Abstract
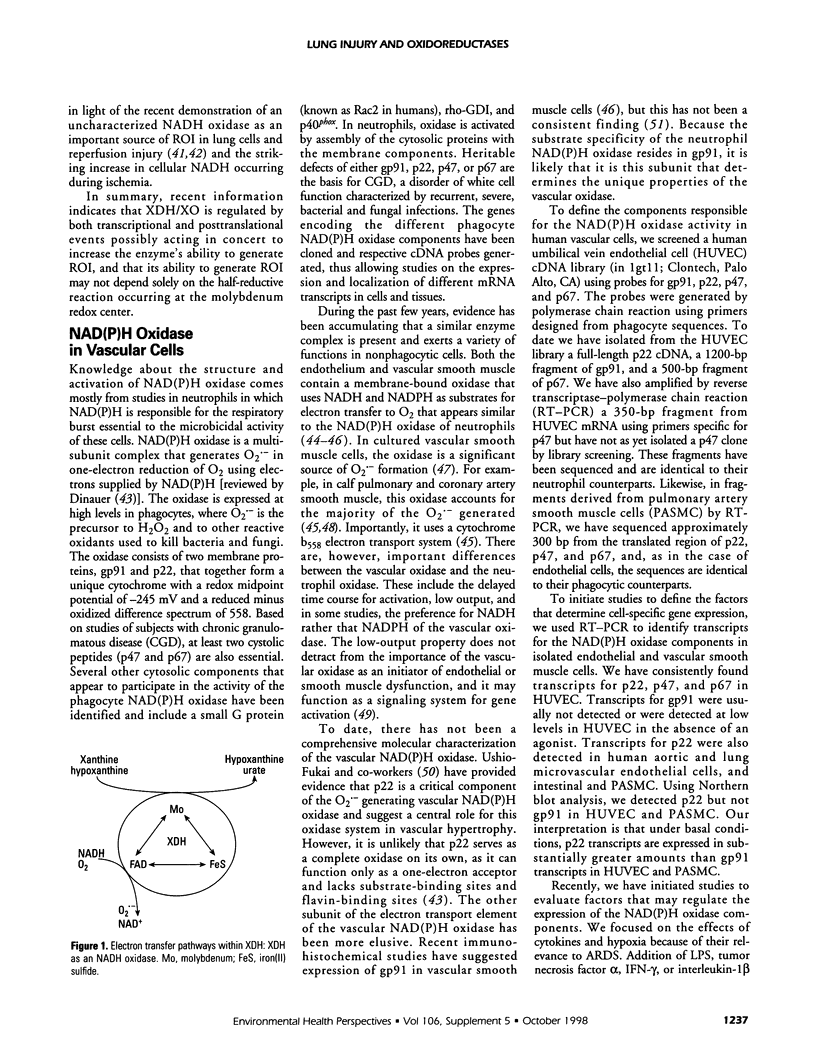
Acute lung injury represents a wide spectrum of pathologic processes, the most severe end of the spectrum being the acute respiratory distress syndrome. Reactive oxygen intermediates have been implicated as important in the pathobiochemistry of acute lung injury. The endogenous sources that contribute to the generation of reactive oxygen intermediates in acute lung injury are poorly defined but probably include the molybdenum hydroxylases, NAD(P)H oxidoreductases, the mitochondrial electron transport chain, and arachidonic acid-metabolizing enzymes. Our laboratory has focused, in particular, on the regulation of two of these enzyme systems, xanthine oxidoreductase (XDH/XO) and NAD(P)H oxidase. We observe that gene expression of XDH/XO is regulatory in a cell-specific manner and is markedly affected by inflammatory cytokines, steroids, and physiologic events such as hypoxia. Posttranslational processing is also important in regulating XDH/XO activity. More recently, the laboratory has characterized an NAD(P)H oxidase in vascular cells. The cytochrome components of the oxidase, gp91 and p22, appear similar to the components present in phagocytic cells that contribute to their respiratory burst. In human vascular endothelial and smooth muscle cells, oncostatin M potently induces gp91 expression. We believe that regulation of gp91 is a central controlling factor in expression of the vascular NAD(P)H oxidase. In summary, the studies support the concept that the oxidoreductases of vascular cells are expressed in a highly regulated and self-specific fashion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Fukushima T., Usami Y., Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem J. 1993 Jan 15;289(Pt 2):523–527. doi: 10.1042/bj2890523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T., Ando M., Oda T., Doi T., Ijiri S., Araki S., Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest. 1990 Mar;85(3):739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya Y., Yamazaki K., Sato M., Noda K., Nishino T., Nishino T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem. 1990 Aug 25;265(24):14170–14175. [PubMed] [Google Scholar]
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. O., Bensard D. D., Brown J. M., Repine J. E., Shanley P. F., Leff J. A., Terada L. S., Banerjee A., Harken A. H. FNLP injures endotoxin-primed rat lung by neutrophil-dependent and -independent mechanisms. Am J Physiol. 1991 Feb;260(2 Pt 2):R413–R420. doi: 10.1152/ajpregu.1991.260.2.R413. [DOI] [PubMed] [Google Scholar]
- Anderson B. O., Moore E. E., Moore F. A., Leff J. A., Terada L. S., Harken A. H., Repine J. E. Hypovolemic shock promotes neutrophil sequestration in lungs by a xanthine oxidase-related mechanism. J Appl Physiol (1985) 1991 Nov;71(5):1862–1865. doi: 10.1152/jappl.1991.71.5.1862. [DOI] [PubMed] [Google Scholar]
- Anderson B. O., Moore E. E., Moore F. A., Leff J. A., Terada L. S., Harken A. H., Repine J. E. Hypovolemic shock promotes neutrophil sequestration in lungs by a xanthine oxidase-related mechanism. J Appl Physiol (1985) 1991 Nov;71(5):1862–1865. doi: 10.1152/jappl.1991.71.5.1862. [DOI] [PubMed] [Google Scholar]
