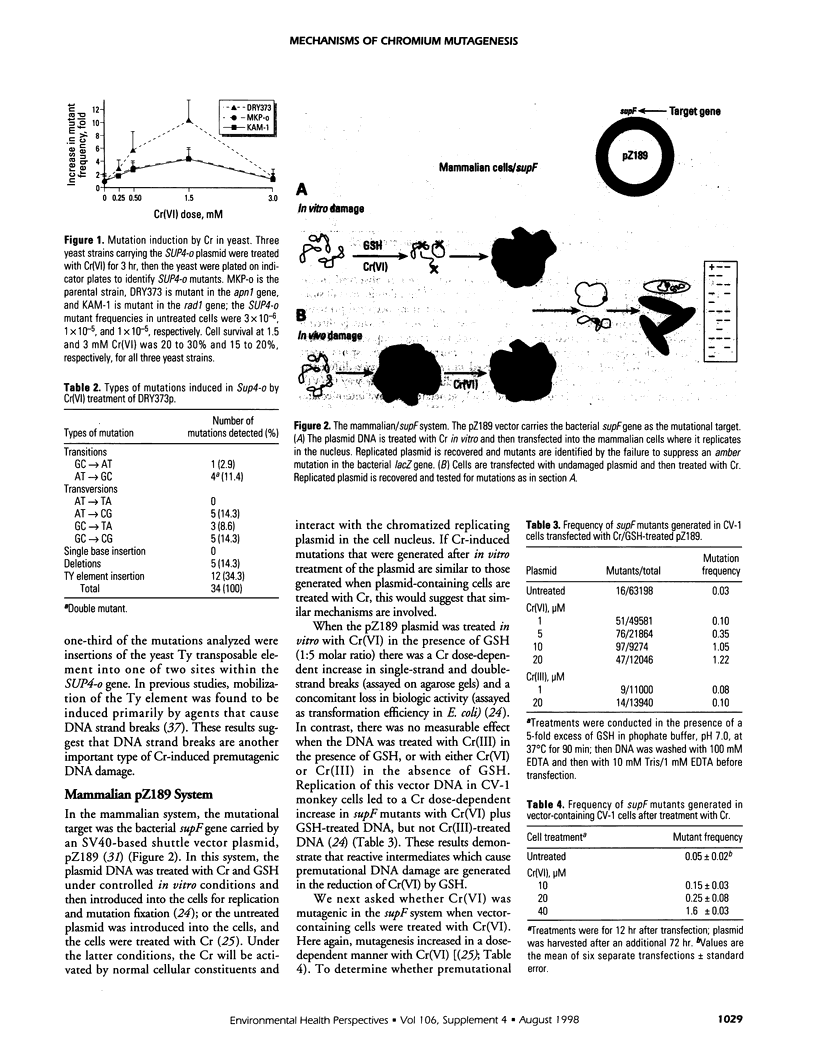
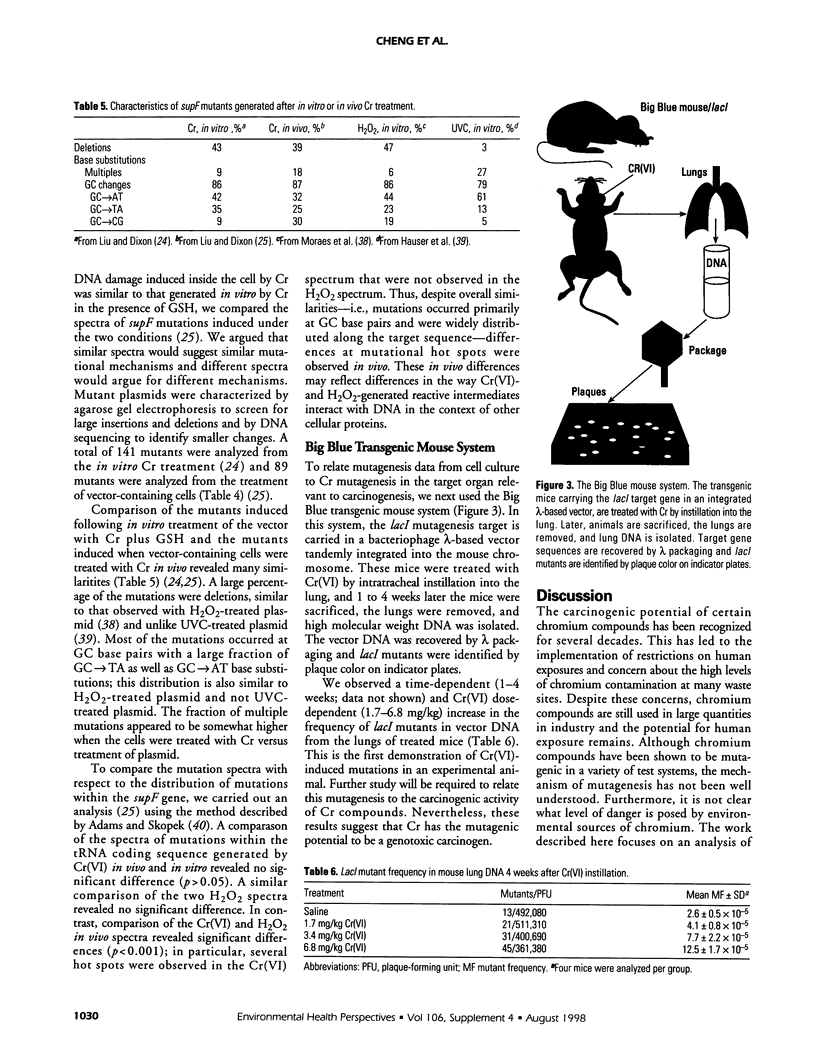
Chromium (Cr) is a widespread environmental contaminant and a known human carcinogen. We have used shuttle vector systems in yeast, mammalian cells, and transgenic mice to characterize the mutational specificity and premutational DNA damage induced by Cr(VI) and its reduction intermediates in order to elucidate the mechanism by which Cr induces mutations. In the yeast system, treatment of vector-containing cells with Cr(VI) results in a dose-dependent increase in mutations in the SUP4-o target gene of the vector; mutagenesis is enhanced in an apn-1 yeast mutant, deficient in the capacity to repair oxidative-type DNA damage. In vector-containing mammalian cells, treatment with Cr(VI) also results in a dose-dependent increase in mutations in the vector target gene supF. The Cr-induced mutations in supF occurred mostly at G:C base pairs and were widely distributed across the gene, a pattern similar to those observed with ionizing radiation or hydrogen peroxide. These results support the hypothesis that Cr(VI)-induced oxidative-type DNA damage is responsible for Cr mutagenesis in the cell. Recently these studies were extended into the Big Blue transgenic mouse system in which Cr-induced mutagenesis was observed in the lung, the target organ for Cr carcinogenesis in humans. Analysis of the spectrum of these mutations will test whether Cr mutagenesis occurs by similar mechanisms in the intact animal as in cell culture systems and yeast.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcedo J. A., Wetterhahn K. E. Chromium toxicity and carcinogenesis. Int Rev Exp Pathol. 1990;31:85–108. doi: 10.1016/b978-0-12-364931-7.50008-2. [DOI] [PubMed] [Google Scholar]
- Bianchi V., Celotti L., Lanfranchi G., Majone F., Marin G., Montaldi A., Sponza G., Tamino G., Venier P., Zantedeschi A. Genetic effects of chromium compounds. Mutat Res. 1983 May-Jun;117(3-4):279–300. doi: 10.1016/0165-1218(83)90128-3. [DOI] [PubMed] [Google Scholar]
- Biedermann K. A., Landolph J. R. Role of valence state and solubility of chromium compounds on induction of cytotoxicity, mutagenesis, and anchorage independence in diploid human fibroblasts. Cancer Res. 1990 Dec 15;50(24):7835–7842. [PubMed] [Google Scholar]
- Casadevall M., Kortenkamp A. The generation of apurinic/apyrimidinic sites in isolated DNA during the reduction of chromate by glutathione. Carcinogenesis. 1994 Feb;15(2):407–409. doi: 10.1093/carcin/15.2.407. [DOI] [PubMed] [Google Scholar]
