Abstract
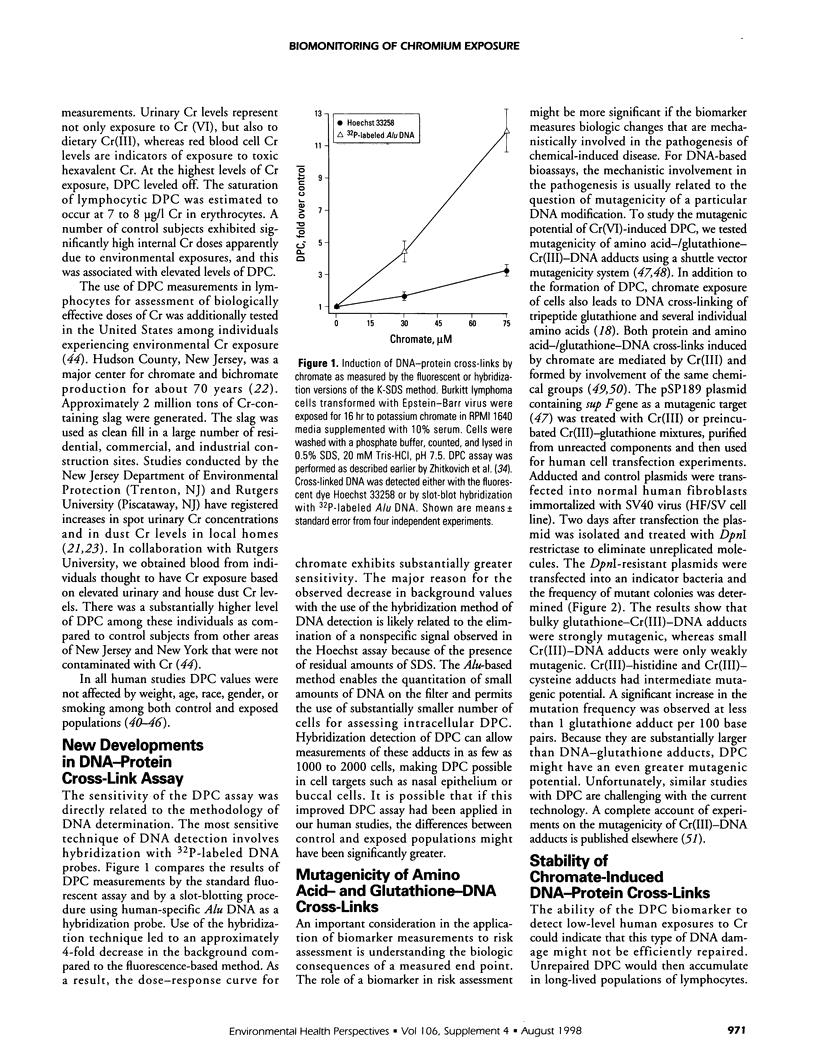
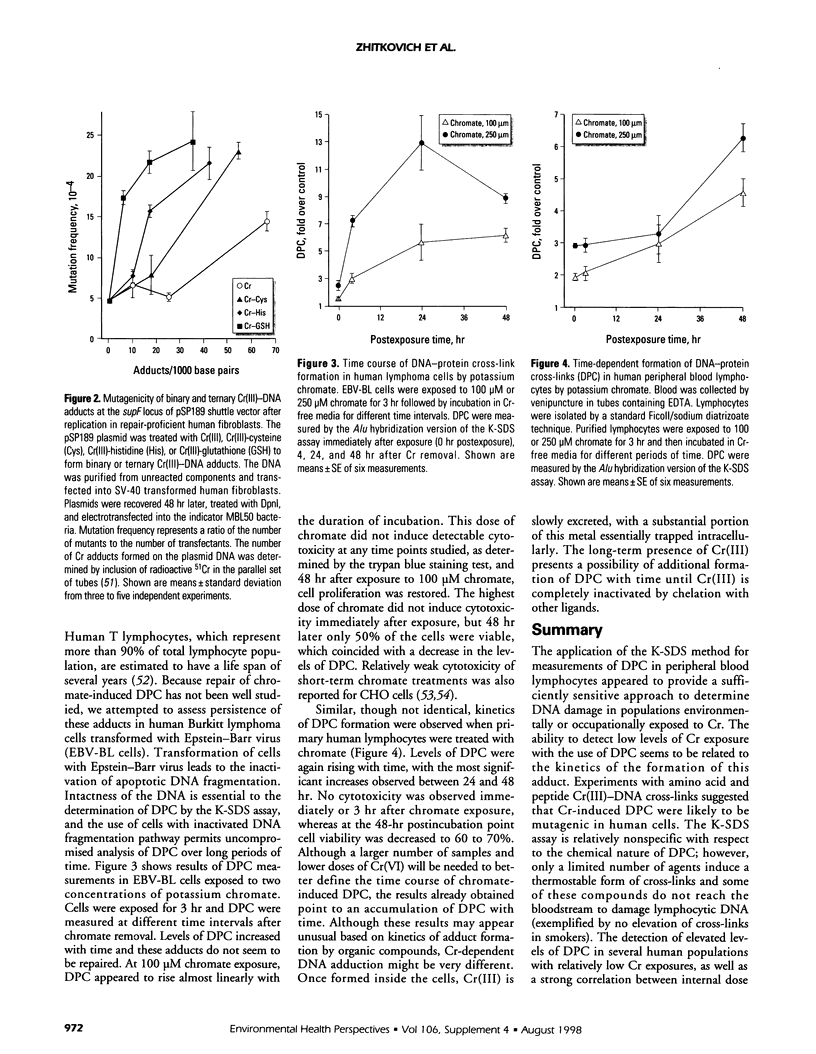
Human exposure to carcinogenic Cr(VI) compounds is found among workers in a large number of professional groups, and it can also occur through environmental pollution. A significant number of toxic waste sites contain Cr as a major contaminant. In this paper we summarize our efforts to apply measurements of DNA-protein cross-links (DPC) as test for biologically active doses of Cr(VI). DPC were found at elevated levels in lymphocytes in several human populations with low to medium Cr exposures. At high exposure to Cr(VI), exemplified by a group of Bulgarian chromeplaters, DPC plateaued and adducts' levels were similar to those found in environmentally exposed individuals. Lymphocytic DPC correlated strongly with Cr levels in erythrocytes that are indicative of Cr(VI) exposure. DPC in lymphocytes were not confounded by such variables as smoking, age, body weight, gender, or ethnicity. A new version of the cross-link assay offers improved sensitivity and requires a small amount of biologic material. Preliminary results indicate that the ability of DPC to reach detectable levels at low levels of Cr exposure could be related to a lack of repair of these lesions in lymphoid cells. Cr(III)-mediated cross-links of DNA with peptide glutathione or single amino acids were mutagenic in human cells, with a relationship of higher molecular weight of the peptide/amino acid correlating with a more potent mutagenic response. We speculate that bulky DPC could also have a significant promutagenic effect. The current methodology does not allow specific determination of Cr-induced DPC; however, demonstrated sensitivity of DPC measurements and the assay's large sample capacity may allow this assay to be used as the initial screening test for the occurrence of DNA damage in Cr(VI)-exposed populations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Berkovits H. J., Floyd R. A., Wetterhahn K. E. Reaction of chromium (VI) with hydrogen peroxide in the presence of glutathione: reactive intermediates and resulting DNA damage. Chem Res Toxicol. 1990 Nov-Dec;3(6):595–603. doi: 10.1021/tx00018a016. [DOI] [PubMed] [Google Scholar]
- Alexander J., Aaseth J. Uptake of chromate in human red blood cells and isolated rat liver cells: the role of the anion carrier. Analyst. 1995 Mar;120(3):931–933. doi: 10.1039/an9952000931. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Bryden N. A., Polansky M. M. Serum chromium of human subjects: effects of chromium supplementation and glucose. Am J Clin Nutr. 1985 Mar;41(3):571–577. doi: 10.1093/ajcn/41.3.571. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Polansky M. M., Bryden N. A., Roginski E. E., Patterson K. Y., Veillon C., Glinsmann W. Urinary chromium excretion of human subjects: effects of chromium supplementation and glucose loading. Am J Clin Nutr. 1982 Dec;36(6):1184–1193. doi: 10.1093/ajcn/36.6.1184. [DOI] [PubMed] [Google Scholar]
