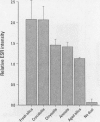
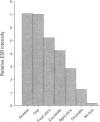
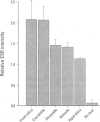
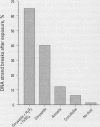
Abstract
Occupational exposures to mineral particles cause pneumoconiosis and other diseases, including cancer. Recent studies have suggested that reactive oxygen species (ROS) may play a key role in the mechanisms of disease initiation and progression following exposure to these particles. ROS-induced primary stimuli result in the increased secretion of proinflammatory cytokines and other mediators, promoting events that appear to be important in the progression of cell injury and pulmonary disease. We have provided evidence supporting the hypothesis that inhalation of insoluble particles such as asbestos, agricultural dusts, coal, crystalline silica, and inorganic dust can be involved in facilitating multiple pathways for persistent generation of ROS, which may lead to a continuum of inflammation leading to progression of disease. This article briefly summarizes some of the recent findings from our laboratories with emphasis on the molecular events by which ROS are involved in promoting pneumoconiosis and carcinogenesis.
Full text
PDF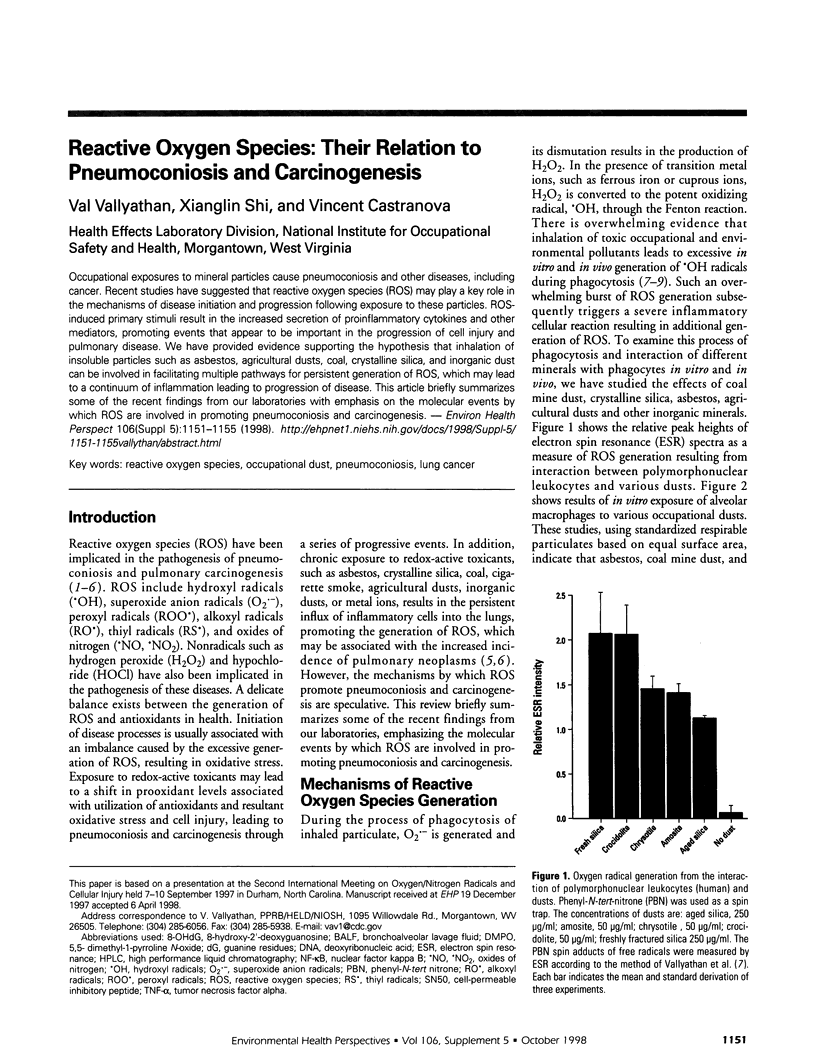
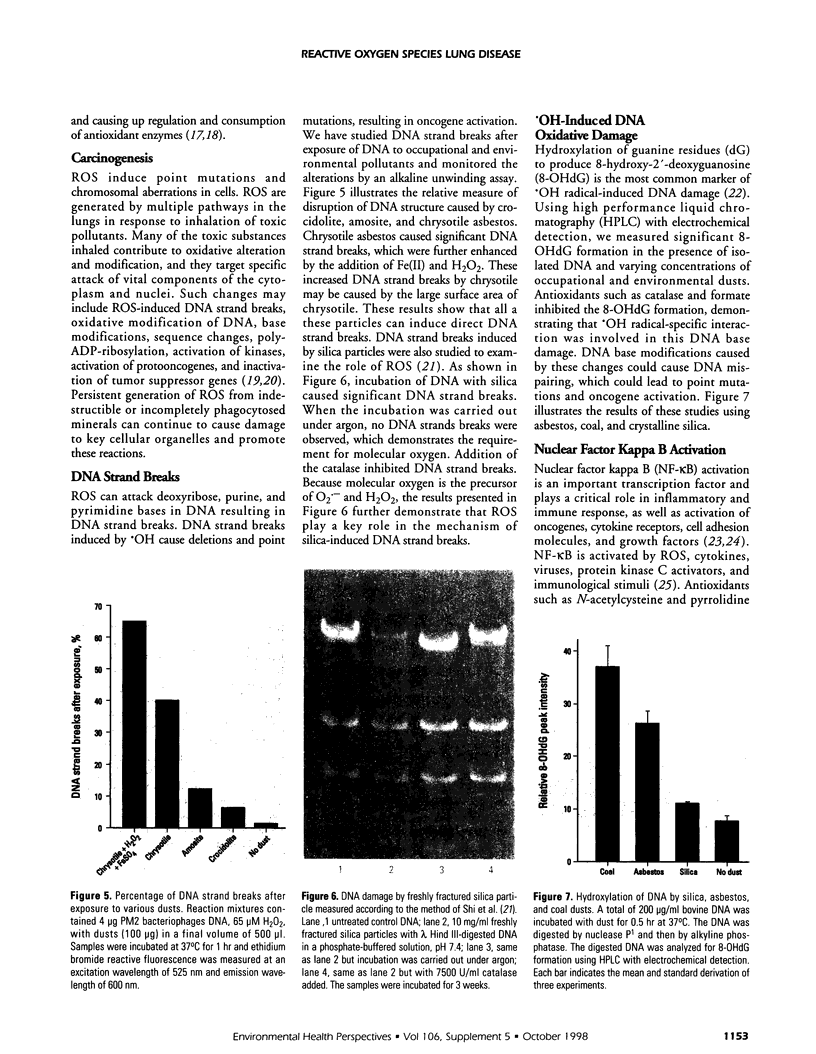
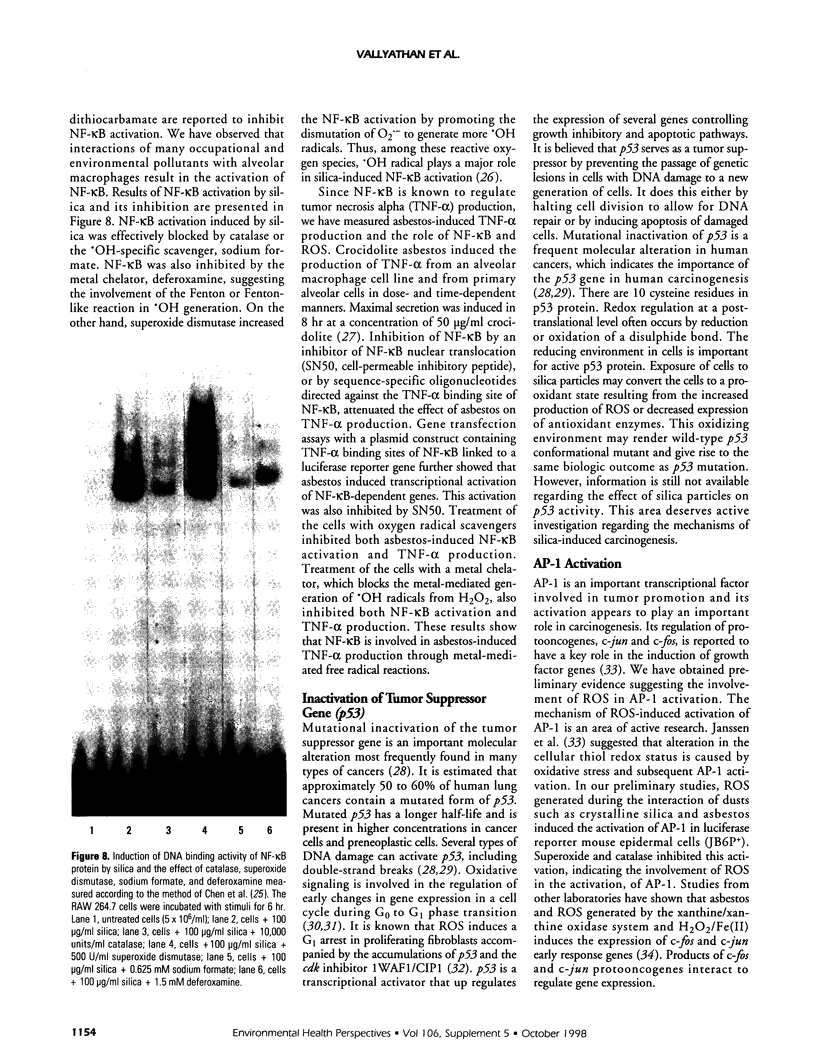

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Brigham K. L. Role of free radicals in lung injury. Chest. 1986 Jun;89(6):859–863. doi: 10.1378/chest.89.6.859. [DOI] [PubMed] [Google Scholar]
