Abstract
Trichloroethylene (TCE) and related hydrocarbons constitute an important class of environmental pollutants whose adverse effects on liver, kidney, and other tissues may, in part, be mediated by peroxisome proliferator-activated receptors (PPARs), ligand-activated transcription factors belonging to the steroid receptor superfamily. Activation of PPAR induces a dramatic proliferation of peroxisomes in rodent hepatocytes and ultimately leads to hepatocellular carcinoma. To elucidate the role of PPAR in the pathophysiologic effects of TCE and its metabolites, it is important to understand the mechanisms whereby PPAR is activated both by TCE and endogenous peroxisome proliferators. The investigations summarized in this article a) help clarify the mechanism by which TCE and its metabolites induce peroxisome proliferation and b) explore the potential role of the adrenal steroid and anticarcinogen dehydroepiandrosterone 3beta-sulfate (DHEA-S) as an endogenous PPAR activator. Transient transfection studies have demonstrated that the TCE metabolites trichloroacetate and dichloroacetate both activate PPAR alpha, a major liver-expressed receptor isoform. TCE itself was inactive when tested over the same concentration range, suggesting that its acidic metabolites mediate the peroxisome proliferative potential of TCE. Although DHEA-S is an active peroxisome proliferator in vivo, this steroid does not stimulate trans-activation of PPAR alpha or of two other PPAR isoforms, gamma and delta/Nuc1, when evaluated in COS-1 cell transfection studies. To test whether PPAR alpha mediates peroxisomal gene induction by DHEA-S in intact animals, DHEA-S has been administered to mice lacking a functional PPAR alpha gene. DHEA-S was thus shown to markedly increase hepatic expression of two microsomal P4504A proteins associated with the peroxisomal proliferative response in wild-type mice. In contrast, DHEA-S did not induce these hepatic proteins in PPAR alpha-deficient mice. Thus, despite its unresponsiveness to steroidal peroxisome proliferators in transfection assays, PPAR alpha is an obligatory mediator of DHEA-S-stimulated hepatic peroxisomal gene induction. DHEA-S, or one of its metabolites, may thus serve as an important endogenous regulator of liver peroxisomal enzyme expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
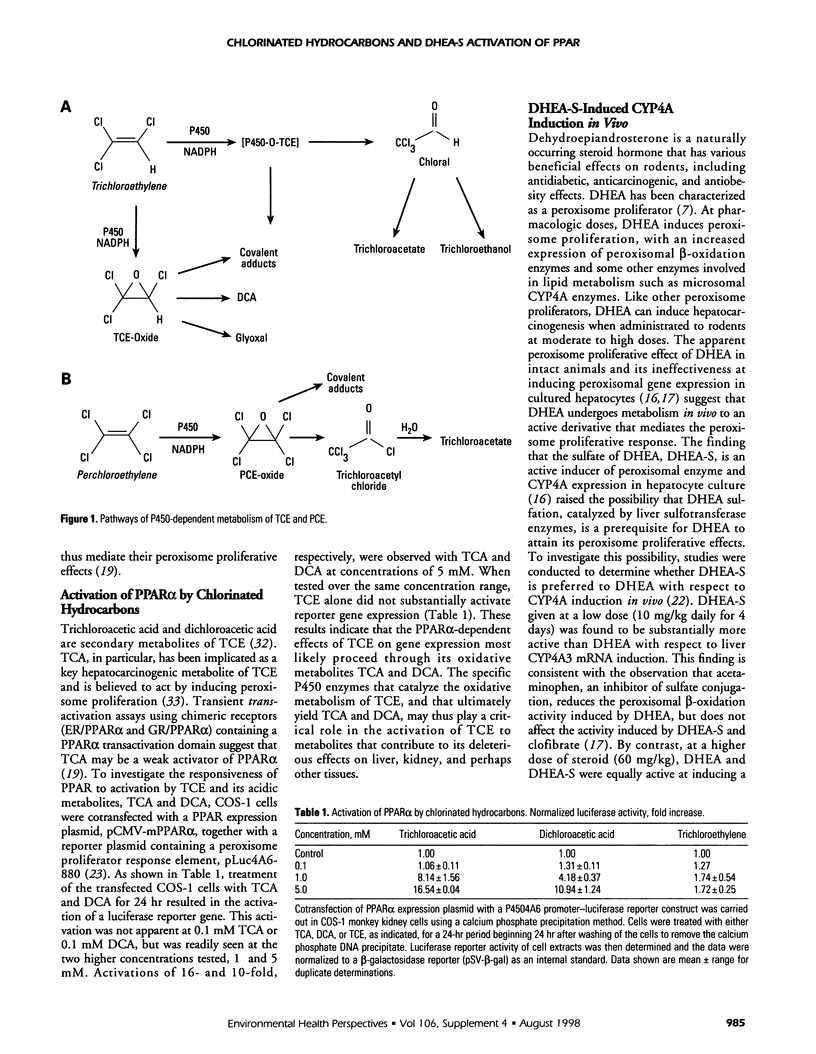
- Bruckner J. V., Davis B. D., Blancato J. N. Metabolism, toxicity, and carcinogenicity of trichloroethylene. Crit Rev Toxicol. 1989;20(1):31–50. doi: 10.3109/10408448909037475. [DOI] [PubMed] [Google Scholar]
