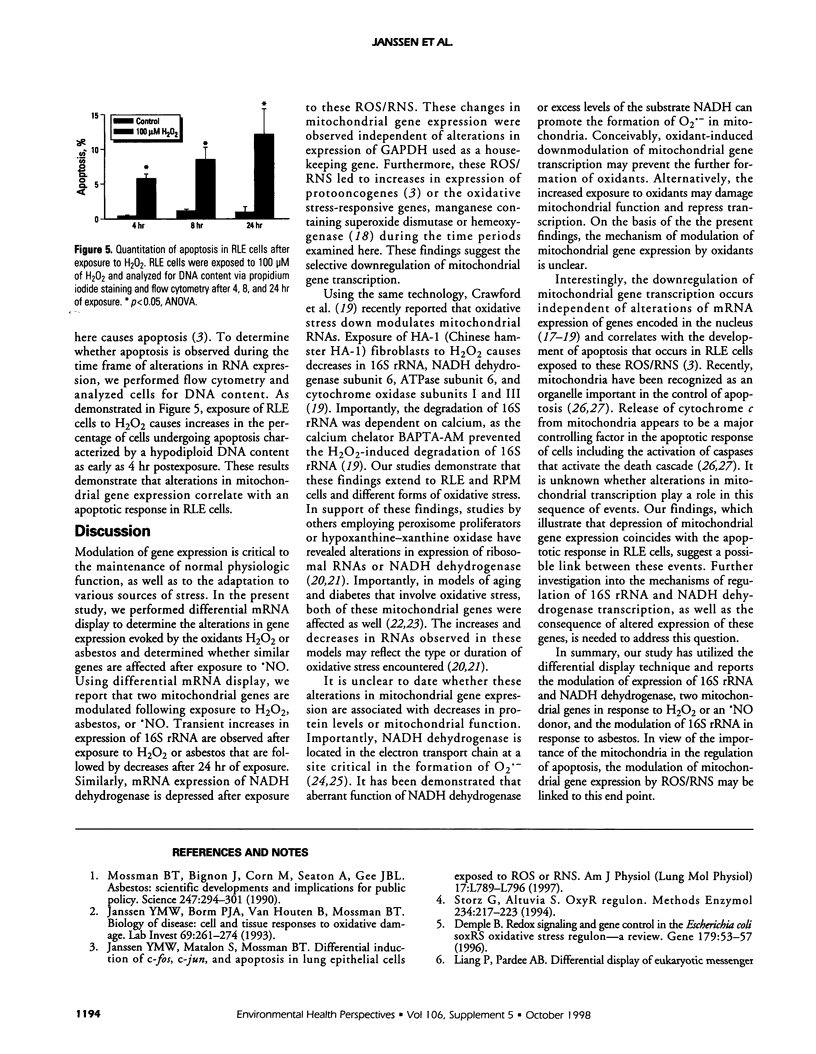
Abstract
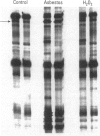
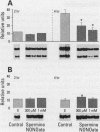
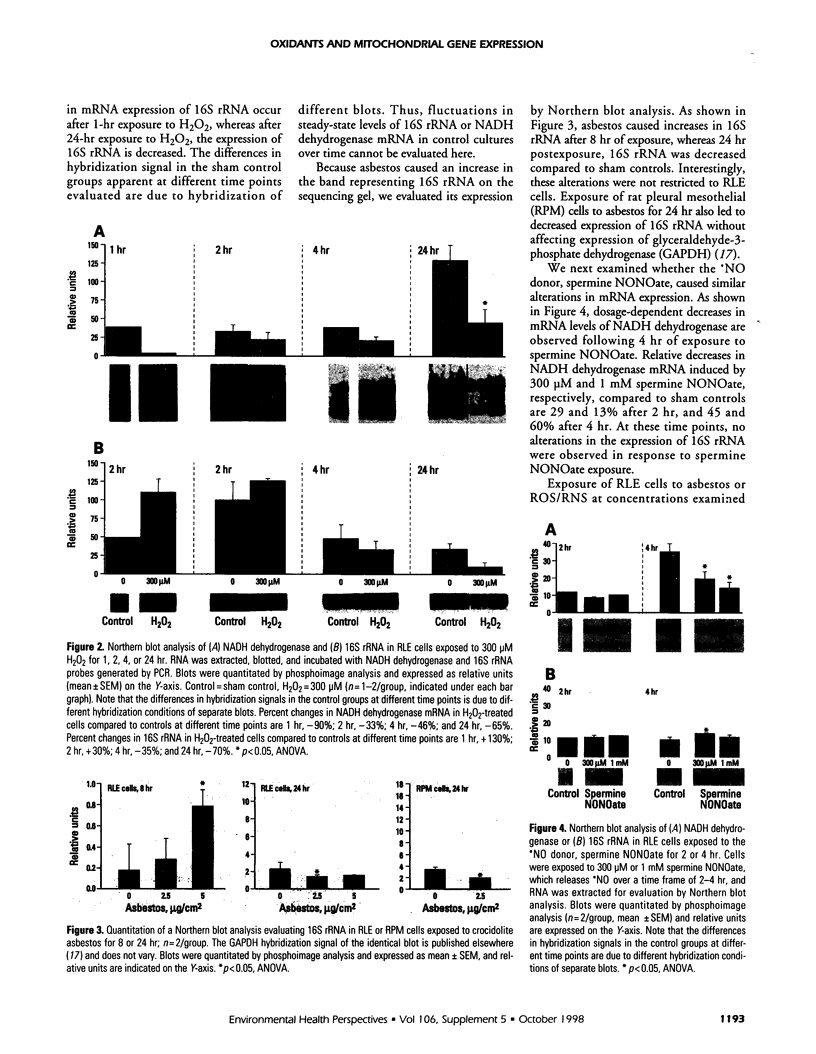
Oxidants are important in the regulation of signal transduction and gene expression. Multiple classes of genes are transcriptionally activated by oxidants and are implicated in different phenotypic responses. In the present study, we performed differential mRNA display to elucidate genes that are induced or repressed after exposure of rat lung epithelial (RLE) cells to H2O2 or crocidolite asbestos, a pathogenic mineral that generates oxidants. After 8 or 24 hr of exposure, RNA was extracted, reverse transcribed, and amplified by polymerase chain reaction with degenerate primers to visualize alterations in gene expression. The seven clones obtained were sequenced and encoded the mitochondrial genes, NADH dehydrogenase subunits ND5 and ND6, and 16S ribosomal RNA. Evaluation of their expression by Northern blot analysis revealed increased expression of 16S rRNA after 1 or 2 hr of exposure to H2O2. At later time periods (4 and 24 hr), mRNA levels of 16S rRNA and NADH dehydrogenase were decreased in H2O2-treated RLE cells when compared to sham controls. Crocidolite asbestos caused increases in 16S rRNA levels after 8 hr of exposure, whereas after 24 hr of exposure to asbestos, 16S rRNA levels were decreased in comparison to sham controls. In addition to these oxidants, the nitric oxide generator spermine NONOate caused similar decreases in NADH dehydrogenase mRNA levels after 4 hr of exposure. The present data and previous studies demonstrated that all oxidants examined resulted in apoptosis in RLE cells during the time frame where alterations of mitochondrial gene expression were observed. As the mitochondrion is a major organelle that controls apoptosis, alterations in expression of mitochondrial genes may be involved in the regulation of apoptosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonetti D. A., Reynet C., Kahn C. R. Increased expression of mitochondrial-encoded genes in skeletal muscle of humans with diabetes mellitus. J Clin Invest. 1995 Mar;95(3):1383–1388. doi: 10.1172/JCI117790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BéruBé K. A., Quinlan T. R., Fung H., Magae J., Vacek P., Taatjes D. J., Mossman B. T. Apoptosis is observed in mesothelial cells after exposure to crocidolite asbestos. Am J Respir Cell Mol Biol. 1996 Jul;15(1):141–147. doi: 10.1165/ajrcmb.15.1.8679218. [DOI] [PubMed] [Google Scholar]
