Abstract
The anterior nares are a primary ecologic niche for Staphylococcus aureus, and nasal colonization by this opportunistic pathogen increases the risk of development of S. aureus infection. Clearance of S. aureus nasal colonization greatly reduces this risk. Mupirocin ointment is the current standard of care for clearance of S. aureus nasal colonization, but resistance to this antibiotic is emerging. Lysostaphin is a glycylglycine endopeptidase which specifically cleaves the cross-linking pentaglycine bridges in the cell walls of staphylococci. Lysostaphin is extremely staphylocidal (MIC at which 90% of isolates are inhibited, 0.001 to 0.064 μg/ml) and rapidly lyses both actively growing and quiescent S. aureus. This study demonstrates that a single application of 0.5% lysostaphin (actual dose, ∼150 μg of lysostaphin), formulated in a petrolatum-based cream, dramatically reduces S. aureus nasal colonization in 100% of animals tested and eradicates S. aureus nasal colonization in 93% of animals in a cotton rat model. A single dose of lysostaphin cream is more effective than a single dose of mupirocin ointment in eradicating S. aureus nasal colonization in this animal model. The lantibiotic peptide nisin, which has potent in vitro antistaphylococcal activity, was ineffective in reducing staphylococcal nasal carriage in this model. Nasal colonization was not reduced after three treatments with 5% nisin (∼1,500 μg/dose) in any of the treated animals. Lysostaphin formulated in cream may prove to be a superior alternative to mupirocin ointment for clearance of S. aureus nasal colonization.
Staphylococcus aureus infection remains one of the most common nosocomial and community-acquired infections (1). With the continuing emergence of methicillin-resistant S. aureus (MRSA) (22) and strains of S. aureus that are intermediately resistant to glycopeptides (45) and the isolation of the first clinical strain of S. aureus that is fully vancomycin resistant (5), S. aureus is becoming an even more difficult health problem to address, particularly in settings such as hospitals and nursing homes.
The anterior nares of humans are a principal ecologic niche for both methicillin-sensitive S. aureus (MSSA) and MRSA (17, 26, 28, 51). Indeed, nasal carriage of S. aureus has been repeatedly shown to have a significant epidemiological link with subsequent development of staphylococcal disease, particularly for those requiring major surgery, implanted devices, hemodialysis, or treatment in intensive care units (6, 7, 18, 33, 37, 49, 53). In healthy humans there appear to be three patterns of S. aureus nasal carriage: noncarriers, intermittent carriers, and chronic carriers. One study (26) estimates the distribution of these carriers to be 20, 60, and 20%, respectively. This suggests that a significant portion of the population is at risk for development of staphylococcal disease linked to nasal carriage of S. aureus. People at greatest risk include those with diminished immunity and those with other chronic diseases (26).
Studies suggest that clearance of S. aureus nasal colonization can reduce the subsequent risk of developing S. aureus infections (27, 52, 53) and also reduce the community spread of drug-resistant S. aureus (28). In one striking example (52), the use of mupirocin ointment reduced the postoperative staphylococcal infection rate of patients undergoing upper gastrointestinal surgery from 11.7% in the control group to 0.71% in the intervention group (P < 0.001). In a recent study (39), prophylactic intranasal mupirocin significantly decreased the rate of all nosocomial S. aureus infections among patients who were S. aureus carriers. Currently, Bactroban Nasal (2% mupirocin calcium ointment; SmithKline Beecham, Bristol, Tenn.) is the most widely prescribed and effective antibacterial agent for eradication of S. aureus nasal colonization (15, 27, 31, 46). Unfortunately, as with many antimicrobials, mupirocin resistance is emerging in S. aureus (8, 11, 13, 29, 30, 50). Furthermore, multiple applications of mupirocin are required to effect a significant reduction of nasal colonization, thus precluding its use in patients requiring rapid clearance for emergency surgical procedures. Several other interventions for the clearance of S. aureus nasal carriage have also been explored, including Neosporin ointment and Polysporin ointment (19), bacitracin (46), and other antibiotics (26, 53). These alternative interventions have proven unreliable for eradication of nasal colonization by S. aureus. New interventions for S. aureus nasal colonization are clearly needed.