- Chen J., Thilly W. G. Mutational spectrum of chromium(VI) in human cells. Mutat Res. 1994 Jan-Feb;323(1-2):21–27. doi: 10.1016/0165-7992(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Cupo D. Y., Wetterhahn K. E. Modification of chromium(VI)-induced DNA damage by glutathione and cytochromes P-450 in chicken embryo hepatocytes. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6755–6759. doi: 10.1073/pnas.82.20.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfle H. L., Walsh D. F., Holcroft J., Hague N., de Boer J. G., Glickman B. W. An efficient laboratory protocol for the sequencing of large numbers of lacI mutants recovered from Big Blue transgenic animals. Environ Mol Mutagen. 1996;28(4):393–396. doi: 10.1002/(SICI)1098-2280(1996)28:4<393::AID-EM13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hauser J., Seidman M. M., Sidur K., Dixon K. Sequence specificity of point mutations induced during passage of a UV-irradiated shuttle vector plasmid in monkey cells. Mol Cell Biol. 1986 Jan;6(1):277–285. doi: 10.1128/mcb.6.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo K., Suzuki S., Furuya N., Meshizuka K. An analysis of chromium, copper, and zinc in organs of a chromate worker. Int Arch Occup Environ Health. 1980;46(2):141–150. doi: 10.1007/BF00378192. [DOI] [PubMed] [Google Scholar]
- Izawa S., Inoue Y., Kimura A. Oxidative stress response in yeast: effect of glutathione on adaptation to hydrogen peroxide stress in Saccharomyces cerevisiae. FEBS Lett. 1995 Jul 10;368(1):73–76. doi: 10.1016/0014-5793(95)00603-7. [DOI] [PubMed] [Google Scholar]
- Kohler S. W., Provost G. S., Fieck A., Kretz P. L., Bullock W. O., Putman D. L., Sorge J. A., Short J. M. Analysis of spontaneous and induced mutations in transgenic mice using a lambda ZAP/lacI shuttle vector. Environ Mol Mutagen. 1991;18(4):316–321. doi: 10.1002/em.2850180421. [DOI] [PubMed] [Google Scholar]
- Langård S. One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am J Ind Med. 1990;17(2):189–215. doi: 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- Liu S., Dixon K. Induction of mutagenic DNA damage by chromium (VI) and glutathione. Environ Mol Mutagen. 1996;28(2):71–79. doi: 10.1002/(SICI)1098-2280(1996)28:2<71::AID-EM2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther. 1991;51(2):155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Mertz W. Chromium occurrence and function in biological systems. Physiol Rev. 1969 Apr;49(2):163–239. doi: 10.1152/physrev.1969.49.2.163. [DOI] [PubMed] [Google Scholar]