- Buben J. A., O'Flaherty E. J. Delineation of the role of metabolism in the hepatotoxicity of trichloroethylene and perchloroethylene: a dose-effect study. Toxicol Appl Pharmacol. 1985 Mar 30;78(1):105–122. doi: 10.1016/0041-008x(85)90310-2. [DOI] [PubMed] [Google Scholar]
- Campos-Outcalt D. Trichloroethylene: environmental and occupational exposure. Am Fam Physician. 1992 Aug;46(2):495–500. [PubMed] [Google Scholar]
- Candura S. M., Faustman E. M. Trichloroethylene: toxicology and health hazards. G Ital Med Lav. 1991 Jan-Nov;13(1-6):17–25. [PubMed] [Google Scholar]
- Chia S. E., Goh V. H., Ong C. N. Endocrine profiles of male workers with exposure to trichloroethylene. Am J Ind Med. 1997 Sep;32(3):217–222. doi: 10.1002/(sici)1097-0274(199709)32:3<217::aid-ajim6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Costa A. K., Ivanetich K. M. Tetrachloroethylene metabolism by the hepatic microsomal cytochrome P-450 system. Biochem Pharmacol. 1980 Oct 15;29(20):2863–2869. doi: 10.1016/0006-2952(80)90023-4. [DOI] [PubMed] [Google Scholar]
- Elcombe C. R. Species differences in carcinogenicity and peroxisome proliferation due to trichloroethylene: a biochemical human hazard assessment. Arch Toxicol Suppl. 1985;8:6–17. doi: 10.1007/978-3-642-69928-3_2. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Chen J., Evans R. M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997 Apr 29;94(9):4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel R. A., Slaughter C. A., Orth K., Moomaw C. R., Hicks S. H., Snyder J. M., Bennett M., Prough R. A., Putnam R. S., Milewich L. Peroxisome proliferation and induction of peroxisomal enzymes in mouse and rat liver by dehydroepiandrosterone feeding. J Steroid Biochem. 1990 Feb;35(2):333–342. doi: 10.1016/0022-4731(90)90293-2. [DOI] [PubMed] [Google Scholar]
- Gordon G. B., Shantz L. M., Talalay P. Modulation of growth, differentiation and carcinogenesis by dehydroepiandrosterone. Adv Enzyme Regul. 1987;26:355–382. doi: 10.1016/0065-2571(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Göttlicher M., Widmark E., Li Q., Gustafsson J. A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F., Tamura H., Yamada J., Kasai H., Suga T. Characteristics of the hepatocarcinogenesis caused by dehydroepiandrosterone, a peroxisome proliferator, in male F-344 rats. Carcinogenesis. 1994 Oct;15(10):2215–2219. doi: 10.1093/carcin/15.10.2215. [DOI] [PubMed] [Google Scholar]
- Herren-Freund S. L., Pereira M. A., Khoury M. D., Olson G. The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicol Appl Pharmacol. 1987 Sep 15;90(2):183–189. doi: 10.1016/0041-008x(87)90325-5. [DOI] [PubMed] [Google Scholar]
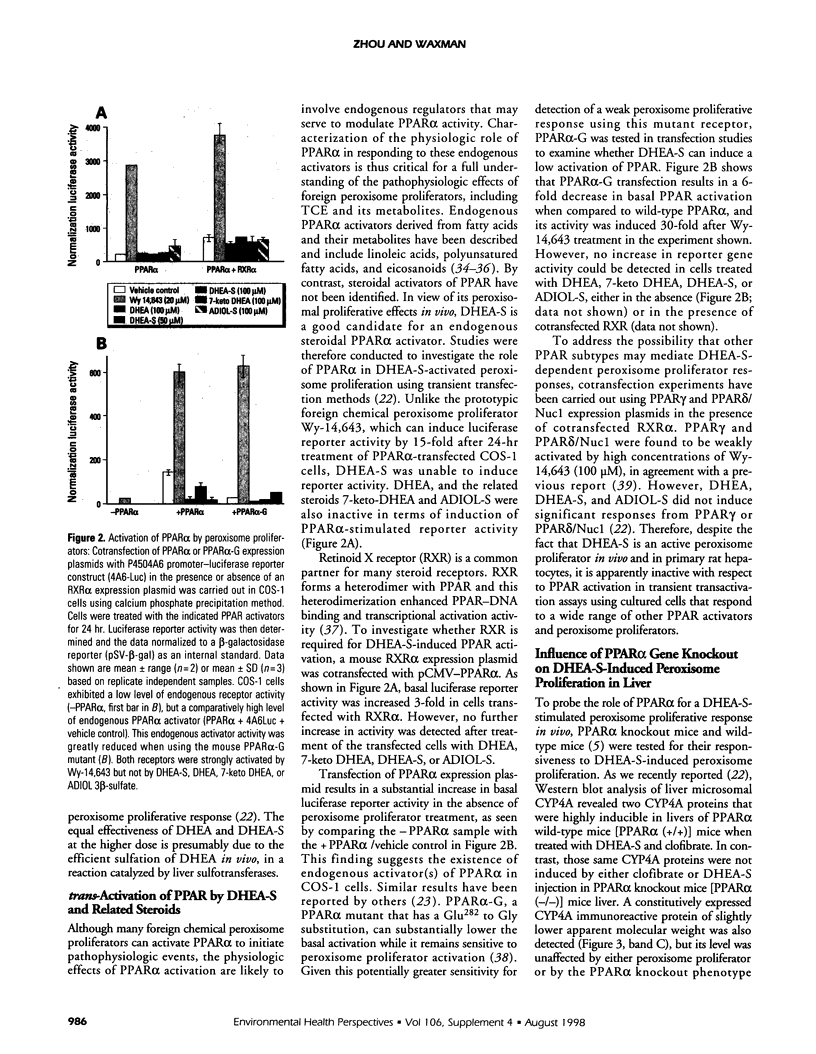