- Beale R., Grover E. R., Smithies M., Bihari D. Acute respiratory distress syndrome ("ARDS"): no more than a severe acute lung injury? BMJ. 1993 Nov 20;307(6915):1335–1339. doi: 10.1136/bmj.307.6915.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R. S., Martin W. Arterial endothelial barrier dysfunction: actions of homocysteine and the hypoxanthine-xanthine oxidase free radical generating system. Br J Pharmacol. 1993 Apr;108(4):920–926. doi: 10.1111/j.1476-5381.1993.tb13487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard G. R., Artigas A., Brigham K. L., Carlet J., Falke K., Hudson L., Lamy M., Legall J. R., Morris A., Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- Brigham K. L. Oxygen radicals--an important mediator of sepsis and septic shock. Klin Wochenschr. 1991 Dec 15;69(21-23):1004–1008. doi: 10.1007/BF01645147. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Benboubetra M., Ellison M., Powell D., Reckless J. D., Harrison R. Molecular activation-deactivation of xanthine oxidase in human milk. Biochim Biophys Acta. 1995 Oct 19;1245(2):248–254. doi: 10.1016/0304-4165(95)00093-q. [DOI] [PubMed] [Google Scholar]
- Bunnell E., Pacht E. R. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1993 Nov;148(5):1174–1178. doi: 10.1164/ajrccm/148.5.1174. [DOI] [PubMed] [Google Scholar]
- Crawford L. E., Milliken E. E., Irani K., Zweier J. L., Becker L. C., Johnson T. M., Eissa N. T., Crystal R. G., Finkel T., Goldschmidt-Clermont P. J. Superoxide-mediated actin response in post-hypoxic endothelial cells. J Biol Chem. 1996 Oct 25;271(43):26863–26867. doi: 10.1074/jbc.271.43.26863. [DOI] [PubMed] [Google Scholar]
- DeForge L. E., Preston A. M., Takeuchi E., Kenney J., Boxer L. A., Remick D. G. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993 Dec 5;268(34):25568–25576. [PubMed] [Google Scholar]
- Dinauer M. C. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit Rev Clin Lab Sci. 1993;30(4):329–369. doi: 10.3109/10408369309082591. [DOI] [PubMed] [Google Scholar]
- Dupont G. P., Huecksteadt T. P., Marshall B. C., Ryan U. S., Michael J. R., Hoidal J. R. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J Clin Invest. 1992 Jan;89(1):197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Turrens J. F., Mirza Z., Crapo J. D., Young S. L. Modulation of oxidant lung injury by using liposome-entrapped superoxide dismutase and catalase. Fed Proc. 1985 Jul;44(10):2591–2595. [PubMed] [Google Scholar]
- Friedl H. P., Till G. O., Trentz O., Ward P. A. Role of oxygen radicals in tourniquet-related ischemia-reperfusion injury of human patients. Klin Wochenschr. 1991 Dec 15;69(21-23):1109–1112. doi: 10.1007/BF01645168. [DOI] [PubMed] [Google Scholar]