Lysostaphin is an antibacterial enzyme first identified in Staphylococcus simulans (formerly known as Staphylococcus staphylolyticus) in 1964 (44). Lysostaphin is a glycylglycine endopeptidase capable of specifically cleaving the cross-linking pentaglycine bridges in the cell walls of staphylococci (4). Since the cell wall bridges of S. aureus contain a high proportion of pentaglycine, lysostaphin is highly effective in lysing both actively growing and quiescent S. aureus cells. Lysostaphin activity against other species of staphylococci has also been demonstrated (54). Three studies from the late 1960s and early 1970s (21, 32, 42) examined the use of lysostaphin to clear S. aureus nasal colonization. These studies found that repeated application of lysostaphin (three or four times per day for 5 to 14 days) administered in saline could reduce or eliminate S. aureus nasal colonization. Eradication of S. aureus lasted for various lengths of time after the termination of treatment, although most patients eventually became recolonized with S. aureus. When those studies were conducted, however, recombinant lysostaphin was not available and manufacture of consistent lots of purified natural lysostaphin was difficult to achieve. No further work using lysostaphin to clear nasal colonization was reported for almost 30 years. Recently, recombinant lysostaphin has become available (43), and studies investigating lysostaphin as a therapy for S. aureus disease have reemerged in the literature (9, 10, 12, 38, 40).
To test the efficacy of lysostaphin for clearance of nasal carriage of S. aureus, we sought to reproduce an existing mouse model (25). This model, however, was not adequate in that we could not achieve consistently high levels of staphylococcal nasal colonization in mice. We therefore have developed an alternative animal model using the cotton rat (Sigmodon hispidus), which has been studied previously as a model for respiratory pathogens (41). The cotton rat model demonstrates consistent and persistent high-level nasal colonization by S. aureus. The capacities of three antibacterial agents, lysostaphin, mupirocin, and nisin, a lantibiotic with potent in vitro antistaphylococcal activity (23), to clear S. aureus nasal colonization have been studied in this model. A single application of 0.5% lysostaphin in a petrolatum-based cream consistently eradicated S. aureus nasal colonization by both MSSA and MRSA in the cotton rat nasal colonization model, while nisin cream did not clear nasal colonization. These animal studies also suggest that lysostaphin formulated in cream may be a superior alternative to the currently available mupirocin ointment for clearance of S. aureus nasal colonization.
MATERIALS AND METHODS
Materials.
Purified lysostaphin and nisin were purchased from Nutrition 21 Inc. (formerly AMBI Inc.; Purchase, N.Y.) or were produced by Biosynexus Inc. (Gaithersburg, Md.). Bactroban Nasal (2% mupirocin calcium ointment; a commercially available form of mupirocin) is produced by SmithKline Beecham and was purchased from a local pharmacy.
S. aureus strains.
Several S. aureus strains were used in these studies. These strains are listed in Table 1. Stocks were maintained frozen at −80°C in tryptic soy broth (Becton Dickinson [BD], Sparks, Md.) before use in the nasal colonization model. Strain SA 3865 MupR contains a conjugative mupirocin resistance plasmid (34). The identities of fresh clinical isolates MBT 5040 and MRSA 12/12 were confirmed by the API STAPH identification system (bioMerieux, Lombard, Ill.). S. aureus strain Newman was one of the strains used in the mouse model of nasal colonization (25). Lysostaphin and nisin MICs were determined in accordance with NCCLS guidelines (36) for broth microdilution susceptibility testing methods with the following modifications: 0.1% bovine serum albumin was added to the media of the lysostaphin MICs to reduce nonspecific adherence to plastic (10), while 0.1% Tween 80 was added to the media of the nisin MICs for the same reason. Since mupirocin is not commercially available as a purified antibiotic, MIC testing with this drug was not conducted.