- Moraes E. C., Keyse S. M., Pidoux M., Tyrrell R. M. The spectrum of mutations generated by passage of a hydrogen peroxide damaged shuttle vector plasmid through a mammalian host. Nucleic Acids Res. 1989 Oct 25;17(20):8301–8312. doi: 10.1093/nar/17.20.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parket A., Inbar O., Kupiec M. Recombination of Ty elements in yeast can be induced by a double-strand break. Genetics. 1995 May;140(1):67–77. doi: 10.1093/genetics/140.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M. K., Giroux C. N., Kunz B. A. Development of a yeast system to assay mutational specificity. Mutat Res. 1987 Apr;182(2):65–74. doi: 10.1016/0165-1161(87)90055-0. [DOI] [PubMed] [Google Scholar]
- Salnikow K., Zhitkovich A., Costa M. Analysis of the binding sites of chromium to DNA and protein in vitro and in intact cells. Carcinogenesis. 1992 Dec;13(12):2341–2346. doi: 10.1093/carcin/13.12.2341. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Standeven A. M., Wetterhahn K. E. Ascorbate is the principal reductant of chromium (VI) in rat liver and kidney ultrafiltrates. Carcinogenesis. 1991 Sep;12(9):1733–1737. doi: 10.1093/carcin/12.9.1733. [DOI] [PubMed] [Google Scholar]
- Tsapakos M. J., Hampton T. H., Wetterhahn K. E. Chromium(VI)-induced DNA lesions and chromium distribution in rat kidney, liver, and lung. Cancer Res. 1983 Dec;43(12 Pt 1):5662–5667. [PubMed] [Google Scholar]
- Wiegand H. J., Ottenwälder H., Bolt H. M. The reduction of chromium (VI) to chromium (III) by glutathione: an intracellular redox pathway in the metabolism of the carcinogen chromate. Toxicology. 1984 Dec;33(3-4):341–348. doi: 10.1016/0300-483x(84)90050-7. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Hsieh Y. C., Wu C. W., Lee T. C. Mutational specificity of chromium(VI) compounds in the hprt locus of Chinese hamster ovary-K1 cells. Carcinogenesis. 1992 Nov;13(11):2053–2057. doi: 10.1093/carcin/13.11.2053. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A., Voitkun V., Costa M. Glutathione and free amino acids form stable complexes with DNA following exposure of intact mammalian cells to chromate. Carcinogenesis. 1995 Apr;16(4):907–913. doi: 10.1093/carcin/16.4.907. [DOI] [PubMed] [Google Scholar]