- Giler S., Sperling O., Brosh S., Urca I., De Vries A. Serum xanthine oxidase in jaundice. Clin Chim Acta. 1975 Aug 18;63(1):37–40. doi: 10.1016/0009-8981(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A. Oxygen radicals, inflammation, and arthritis: pathophysiological considerations and implications for treatment. Semin Arthritis Rheum. 1991 Feb;20(4):219–240. doi: 10.1016/0049-0172(91)90018-u. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Minieri C. A., Ollerenshaw J. D., Alexander R. W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994 Jun;74(6):1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Haddad I. Y., Pataki G., Hu P., Galliani C., Beckman J. S., Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994 Dec;94(6):2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun P. M., Yu F. S., Shedd A. L., Zulueta J. J., Thannickal V. J., Lanzillo J. J., Fanburg B. L. Regulation of endothelial cell xanthine dehydrogenase xanthine oxidase gene expression by oxygen tension. Am J Physiol. 1994 Feb;266(2 Pt 1):L163–L171. doi: 10.1152/ajplung.1994.266.2.L163. [DOI] [PubMed] [Google Scholar]
- Kennedy T. P., Rao N. V., Hopkins C., Pennington L., Tolley E., Hoidal J. R. Role of reactive oxygen species in reperfusion injury of the rabbit lung. J Clin Invest. 1989 Apr;83(4):1326–1335. doi: 10.1172/JCI114019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki M., Li Calzi M., Scanziani E., Garattini E., Terao M. Tissue- and cell-specific expression of mouse xanthine oxidoreductase gene in vivo: regulation by bacterial lipopolysaccharide. Biochem J. 1995 Feb 15;306(Pt 1):225–234. doi: 10.1042/bj3060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon S. V., Martin S. J., Cotter T. G. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991 Mar;24(2):203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Marshall C., Mamary A. J., Verhoeven A. J., Marshall B. E. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996 Nov;15(5):633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- Marui N., Offermann M. K., Swerlick R., Kunsch C., Rosen C. A., Ahmad M., Alexander R. W., Medford R. M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993 Oct;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohazzab-H K. M., Kaminski P. M., Fayngersh R. P., Wolin M. S. Oxygen-elicited responses in calf coronary arteries: role of H2O2 production via NADH-derived superoxide. Am J Physiol. 1996 Mar;270(3 Pt 2):H1044–H1053. doi: 10.1152/ajpheart.1996.270.3.H1044. [DOI] [PubMed] [Google Scholar]
- Mohazzab-H K. M., Kaminski P. M., Wolin M. S. Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation. 1997 Jul 15;96(2):614–620. doi: 10.1161/01.cir.96.2.614. [DOI] [PubMed] [Google Scholar]
- Mohazzab K. M., Kaminski P. M., Wolin M. S. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994 Jun;266(6 Pt 2):H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Minton T. A., Mukundan C. R., Campbell M. D., Zackert W. E., Daniel V. C., Badr K. F., Blair I. A., Roberts L. J., 2nd Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J Biol Chem. 1994 Feb 11;269(6):4317–4326. [PubMed] [Google Scholar]
- Oda T., Akaike T., Hamamoto T., Suzuki F., Hirano T., Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989 May 26;244(4907):974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- Pagano P. J., Ito Y., Tornheim K., Gallop P. M., Tauber A. I., Cohen R. A. An NADPH oxidase superoxide-generating system in the rabbit aorta. Am J Physiol. 1995 Jun;268(6 Pt 2):H2274–H2280. doi: 10.1152/ajpheart.1995.268.6.H2274. [DOI] [PubMed] [Google Scholar]