TABLE 1.
Strains of S. aureus antibiotic resistance, and origins
| S. aureus strain | Antibiotic resistancea and capsule type | MIC (μg/ml)
|
Origin or reference | |
|---|---|---|---|---|
| Lysostaphin | Nisin | |||
| MBT 5040b | MRSA, streptomycin resistant | 0.004 | 3.2 | Fresh clinical isolate obtained from WRAMCc |
| MBT 5040 LysoR | Lysostaphin resistant (reverted to methicillin sensitivity phenotype [9]) | >32 | 3.2 | Isolated in vitro |
| Newman | MSSA | 0.004 | NDd | 25 |
| ATCC 49521 | MSSA, capsule type 5 | 0.016 | 0.4 | Direct from ATCC |
| ATCC 12605 | MSSA, capsule type 8 | 0.032 | 0.2 | Direct from ATCC |
| SA5-strR | Streptomycin resistant | 0.004 | 0.2 | Isolated in vitro |
| SA 3865 MupR | Mupirocin resistante | 0.004 | ND | 34 |
| MRSA 12/12b | MRSA | 0.002 | ND | Fresh clinical isolate obtained from WRAMC |
Confirmed by disk diffusion assay (36).
Identity confirmed by API STAPH identification system (bioMerieux).
WRAMC, Walter Reed Army Medical Center.
ND, not determined.
Cotton rat nasal colonization model.
The cotton rat nasal colonization model is an adaptation of the mouse nasal colonization model described by Kiser et al. (25). Briefly, various strains of S. aureus were grown overnight on Columbia agar (BD) supplemented with 2% NaCl (Sigma, St. Louis, Mo.) to induce capsule formation, which may enhance nasal colonization (25). Plate-grown bacteria were washed by suspension in phosphate-buffered saline (PBS; BioWhittaker) so that the percent transmittance of the suspension at 650 nM was 10% in a 10-mm path length. This percent transmittance is equivalent to ∼109 CFU/ml. A volume of suspended bacteria equivalent to 109 CFU per animal to be instilled was pelleted by centrifugation and then resuspended in 10 μl of PBS per animal to be instilled. Six-week-old female cotton rats (Sigmodon hispidus; bred at the Biosynexus Inc. breeding facility; U.S. Department of Agriculture certificate 51-R-0075) or 6-week-old female ICR mice (Harlan, Indianapolis, Ind.) were anesthetized with a combination of xylazine hydrochloride (Rompun), acepromazine maleate, and ketamine (2.5, 2.5, and 25 mg/kg of body weight, respectively) or Rompun (16 mg/kg) and ketamine (85 mg/kg), respectively. A 10-μl aliquot of resuspended S. aureus (∼109 CFU) was intranasally instilled in a dropwise fashion distributed equally in each nostril of the anesthetized animals.
Four to five days after nasal instillation of S. aureus, intranasal treatments were initiated if appropriate. These treatments, administered to anaesthetized cotton rats, included once-a-day treatment for 1 to 3 days with various concentrations of lysostaphin or other antibacterials in either PBS (10 μl) or a petrolatum-based cream (∼30 μl/nose) of the following composition: MIGLYOL 812 N EP (caprylic/capric triglyceride; Sasol, Witten, Germany), 36.0% (wt/wt); SOFTISAN 649 (bis-diglyceryl polyacyladipate-2; Sasol), 24.2% (wt/wt); white petrolatum, USP (Ultra Chemical Inc., Red Bank, N.J.), 27.5% (wt/wt); paraffin, USP (Sigma), 3.4% (wt/wt); white bleached beeswax, USP (Kosterkeunen, Watertown, Conn.), 3.4% (wt/wt); zinc stearate, USP (Sigma), 0.5% (wt/wt); aqueous solution of lysostaphin (Nutrition 21 or Biosynexus, Inc.), 5.0% (wt/wt). Mupirocin was available only as a commercial formulation (Bactroban Nasal); the 2% ointment used in these studies was used as provided by the manufacturer. Cream formulations were administered to anaesthetized cotton rats with a 1-ml syringe fitted with a flexible 23-gauge Angiocath (BD). The catheter was inserted 2 to 3 mm into each naris and then drawn back slowly as the cream was injected into the naris. The nose of the animal was massaged well to ensure even distribution of the cream throughout the nares and to prevent obstruction of the airway. Each animal received ∼30 μl of cream per nose divided equally in each nostril.