- Ariza R. R., Roldán-Arjona T., Hera C., Pueyo C. A method for selection of forward mutations in supF gene carried by shuttle-vector plasmids. Carcinogenesis. 1993 Feb;14(2):303–305. doi: 10.1093/carcin/14.2.303. [DOI] [PubMed] [Google Scholar]
- Blankenship L. J., Carlisle D. L., Wise J. P., Orenstein J. M., Dye L. E., 3rd, Patierno S. R. Induction of apoptotic cell death by particulate lead chromate: differential effects of vitamins C and E on genotoxicity and survival. Toxicol Appl Pharmacol. 1997 Oct;146(2):270–280. doi: 10.1006/taap.1997.8237. [DOI] [PubMed] [Google Scholar]
- Blankenship L. J., Manning F. C., Orenstein J. M., Patierno S. R. Apoptosis is the mode of cell death caused by carcinogenic chromium. Toxicol Appl Pharmacol. 1994 May;126(1):75–83. doi: 10.1006/taap.1994.1092. [DOI] [PubMed] [Google Scholar]
- Bonde J. P., Christensen J. M. Chromium in biological samples from low-level exposed stainless steel and mild steel welders. Arch Environ Health. 1991 Jul-Aug;46(4):225–229. doi: 10.1080/00039896.1991.9937453. [DOI] [PubMed] [Google Scholar]
- Braselmann H., Schmid E., Bauchinger M. Chromosome aberrations in nuclear power plant workers: the influence of dose accumulation and lymphocyte life-time. Mutat Res. 1994 Apr 15;306(2):197–202. doi: 10.1016/0027-5107(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Burke T., Fagliano J., Goldoft M., Hazen R. E., Iglewicz R., McKee T. Chromite ore processing residue in Hudson County, New Jersey. Environ Health Perspect. 1991 May;92:131–137. doi: 10.1289/ehp.9192131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan T. P., Motz J., Snyder C. A., Squibb K. S., Costa M. Differential DNA-protein crosslinking in lymphocytes and liver following chronic drinking water exposure of rats to potassium chromate. Toxicol Appl Pharmacol. 1991 Jun 1;109(1):60–72. doi: 10.1016/0041-008x(91)90191-g. [DOI] [PubMed] [Google Scholar]
- Costa M., Zhitkovich A., Gargas M., Paustenbach D., Finley B., Kuykendall J., Billings R., Carlson T. J., Wetterhahn K., Xu J. Interlaboratory validation of a new assay for DNA-protein crosslinks. Mutat Res. 1996 Jul 10;369(1-2):13–21. doi: 10.1016/s0165-1218(96)90043-9. [DOI] [PubMed] [Google Scholar]
- Costa M., Zhitkovich A., Toniolo P. DNA-protein cross-links in welders: molecular implications. Cancer Res. 1993 Feb 1;53(3):460–463. [PubMed] [Google Scholar]