- Cai Y., Nelson B. D., Li R., Luciakova K., dePierre J. W. Thyromimetic action of the peroxisome proliferators clofibrate, perfluorooctanoic acid, and acetylsalicylic acid includes changes in mRNA levels for certain genes involved in mitochondrial biogenesis. Arch Biochem Biophys. 1996 Jan 1;325(1):107–112. doi: 10.1006/abbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crawford D. R., Wang Y., Schools G. P., Kochheiser J., Davies K. J. Down-regulation of mammalian mitochondrial RNAs during oxidative stress. Free Radic Biol Med. 1997;22(3):551–559. doi: 10.1016/s0891-5849(96)00380-2. [DOI] [PubMed] [Google Scholar]
- Demple B. Redox signaling and gene control in the Escherichia coli soxRS oxidative stress regulon--a review. Gene. 1996 Nov 7;179(1):53–57. doi: 10.1016/s0378-1119(96)00329-0. [DOI] [PubMed] [Google Scholar]
- Doggett D. L., Rotenberg M. O., Pignolo R. J., Phillips P. D., Cristofalo V. J. Differential gene expression between young and senescent, quiescent WI-38 cells. Mech Ageing Dev. 1992 Sep;65(2-3):239–255. doi: 10.1016/0047-6374(92)90039-g. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Carter J. M., Iype P. T., Kumari H. L., Crosby L. L., Aardema M. J., Isfort R. J., Cody D., Chestnut M. H., Burns J. L. Establishment of immortalized alveolar type II epithelial cell lines from adult rats. In Vitro Cell Dev Biol Anim. 1995 Jul-Aug;31(7):516–527. doi: 10.1007/BF02634029. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Janssen Y. M., Mossman B. T. Persistent induction of c-fos and c-jun expression by asbestos. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3299–3303. doi: 10.1073/pnas.90.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen Y. M., Heintz N. H., Marsh J. P., Borm P. J., Mossman B. T. Induction of c-fos and c-jun proto-oncogenes in target cells of the lung and pleura by carcinogenic fibers. Am J Respir Cell Mol Biol. 1994 Nov;11(5):522–530. doi: 10.1165/ajrcmb.11.5.7946382. [DOI] [PubMed] [Google Scholar]
- Janssen Y. M., Heintz N. H., Mossman B. T. Induction of c-fos and c-jun proto-oncogene expression by asbestos is ameliorated by N-acetyl-L-cysteine in mesothelial cells. Cancer Res. 1995 May 15;55(10):2085–2089. [PubMed] [Google Scholar]
- Janssen Y. M., Van Houten B., Borm P. J., Mossman B. T. Cell and tissue responses to oxidative damage. Lab Invest. 1993 Sep;69(3):261–274. [PubMed] [Google Scholar]
- Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997 Feb 21;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G., Hinkle P. C. Studies on the electron transfer pathway, topography of iron-sulfur centers, and site of coupling in NADH-Q oxidoreductase. J Biol Chem. 1988 Nov 25;263(33):17566–17575. [PubMed] [Google Scholar]
- Liang P., Averboukh L., Keyomarsi K., Sager R., Pardee A. B. Differential display and cloning of messenger RNAs from human breast cancer versus mammary epithelial cells. Cancer Res. 1992 Dec 15;52(24):6966–6968. [PubMed] [Google Scholar]
- Liang P., Averboukh L., Pardee A. B. Distribution and cloning of eukaryotic mRNAs by means of differential display: refinements and optimization. Nucleic Acids Res. 1993 Jul 11;21(14):3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Bignon J., Corn M., Seaton A., Gee J. B. Asbestos: scientific developments and implications for public policy. Science. 1990 Jan 19;247(4940):294–301. doi: 10.1126/science.2153315. [DOI] [PubMed] [Google Scholar]
- Nohl H. Generation of superoxide radicals as byproduct of cellular respiration. Ann Biol Clin (Paris) 1994;52(3):199–204. [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Neveu M., Liang P., Sotiropoulou G. Identification by differential display of alpha 6 integrin as a candidate tumor suppressor gene. FASEB J. 1993 Jul;7(10):964–970. doi: 10.1096/fasebj.7.10.8344495. [DOI] [PubMed] [Google Scholar]
- Storz G., Altuvia S. OxyR regulon. Methods Enzymol. 1994;234:217–223. doi: 10.1016/0076-6879(94)34088-9. [DOI] [PubMed] [Google Scholar]
- Sun Y., Hegamyer G., Colburn N. H. Molecular cloning of five messenger RNAs differentially expressed in preneoplastic or neoplastic JB6 mouse epidermal cells: one is homologous to human tissue inhibitor of metalloproteinases-3. Cancer Res. 1994 Mar 1;54(5):1139–1144. [PubMed] [Google Scholar]
- Utans U., Liang P., Wyner L. R., Karnovsky M. J., Russell M. E. Chronic cardiac rejection: identification of five upregulated genes in transplanted hearts by differential mRNA display. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6463–6467. doi: 10.1073/pnas.91.14.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F., Corral-Debrinski M., Adolphe M. Transient mitochondrial transcript level decay in oxidative stressed chondrocytes. J Cell Physiol. 1994 Jan;158(1):128–132. doi: 10.1002/jcp.1041580116. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997 Feb 21;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]