Four or 24 h or up to 7 days after nasal instillation of the final treatment, animals were sacrificed, the nose area was cleansed thoroughly with a sterile 70% ethanol wipe to eliminate external skin colonization by S. aureus, and the noses were surgically removed. The nostrils were bisected with scissors, and then the excised nose was placed in 500 μl of PBS containing 0.5% Tween 20 (Sigma) to aid in the release of colonizing bacteria. In experiments to neutralize any residual lysostaphin activity remaining in noses at the time of sacrifice, bisected noses were placed in 500 μl of PBS plus Tween 20 and 10 mg of proteinase K (Sigma)/ml. The noses were vortexed vigorously three times for 20 s each to release colonizing bacteria, and 50 to 100 μl of supernatant was plated on blood agar (Remel, Lenexa, Kans.) or tryptic soy agar (TSA; BD) supplemented with 7.5% NaCl to inhibit growth of nonstaphylococcal bacteria normally found in cotton rat noses. Since cotton rat noses were also colonized by naturally occurring coagulase-negative staphylococci (CoNS) which were not inhibited by agar containing 7.5% NaCl, an antibiotic (streptomycin at 500 μg/ml or nafcillin at 10 μg/ml; Sigma) was added to the TSA-NaCl in some experiments to aid in isolation of any antibiotic-resistant strain of S. aureus instilled in a particular experiment. In experiments using MRSA treated with lysostaphin, supernatants were also plated on TSA-NaCl without antibiotics to ensure that any S. aureus that became lysostaphin resistant and consequentially reverted to a methicillin sensitive phenotype (9) was not missed. TSA plates supplemented with NaCl were incubated for 48 h at 37°C to allow S. aureus colonies to grow to a size where they could be accurately counted.
To enumerate nasal colonization by MSSA instilled in cotton rat noses in some experiments and to differentiate these S. aureus strains from indigenous CoNS naturally found in cotton rat noses, a subtractive technique was used. Supernatant from excised noses was plated on TSA plus 7.5% NaCl in the presence or absence of lysostaphin (1 μg/ml). This concentration of lysostaphin inhibited growth of S. aureus but not naturally occurring CoNS from cotton rat noses (data not shown). Nasal colonization by instilled MSSA was determined as the CFU on TSA-7.5% NaCl in the absence of lysostaphin minus the CFU on TSA-7.5% NaCl in the presence of 1 μg of lysostaphin/ml. Any colonies from the noses of animals treated with lysostaphin that grew on agar containing 1 μg of lysostaphin/ml were further tested to ensure that they were not the instilled MSSA strain that had converted to lysostaphin resistance (see below). All guidelines of both the U.S. Department of Agriculture and the Biosynexus Inc. Institutional Animal Care and Use Committee were followed during the animal studies described in this paper.
Determination of the duration of antistaphylococcal activity of lysostaphin and nisin creams in the nose.
To determine the persistence of the antistaphylococcal activity of lysostaphin and nisin in cream in the nose, naive cotton rats were intranasally instilled with petrolatum-based cream containing either a placebo control, 0.5 (5 mg/ml; actual dose, ∼150 μg) or 0.125% (1.25 mg/ml; actual dose, ∼37.5 μg) (wt/wt) lysostaphin, or 5% (wt/wt) (50 mg/ml; actual dose, ∼1,500 μg) nisin. Two to 24 h after instillation of the cream, animals were sacrificed and the noses were surgically removed and placed in 500 μl of PBS-0.5% Tween 20 and vortexed well to release the remaining drug. In neutralization experiments, 10 mg of proteinase K/ml was added to the buffer in some samples. S. aureus strain ATCC 49521 (103 CFU) or MBT 5040 (105 CFU) was incubated with the excised noses in buffer for 30 min and then 100 μl of supernatant was plated on TSA-7.5% NaCl in the presence or absence of streptomycin (500 μg/ml), as appropriate.
