Abstract
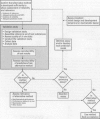
Before nonanimal toxicity tests may be officially accepted by regulatory agencies, it is generally agreed that the validity of the new methods must be demonstrated in an independent, scientifically sound validation program. Validation has been defined as the demonstration of the reliability and relevance of a test method for a particular purpose. This paper provides a brief review of the development of the theoretical aspects of the validation process and updates current thinking about objectively testing the performance of an alternative method in a validation study. Validation of alternative methods for eye irritation testing is a specific example illustrating important concepts. Although discussion focuses on the validation of alternative methods intended to replace current in vivo toxicity tests, the procedures can be used to assess the performance of alternative methods intended for other uses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balls M. In vitro methods in regulatory toxicology: the crucial significance of validation. Arch Toxicol Suppl. 1995;17:155–162. doi: 10.1007/978-3-642-79451-3_13. [DOI] [PubMed] [Google Scholar]
- Balls M. Replacement of animal procedures: alternatives in research, education and testing. Lab Anim. 1994 Jul;28(3):193–211. doi: 10.1258/002367794780681714. [DOI] [PubMed] [Google Scholar]
- Balls Michael, Goldberg Alan M., Fentem Julia H., Broadhead Caren L., Burch Rex L., Festing Michael F. W., Frazier John M., Hendriksen Coenraad F. M., Jennings Margaret, van der Kamp Margot D. O. The three Rs: the way forward: the report and recommendations of ECVAM Workshop 11. Altern Lab Anim. 1995 Nov-Dec;23(6):838–866. [PubMed] [Google Scholar]
- Frazier J. M. In vitro models for toxicological research and testing. Toxicol Lett. 1993 May;68(1-2):73–90. doi: 10.1016/0378-4274(93)90121-d. [DOI] [PubMed] [Google Scholar]
- Goldberg A. M., Frazier J. M. Alternatives to animals in toxicity testing. Sci Am. 1989 Aug;261(2):24–30. doi: 10.1038/scientificamerican0889-24. [DOI] [PubMed] [Google Scholar]
- Goldberg A. M., Frazier J. M., Brusick D., Dickens M. S., Flint O., Gettings S. D., Hill R. N., Lipnick R. L., Renskers K. J., Bradlaw J. A. Framework for validation and implementation of in vitro toxicity tests. In Vitro Cell Dev Biol Anim. 1993 Sep;29A(9):688–692. doi: 10.1007/BF02631424. [DOI] [PubMed] [Google Scholar]
- Hunter W. J., Lingk W., Recht P. Intercomparison study on the determination of single administration toxicity in rats. J Assoc Off Anal Chem. 1979 Jul;62(4):864–873. [PubMed] [Google Scholar]
- Weil C. S., Scala R. A. Study of intra- and interlaboratory variability in the results of rabbit eye and skin irritation tests. Toxicol Appl Pharmacol. 1971 Jun;19(2):276–360. doi: 10.1016/0041-008x(71)90112-8. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M. J., Clark D. G., Fielder R. J., Koundakjian P. P., Oliver G. J., Pelling D., Tomlinson N. J., Walker A. P. The international validation of a fixed-dose procedure as an alternative to the classical LD50 test. Food Chem Toxicol. 1990 Jul;28(7):469–482. doi: 10.1016/0278-6915(90)90117-6. [DOI] [PubMed] [Google Scholar]