- Hess R., Stäubli W., Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965 Nov 27;208(5013):856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- Hsu M. H., Palmer C. N., Griffin K. J., Johnson E. F. A single amino acid change in the mouse peroxisome proliferator-activated receptor alpha alters transcriptional responses to peroxisome proliferators. Mol Pharmacol. 1995 Sep;48(3):559–567. [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990 Oct 18;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Forman B. M., Blumberg B., Ong E. S., Borgmeyer U., Mangelsdorf D. J., Umesono K., Evans R. M. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992 Aug 27;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S., Pineau T., Drago J., Lee E. J., Owens J. W., Kroetz D. L., Fernandez-Salguero P. M., Westphal H., Gonzalez F. J. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995 Jun;15(6):3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J. M., Moore L. B., Smith-Oliver T. A., Wilkison W. O., Willson T. M., Kliewer S. A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995 Jun 2;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Muerhoff A. S., Griffin K. J., Johnson E. F. The peroxisome proliferator-activated receptor mediates the induction of CYP4A6, a cytochrome P450 fatty acid omega-hydroxylase, by clofibric acid. J Biol Chem. 1992 Sep 25;267(27):19051–19053. [PubMed] [Google Scholar]
- Okita R. T., Okita J. R. Characterization of a cytochrome P450 from di(2-ethylhexyl) phthalate-treated rats which hydroxylates fatty acids. Arch Biochem Biophys. 1992 May 1;294(2):475–481. doi: 10.1016/0003-9861(92)90714-8. [DOI] [PubMed] [Google Scholar]
- Peters J. M., Zhou Y. C., Ram P. A., Lee S. S., Gonzalez F. J., Waxman D. J. Peroxisome proliferator-activated receptor alpha required for gene induction by dehydroepiandrosterone-3 beta-sulfate. Mol Pharmacol. 1996 Jul;50(1):67–74. [PubMed] [Google Scholar]
- Prough R. A., Webb S. J., Wu H. Q., Lapenson D. P., Waxman D. J. Induction of microsomal and peroxisomal enzymes by dehydroepiandrosterone and its reduced metabolite in rats. Cancer Res. 1994 Jun 1;54(11):2878–2886. [PubMed] [Google Scholar]
- Rao M. S., Reid B., Ide H., Subbarao V., Reddy J. K. Dehydroepiandrosterone-induced peroxisome proliferation in the rat: evaluation of sex differences. Proc Soc Exp Biol Med. 1994 Nov;207(2):186–190. doi: 10.3181/00379727-207-43805. [DOI] [PubMed] [Google Scholar]
- Reuter S., Mayer D. Transport of dehydroepiandrosterone and dehydroepiandrosterone sulphate into rat hepatocytes. J Steroid Biochem Mol Biol. 1995 Sep;54(5-6):227–235. doi: 10.1016/0960-0760(95)00132-j. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Endo N., Rutledge S. J., Vogel R., Shinar D., Rodan G. A. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Mol Endocrinol. 1992 Oct;6(10):1634–1641. doi: 10.1210/mend.6.10.1333051. [DOI] [PubMed] [Google Scholar]