Determination of lysostaphin resistance.
Various S. aureus strains, as identified by the BBL Staphyloslide latex test (BD), isolated from cotton rat noses were tested for lysostaphin resistance by one of two methods. The first method was based on the broth microdilution susceptibility testing method (36). Briefly, the highest concentration of lysostaphin was 0.25 μg/ml; serial twofold dilutions resulted in a low concentration of 0.25 ng/ml. Dilutions were performed in cation-adjusted Mueller-Hinton broth supplemented with 2% NaCl and 0.1% bovine serum albumin (10). Isolates were considered more lysostaphin resistant than the parental strain if the lysostaphin MIC for them increased by more than fourfold (2 dilutions) over that for their parental strain. The second method for determination of lysostaphin resistance was a disk diffusion method. In this method 6-mm-diameter filter paper disks were sterilized and then impregnated with 50 μg of lysostaphin in 7 μl of PBS. The disks were allowed to air dry and were stored at −20°C until used. Susceptibility testing was conducted on cation-adjusted Mueller-Hinton agar supplemented with 2% NaCl. S. aureus isolates were considered lysostaphin resistant if the diameter of the zone of inhibition was less than 12 mm.
RESULTS
Nasal colonization of cotton rats by S. aureus.
To directly compare S. aureus nasal colonization in mice with that in cotton rats, 10 6-week-old females of each species were intranasally instilled with ∼109 CFU of a spontaneously streptomycin-resistant S. aureus type 5 strain isolated in vitro. Five days postinstillation, the animals were sacrificed and nasal colonization was determined on TSA plates supplemented with streptomycin. In this experiment, cotton rats had an average nasal colonization of ∼2,000 CFU/nose (range, 1,400 to 3,000 CFU/nose) while ICR mice had an average of 16 CFU/nose (range, 0 to 40 CFU/nose). This streptomycin-resistant S. aureus strain, however, did not consistently yield high nasal colonization in other experiments (data not shown), so a fresh clinical isolate of MRSA (MBT 5040), which allowed isolation on nafcillin- or streptomycin-containing agar, was examined for its capacity to colonize the cotton rat nares. A second experiment involving direct comparison of ICR mice and cotton rats was performed. In this experiment, mice or cotton rats were intranasally instilled with either 5 × 108 CFU of MBT 5040 or 5 × 107 CFU of S. aureus strain Newman (one of the strains used in the original mouse nasal colonization study [25]). One week after instillation, the animals were sacrificed and nasal colonization was determined. All five cotton rats were nasally colonized with either MBT 5040 (average of 3,416 CFU/nose; range, 1,980 to 6,730 CFU/nose) or S. aureus Newman (average of 4,021 CFU/nose; range, 1,605 to 7,872 CFU/nose). All five mice were also colonized with either MBT 5040 (average of 70 CFU/nose; range, 8 to 194 CFU/nose; Fig. 1) or S. aureus Newman (average of 920 CFU/nose; range, 256 to 1,356 CFU/nose), but at lower levels. These results and our observations over many experiments conducted initially with mice and then cotton rats indicated that cotton rat nasal colonization was more consistent and was at a higher level than mouse nasal colonization (data not shown); because of this, cotton rats were chosen for the experiments described below.
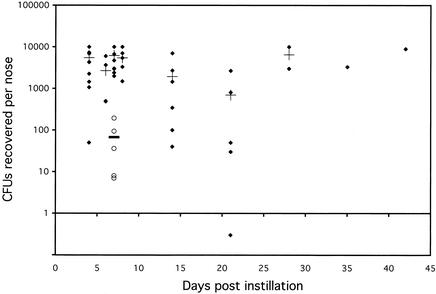
FIG. 1.