- Chen F., Lu Y., Demers L. M., Rojanasakul Y., Shi X., Vallyathan V., Castranova V. Role of hydroxyl radical in silica-induced NF-kappa B activation in macrophages. Ann Clin Lab Sci. 1998 Jan-Feb;28(1):1–13. [PubMed] [Google Scholar]
- Dizdaroglu M. Chemical determination of free radical-induced damage to DNA. Free Radic Biol Med. 1991;10(3-4):225–242. doi: 10.1016/0891-5849(91)90080-m. [DOI] [PubMed] [Google Scholar]
- Gansauge S., Gansauge F., Gause H., Poch B., Schoenberg M. H., Beger H. G. The induction of apoptosis in proliferating human fibroblasts by oxygen radicals is associated with a p53- and p21WAF1CIP1 induction. FEBS Lett. 1997 Mar 3;404(1):6–10. doi: 10.1016/s0014-5793(97)00059-8. [DOI] [PubMed] [Google Scholar]
- Goldstone S. D., Hunt N. H. Redox regulation of the mitogen-activated protein kinase pathway during lymphocyte activation. Biochim Biophys Acta. 1997 Mar 1;1355(3):353–360. doi: 10.1016/s0167-4889(96)00150-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993 Oct 28;329(18):1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- Harris C. C. p53: at the crossroads of molecular carcinogenesis and risk assessment. Science. 1993 Dec 24;262(5142):1980–1981. doi: 10.1126/science.8266092. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Recio L., Katz D. S., Lee C. Q., Wagner M., Schenley R. L. Evidence for reactive oxygen species inducing mutations in mammalian cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9616–9620. doi: 10.1073/pnas.83.24.