- De Flora S., Bagnasco M., Serra D., Zanacchi P. Genotoxicity of chromium compounds. A review. Mutat Res. 1990 Mar;238(2):99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- Dillon C. T., Lay P. A., Cholewa M., Legge G. J., Bonin A. M., Collins T. J., Kostka K. L., Shea-McCarthy G. Microprobe X-ray absorption spectroscopic determination of the oxidation state of intracellular chromium following exposure of V79 Chinese hamster lung cells to genotoxic chromium complexes. Chem Res Toxicol. 1997 May;10(5):533–535. doi: 10.1021/tx970010m. [DOI] [PubMed] [Google Scholar]
- Elias Z., Mur J. M., Pierre F., Gilgenkrantz S., Schneider O., Baruthio F., Danière M. C., Fontana J. M. Chromosome aberrations in peripheral blood lymphocytes of welders and characterization of their exposure by biological samples analysis. J Occup Med. 1989 May;31(5):477–483. [PubMed] [Google Scholar]
- Freeman N. C., Stern A. H., Lioy P. J. Exposure to chromium dust from homes in a Chromium Surveillance Project. Arch Environ Health. 1997 May-Jun;52(3):213–219. doi: 10.1080/00039899709602889. [DOI] [PubMed] [Google Scholar]
- Jelmert O., Hansteen I. L., Langård S. Cytogenetic studies of stainless steel welders using the tungsten inert gas and metal inert gas methods for welding. Mutat Res. 1995 Mar;342(1-2):77–85. doi: 10.1016/0165-1218(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Kilburn K. H., Warshaw R., Boylen C. T., Thornton J. C., Hopfer S. M., Sunderman F. W., Jr, Finklea J. Cross-shift and chronic effects of stainless-steel welding related to internal dosimetry of chromium and nickel. Am J Ind Med. 1990;17(5):607–615. doi: 10.1002/ajim.4700170506. [DOI] [PubMed] [Google Scholar]
- Knudsen L. E., Boisen T., Christensen J. M., Jelnes J. E., Jensen G. E., Jensen J. C., Lundgren K., Lundsteen C., Pedersen B., Wassermann K. Biomonitoring of genotoxic exposure among stainless steel welders. Mutat Res. 1992 May 16;279(2):129–143. doi: 10.1016/0165-1218(92)90255-x. [DOI] [PubMed] [Google Scholar]
- Langård S. One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am J Ind Med. 1990;17(2):189–215. doi: 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- Lewalter J., Korallus U., Harzdorf C., Weidemann H. Chromium bond detection in isolated erythrocytes: a new principle of biological monitoring of exposure to hexavalent chromium. Int Arch Occup Environ Health. 1985;55(4):305–318. doi: 10.1007/BF00377689. [DOI] [PubMed] [Google Scholar]
- Lu C., Anderson L. C., Fenske R. A. Determination of atrazine levels in whole saliva and plasma in rats: potential of salivary monitoring for occupational exposure. J Toxicol Environ Health. 1997 Feb 7;50(2):101–111. doi: 10.1080/009841097160519. [DOI] [PubMed] [Google Scholar]
- Nagaya T., Ishikawa N., Hata H., Otobe T. Sister-chromatid exchanges in lymphocytes from 12 chromium platers: a 5-year follow-up study. Toxicol Lett. 1991 Nov;58(3):329–335. doi: 10.1016/0378-4274(91)90045-8. [DOI] [PubMed] [Google Scholar]