Persistence of nasal colonization of cotton rats by S. aureus strain MBT 5040 over time and comparison with nasal colonization in mice on day 7. At various time points (4 to 42 days) after nasal instillation of ∼109 CFU of S. aureus MBT 5040, animals were sacrificed and nasal colonization was determined. ♦, number of CFU recovered from the noses of individual cotton rats (the solid diamond below the line at 1 CFU represents a cotton rat that was not nasally colonized with S. aureus on the day of sacrifice); +, mean colonization for cotton rats for each time point; ○ (day 7), numbers of CFU recovered from the noses of individual ICR mice; solid bar, mean colonization for ICR mice.
Unlike the mouse nares (25), the normal, untreated cotton rat nares can demonstrate significant natural bacterial colonization by many types of bacteria. These bacteria included several species of CoNS and a number other unidentified gram-positive and gram-negative bacteria. The use of TSA supplemented with 7.5% NaCl inhibited the growth of most of the nonstaphylococcal flora of the cotton rat nares; however, the native CoNS of the cotton rat nares were able to grow on this media. These CoNS belong to numerous species and included a fairly prevalent strain of S. xylosus (as identified by the API STAPH identification system), which appeared weakly positive on the Staphyloslide latex test. Occasionally there were even a few native S. aureus colonies found in cotton rat noses. To enumerate the specific S. aureus strain instilled in each experiment, TSA-7.5% NaCl was usually supplemented with an antibiotic (streptomycin or nafcillin) based on the antibiotic resistance of the instilled S. aureus strain. Since MBT 5040 yielded persistent high-level nasal colonization (Fig. 1) and is methicillin and streptomycin resistant, many of the further experiments were performed with this strain.
Heavily anesthetized cotton rats retain a strong reflex nasal expiration response when drops of liquid are applied to their noses. To ensure consistent nasal colonization in cotton rats, a high titer of S. aureus (∼109 CFU) was routinely instilled in each animal so that, even if a significant fraction of the initial volume was forcefully expelled, nasal colonization was consistently achieved by the remaining volume. Figure 1 shows that, while there was some variability in the level of nasal colonization in cotton rats, the average colonization ranged between 103 and 104 CFU per nose on the day of sacrifice and didn't exceed 104 CFU/nose as early as day 4 even if 109 CFU of S. aureus were initially instilled.
When cotton rats were intranasally instilled with 109 CFU of MBT 5040, virtually all of animals became nasally colonized with the bacteria. Untreated animals sacrificed 4 to 42 days postinstillation exhibited >98% S. aureus nasal colonization, ranging between 40 and ∼10,000 CFU/nose (Fig. 1). Only one animal, on day 21, was not nasally colonized on the day of sacrifice. Nasal colonization experiments were also conducted with another species of cotton rat (Sigmodon fulviventer), but this species did not demonstrate the consistent high-level nasal colonization by S. aureus found in Sigmodon hispidus (data not shown).
Lysostaphin delivered in PBS did not eradicate nasal colonization by MBT 5040 in all animals.
In experiments conducted 30 years ago, lysostaphin was administered in saline in order to reduce or clear nasal colonization by S. aureus in humans (21, 32, 42) and eradication was incomplete. To reproduce these findings in an animal model, cotton rats were instilled with ∼109 CFU of S. aureus MBT 5040. On days 4 and 5 after bacterial instillation, 110 μg of lysostaphin was administered to each cotton rat in 10 μl of PBS in a dropwise fashion once a day for the 2 days. This treatment eradicated nasal colonization by S. aureus in three of nine cotton rats treated in two separate experiments (Table 2). The other six cotton rats remained colonized after treatment. Administration of PBS alone had no effect on nasal colonization by S. aureus (data not shown).
TABLE 2.