- Panus P. C., Burgess B., Freeman B. A. Characterization of cultured alveolar epithelial cell xanthine dehydrogenase/oxidase. Biochim Biophys Acta. 1991 Feb 19;1091(3):303–309. doi: 10.1016/0167-4889(91)90193-2. [DOI] [PubMed] [Google Scholar]
- Pfeffer K. D., Huecksteadt T. P., Hoidal J. R. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulation. J Immunol. 1994 Aug 15;153(4):1789–1797. [PubMed] [Google Scholar]
- Poss W. B., Huecksteadt T. P., Panus P. C., Freeman B. A., Hoidal J. R. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am J Physiol. 1996 Jun;270(6 Pt 1):L941–L946. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- Radi R., Bush K. M., Cosgrove T. P., Freeman B. A. Reaction of xanthine oxidase-derived oxidants with lipid and protein of human plasma. Arch Biochem Biophys. 1991 Apr;286(1):117–125. doi: 10.1016/0003-9861(91)90016-c. [DOI] [PubMed] [Google Scholar]
- Rinaldo J. E., Clark M., Parinello J., Shepherd V. L. Nitric oxide inactivates xanthine dehydrogenase and xanthine oxidase in interferon-gamma-stimulated macrophages. Am J Respir Cell Mol Biol. 1994 Nov;11(5):625–630. doi: 10.1165/ajrcmb.11.5.7524568. [DOI] [PubMed] [Google Scholar]
- Rinaldo J. E., Gorry M. Protection by deferoxamine from endothelial injury: a possible link with inhibition of intracellular xanthine oxidase. Am J Respir Cell Mol Biol. 1990 Dec;3(6):525–533. doi: 10.1165/ajrcmb/3.6.525. [DOI] [PubMed] [Google Scholar]
- Sanders S. A., Eisenthal R., Harrison R. NADH oxidase activity of human xanthine oxidoreductase--generation of superoxide anion. Eur J Biochem. 1997 May 1;245(3):541–548. doi: 10.1111/j.1432-1033.1997.00541.x. [DOI] [PubMed] [Google Scholar]
- Satriano J. A., Shuldiner M., Hora K., Xing Y., Shan Z., Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J Clin Invest. 1993 Sep;92(3):1564–1571. doi: 10.1172/JCI116737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellak H., Franzini E., Hakim J., Pasquier C. Reactive oxygen species rapidly increase endothelial ICAM-1 ability to bind neutrophils without detectable upregulation. Blood. 1994 May 1;83(9):2669–2677. [PubMed] [Google Scholar]
- Thannickal V. J., Fanburg B. L. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995 Dec 22;270(51):30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M., Zafari A. M., Fukui T., Ishizaka N., Griendling K. K. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996 Sep 20;271(38):23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- Weinbroum A., Nielsen V. G., Tan S., Gelman S., Matalon S., Skinner K. A., Bradley E., Jr, Parks D. A. Liver ischemia-reperfusion increases pulmonary permeability in rat: role of circulating xanthine oxidase. Am J Physiol. 1995 Jun;268(6 Pt 1):G988–G996. doi: 10.1152/ajpgi.1995.268.6.G988. [DOI] [PubMed] [Google Scholar]
- Whorton A. R., Montgomery M. E., Kent R. S. Effect of hydrogen peroxide on prostaglandin production and cellular integrity in cultured porcine aortic endothelial cells. J Clin Invest. 1985 Jul;76(1):295–302. doi: 10.1172/JCI111960. [DOI] [PMC free article] [PubMed] [Google Scholar]