- Oesch F., Hengstler J. G., Fuchs J. Cigarette smoking protects mononuclear blood cells of carcinogen exposed workers from additional work exposure-induced DNA single strand breaks. Mutat Res. 1994 May;321(3):175–185. doi: 10.1016/0165-1218(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Olin K. L., Cherr G. N., Rifkin E., Keen C. L. The effects of some redox-active metals and reactive aldehydes on DNA-protein cross-links in vitro. Toxicology. 1996 Jun 17;110(1-3):1–8. doi: 10.1016/0300-483x(96)03318-5. [DOI] [PubMed] [Google Scholar]
- Parris C. N., Seidman M. M. A signature element distinguishes sibling and independent mutations in a shuttle vector plasmid. Gene. 1992 Aug 1;117(1):1–5. doi: 10.1016/0378-1119(92)90482-5. [DOI] [PubMed] [Google Scholar]
- Popp W., Vahrenholz C., Schmieding W., Krewet E., Norpoth K. Investigations of the frequency of DNA strand breakage and cross-linking and of sister chromatid exchange in the lymphocytes of electric welders exposed to chromium- and nickel-containing fumes. Int Arch Occup Environ Health. 1991;63(2):115–120. doi: 10.1007/BF00379074. [DOI] [PubMed] [Google Scholar]
- Salnikow K., Zhitkovich A., Costa M. Analysis of the binding sites of chromium to DNA and protein in vitro and in intact cells. Carcinogenesis. 1992 Dec;13(12):2341–2346. doi: 10.1093/carcin/13.12.2341. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer U., Hechtenberg S., Klyszcz H., Beyersmann D. Accumulation of chromium in Chinese hamster V79-cells and nuclei. Arch Toxicol. 1990;64(6):506–508. doi: 10.1007/BF01977636. [DOI] [PubMed] [Google Scholar]
- Snow E. T. Metal carcinogenesis: mechanistic implications. Pharmacol Ther. 1992;53(1):31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- Standeven A. M., Wetterhahn K. E. Ascorbate is the principal reductant of chromium(VI) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis. 1992 Aug;13(8):1319–1324. doi: 10.1093/carcin/13.8.1319. [DOI] [PubMed] [Google Scholar]
- Stearns D. M., Wetterhahn K. E. Reaction of chromium(VI) with ascorbate produces chromium(V), chromium(IV), and carbon-based radicals. Chem Res Toxicol. 1994 Mar-Apr;7(2):219–230. doi: 10.1021/tx00038a016. [DOI] [PubMed] [Google Scholar]
- Stern A. H., Freeman N. C., Pleban P., Boesch R. R., Wainman T., Howell T., Shupack S. I., Johnson B. B., Lioy P. J. Residential exposure to chromium waste--urine biological monitoring in conjunction with environmental exposure monitoring. Environ Res. 1992 Aug;58(2):147–162. doi: 10.1016/s0013-9351(05)80211-7. [DOI] [PubMed] [Google Scholar]