S. aureus nasal colonization of cotton rats following various intranasal treatments
| Nasal treatment | No. of rats colonized/no. tested | % Eradicated | No. of expts | CFU recovered/colonized naris
|
|
|---|---|---|---|---|---|
| Mean | Median | ||||
| Control (no treatment)a | 41/41 | 0 | 10 | 5,262 | 4,715 |
| Placebo creamb | |||||
| 3 doses | 23/23 | 0 | 5 | 3,372 | 2,010 |
| 1 dose | 23/23 | 0 | 5 | 6,354 | 6,790 |
| Lysostaphin, 110 μg in 10 μl of PBS, 2 dosesb | 6/9 | 33 | 2 | 3,303 | 2,012 |
| 0.5% lysostaphin cream (∼150 μg/dosec)b | |||||
| 3 doses | 0/14 | 100 | 3 | 0 | 0 |
| 1 dose | 5/71 | 93 | 11 | 8 | 5 |
| 0.125% lysostaphin cream (∼37.5 μg/dose)b | |||||
| 3 doses | 9/20 | 55 | 3 | 672 | 165 |
| 1 dose | 8/10 | 20 | 2 | 1,006 | 1,071 |
Control nares harvested between 4 and 8 days after bacterial instillation.
Nares treated 4 to 7 days after bacterial instillation and harvested 24 h posttreatment
Doses of cream were ∼30 μl/nose split equally between two nostrils.
A single treatment of 0.5% lysostaphin in cream eradicates nasal colonization by MBT 5040.
On the assumption that increased retention time of lysostaphin in the nares should improve its effectiveness, the enzyme was formulated in a petrolatum-based cream at 0.5 and 0.125% (wt/wt). In vitro studies showed that lysostaphin-containing cream had potent antistaphylococcal activity while the cream base itself had only slight antistaphylococcal activity (data not shown). In a series of experiments, cotton rats were instilled with ∼109 CFU of MBT 5040. After 4 to 7 days, animals were treated with either 0.5% (actual dose, ∼150 μg), 0.125% (actual dose, ∼37.5 μg), or 0% (placebo) lysostaphin cream once a day for 1 or 3 days. Twenty-four hours after the final instillation of cream, nasal colonization was determined as described in Materials and Methods. Three doses of 0.5% lysostaphin cream over 3 days eradicated nasal colonization in all animals tested (Table 2). More impressively, a single treatment with 0.5% lysostaphin cream eradicated nasal colonization by MBT 5040 in 93% of the cotton rats (Table 2). The five cotton rats that remained nasally colonized after the single treatment with 0.5% lysostaphin cream, no more than one animal per experiment spread over 11 experiments, had very low remaining colonization (mean, 8 CFU/nose). Four animals had <5 CFU/nose, and one animal had ∼20 CFU/nose, recovered from their nares. Single or multiple treatment of colonized cotton rat nares with the placebo (cream without lysostaphin) produced only a small reduction of S. aureus nasal colonization in this model (Table 2).
To ensure that these results were not unique to one strain of S. aureus, additional strains were tested in this model. As shown in Table 3, a single dose of 0.5% lysostaphin cream also eradicated nasal colonization by all S. aureus strains examined. These included two MSSA strains (type 5, ATCC 49521, and type 8, ATCC 12605), a second MRSA strain (MRSA 12/12), and a mupirocin-resistant strain (SA 3865 MupR).
TABLE 3.