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiiska M. B., Mason R. P., Dreher K. L., Costa D. L., Ghio A. J. In vivo evidence of free radical formation in the rat lung after exposure to an emission source air pollution particle. Chem Res Toxicol. 1997 Oct;10(10):1104–1108. doi: 10.1021/tx970049r. [DOI] [PubMed] [Google Scholar]
- Kehrer J. P. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23(1):21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- Levine A. J. p53, the cellular gatekeeper for growth and division. Cell. 1997 Feb 7;88(3):323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Meyer M., Pahl H. L., Baeuerle P. A. Regulation of the transcription factors NF-kappa B and AP-1 by redox changes. Chem Biol Interact. 1994 Jun;91(2-3):91–100. doi: 10.1016/0009-2797(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S., Hu X. N., Vallyathan V. The chemical properties of silica particle surface in relation to silica-cell interactions. J Toxicol Environ Health. 1989;27(4):435–454. doi: 10.1080/15287398909531314. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S., Vallyathan V. ESR evidence for the hydroxyl radical formation in aqueous suspension of quartz particles and its possible significance to lipid peroxidation in silicosis. J Toxicol Environ Health. 1988;25(2):237–245. doi: 10.1080/15287398809531205. [DOI] [PubMed] [Google Scholar]
- Shi X., Castranova V., Halliwell B., Vallyathan V. Reactive oxygen species and silica-induced carcinogenesis. J Toxicol Environ Health B Crit Rev. 1998 Jul-Sep;1(3):181–197. doi: 10.1080/10937409809524551. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Franzoso G., Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Castranova V., Pack D., Leonard S., Shumaker J., Hubbs A. F., Shoemaker D. A., Ramsey D. M., Pretty J. R., McLaurin J. L. Freshly fractured quartz inhalation leads to enhanced lung injury and inflammation. Potential role of free radicals. Am J Respir Crit Care Med. 1995 Sep;152(3):1003–1009. doi: 10.1164/ajrccm.152.3.7663775. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Mega J. F., Shi X., Dalal N. S. Enhanced generation of free radicals from phagocytes induced by mineral dusts. Am J Respir Cell Mol Biol. 1992 Apr;6(4):404–413. doi: 10.1165/ajrcmb/6.4.404. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Shi X. L., Dalal N. S., Irr W., Castranova V. Generation of free radicals from freshly fractured silica dust. Potential role in acute silica-induced lung injury. Am Rev Respir Dis. 1988 Nov;138(5):1213–1219. doi: 10.1164/ajrccm/138.5.1213. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Shi X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ Health Perspect. 1997 Feb;105 (Suppl 1):165–177. doi: 10.1289/ehp.97105s1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Mutation caused by human phagocytes. Science. 1981 May 1;212(4494):546–547. doi: 10.1126/science.6259738. [DOI] [PubMed] [Google Scholar]
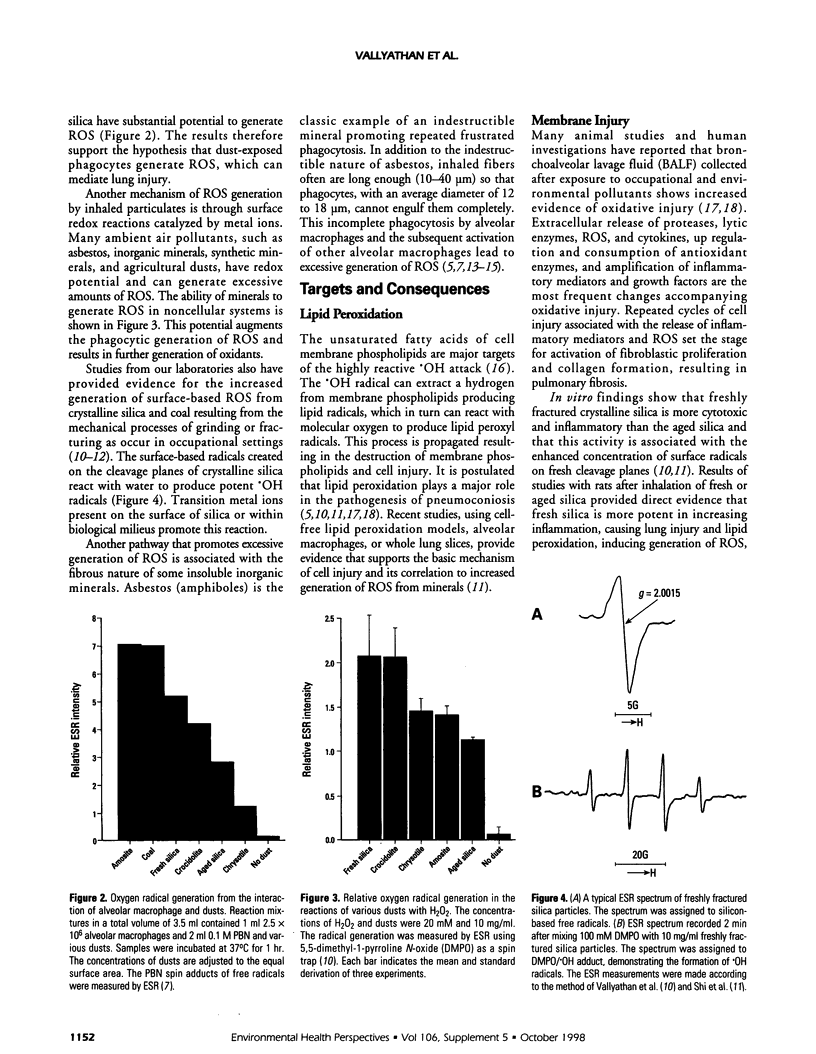
- Wiseman H., Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996 Jan 1;313(Pt 1):17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]