- Stridsklev I. C., Hemmingsen B., Karlsen J. T., Schaller K. H., Raithel H. J., Langård S. Biologic monitoring of chromium and nickel among stainless steel welders using the manual mental arc method. Int Arch Occup Environ Health. 1993;65(4):209–219. doi: 10.1007/BF00381193. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Fukuda K. Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch Toxicol. 1990;64(3):169–176. doi: 10.1007/BF02010721. [DOI] [PubMed] [Google Scholar]
- Taioli E., Kinney P., Zhitkovich A., Fulton H., Voitkun V., Cosma G., Frenkel K., Toniolo P., Garte S., Costa M. Application of reliability models to studies of biomarker validation. Environ Health Perspect. 1994 Mar;102(3):306–309. doi: 10.1289/ehp.94102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taioli E., Zhitkovich A., Kinney P., Udasin I., Toniolo P., Costa M. Increased DNA-protein crosslinks in lymphocytes of residents living in chromium-contaminated areas. Biol Trace Elem Res. 1995 Dec;50(3):175–180. doi: 10.1007/BF02785408. [DOI] [PubMed] [Google Scholar]
- Voitkun V., Zhitkovich A., Costa M. Complexing of amino acids to DNA by chromate in intact cells. Environ Health Perspect. 1994 Sep;102 (Suppl 3):251–255. doi: 10.1289/ehp.94102s3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voitkun V., Zhitkovich A., Costa M. Cr(III)-mediated crosslinks of glutathione or amino acids to the DNA phosphate backbone are mutagenic in human cells. Nucleic Acids Res. 1998 Apr 15;26(8):2024–2030. doi: 10.1093/nar/26.8.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedrychowski A., Ward W. S., Schmidt W. N., Hnilica L. S. Chromium-induced cross-linking of nuclear proteins and DNA. J Biol Chem. 1985 Jun 10;260(11):7150–7155. [PubMed] [Google Scholar]
- Werfel U., Langen V., Eickhoff I., Schoonbrood J., Vahrenholz C., Brauksiepe A., Popp W., Norpoth K. Elevated DNA single-strand breakage frequencies in lymphocytes of welders exposed to chromium and nickel. Carcinogenesis. 1998 Mar;19(3):413–418. doi: 10.1093/carcin/19.3.413. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A., Costa M. A simple, sensitive assay to detect DNA-protein crosslinks in intact cells and in vivo. Carcinogenesis. 1992 Aug;13(8):1485–1489. doi: 10.1093/carcin/13.8.1485. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A., Voitkun V., Costa M. Formation of the amino acid-DNA complexes by hexavalent and trivalent chromium in vitro: importance of trivalent chromium and the phosphate group. Biochemistry. 1996 Jun 4;35(22):7275–7282. doi: 10.1021/bi960147w. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A., Voitkun V., Costa M. Glutathione and free amino acids form stable complexes with DNA following exposure of intact mammalian cells to chromate. Carcinogenesis. 1995 Apr;16(4):907–913. doi: 10.1093/carcin/16.4.907. [DOI] [PubMed] [Google Scholar]