- Schoonjans K., Martin G., Staels B., Auwerx J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr Opin Lipidol. 1997 Jun;8(3):159–166. doi: 10.1097/00041433-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Schwartz A. G., Whitcomb J. M., Nyce J. W., Lewbart M. L., Pashko L. L. Dehydroepiandrosterone and structural analogs: a new class of cancer chemopreventive agents. Adv Cancer Res. 1988;51:391–424. doi: 10.1016/s0065-230x(08)60227-4. [DOI] [PubMed] [Google Scholar]
- Sundseth S. S., Waxman D. J. Sex-dependent expression and clofibrate inducibility of cytochrome P450 4A fatty acid omega-hydroxylases. Male specificity of liver and kidney CYP4A2 mRNA and tissue-specific regulation by growth hormone and testosterone. J Biol Chem. 1992 Feb 25;267(6):3915–3921. [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Spiegelman B. M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994 Dec 30;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Warren J. R., Simmon V. F., Reddy J. K. Properties of hypolipidemic peroxisome proliferators in the lymphocyte [3H]thymidine and Salmonella mutagenesis assays. Cancer Res. 1980 Jan;40(1):36–41. [PubMed] [Google Scholar]
- Waxman D. J. Role of metabolism in the activation of dehydroepiandrosterone as a peroxisome proliferator. J Endocrinol. 1996 Sep;150 (Suppl):S129–S147. [PubMed] [Google Scholar]
- Wu H. Q., Masset-Brown J., Tweedie D. J., Milewich L., Frenkel R. A., Martin-Wixtrom C., Estabrook R. W., Prough R. A. Induction of microsomal NADPH-cytochrome P-450 reductase and cytochrome P-450IVA1 (P-450LA omega) by dehydroepiandrosterone in rats: a possible peroxisomal proliferator. Cancer Res. 1989 May 1;49(9):2337–2343. [PubMed] [Google Scholar]
- Yamada J., Sakuma M., Ikeda T., Fukuda K., Suga T. Characteristics of dehydroepiandrosterone as a peroxisome proliferator. Biochim Biophys Acta. 1991 Apr 17;1092(2):233–243. doi: 10.1016/0167-4889(91)90162-q. [DOI] [PubMed] [Google Scholar]
- Yamada J., Sakuma M., Ikeda T., Suga T. Activation of dehydroepiandrosterone as a peroxisome proliferator by sulfate conjugation. Arch Biochem Biophys. 1994 Sep;313(2):379–381. doi: 10.1006/abbi.1994.1402. [DOI] [PubMed] [Google Scholar]
- Yu K., Bayona W., Kallen C. B., Harding H. P., Ravera C. P., McMahon G., Brown M., Lazar M. A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995 Oct 13;270(41):23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Alvares K., Huang Q., Rao M. S., Reddy J. K. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem. 1993 Dec 25;268(36):26817–26820. [PubMed] [Google Scholar]