A single dose of 0.5% lysostaphin cream (∼150 μg of lysostaphin) eradicates S. aureus nasal colonization of cotton rats by all strains of S. aureus tested
| S. aureus strain | Control group
|
Lysostaphin-treated group
|
||
|---|---|---|---|---|
| No. of rats colonized/no. tested | Mean colonization (CFU) | No. of rats colonized/no. tested | Mean colonization (CFU) | |
| ATCC 49521 (type 5) | 5/5 | >10,000 | 0/5 | 0 |
| ATCC 12605 (type 8) | 5/5 | 5,418 | 0/5 | 0 |
| MRSA 12/12 | 5/5 | 567 | 0/5 | 0 |
| SA 3865 MupR | 5/5 | 268 | 0/5 | 0 |
To determine whether nasal colonization by S. aureus might reemerge after discontinuing lysostaphin treatment, three animals nasally colonized with MBT 5040 and treated 5 days after bacterial instillation with a single dose of 0.5% lysostaphin cream were held for 1 week after treatment before nasal colonization was determined. All three animals remained free of S. aureus nasal colonization at this time, which suggested that the clearance of S. aureus nasal colonization was complete and that there was no S. aureus remaining sequestered in the nasal mucosa that could grow and recolonize the nares. This result also suggested that the eradication of S. aureus occurred in the nose of the living animal and not in buffer since there was no residual lysostaphin found in the nose 7 days after instillation (data not shown) that could affect S. aureus ex vivo.
A lower-concentration lysostaphin cream (0.125%) was not as effective for clearance of nasal colonization by S. aureus. Three doses of 0.125% cream eradicated S. aureus nasal colonization in 55% of animals tested (Table 2), while a single dose of 0.125% lysostaphin cream eradicated nasal colonization by S. aureus in only 2 of 10 animals (Table 2).
A single dose of 0.5% lysostaphin cream is more effective than a single dose of 2% mupirocin ointment.
To directly compare 0.5% lysostaphin cream with 2% mupirocin ointment (Bactroban Nasal; actual dose, ∼600 μg), groups of five nasally colonized cotton rats were treated with one dose per day of either treatment over 3 days. Three days of either treatment eradicated S. aureus nasal colonization in all five cotton rats (Table 4). However, when nasally colonized cotton rats were treated with either a single application of 0.5% lysostaphin cream or 2% mupirocin ointment, S. aureus nasal colonization was eradicated only in the group of five animals treated with 0.5% lysostaphin cream. A single application of 2% mupirocin ointment eradicated nasal colonization in only two of five cotton rats (Table 4), leaving low-level colonization in the other three animals.
TABLE 4.
Lysostaphin cream versus mupirocin ointment for eradication of S. aureus nasal colonization of cotton rats
| Treatment | No. of rats colonized after treatment/no. of rats instilled with S. aureus strain MBT 5040 | Mean CFU recovered/colonize naris |
|---|---|---|
| Placebo cream, 3 doses | 8/8 | 3,740 |
| 0.5% lysostaphin cream (∼150 μg/dose) | ||
| 3 doses | 0/5 | 0 |
| 1 dose | 0/5 | 0 |
| 2% mupirocin ointment (∼600 μg/dose) | ||
| 3 doses | 0/5 | 0 |
| 1 dose | 3/5 | 35 |
Intranasal lysostaphin-resistant colonies were not found.
Lysostaphin resistance in S. aureus can occur both in vitro (14, 16, 47, 48) and in vivo (10). To determine whether lysostaphin resistance could emerge in the nares of cotton rats treated with lysostaphin cream, 91 S. aureus colonies isolated from 22 cotton rat noses treated with either 0.5 or 0.125% lysostaphin cream were tested for resistance to lysostaphin by either the MIC or the disk diffusion method. In these assays, none of the 91 MBT 5040 isolates from noses treated with lysostaphin cream (0.5 or 0.125%) was found to be lysostaphin resistant. The MICs for the isolated colonies matched those for the parental strains, or the zone of inhibition in a disk diffusion assay was >12 mm (data not shown). This finding suggested that perhaps mutations that lead to lysostaphin resistance in S. aureus adversely affect the bacterium's capacity to colonize the nares. To test this theory, a lysostaphin-resistant variant of MBT 5040 (MBT 5040 LysoR; MIC, >32 μg/ml) that was isolated in vitro was instilled in cotton rat nares at ∼109 CFU. Only one of five cotton rats instilled with MBT 5040 LysoR became colonized with this lysostaphin-resistant variant, and this animal only had 200 CFU recovered from its nose on day 7 compared to an average of ∼5,000 CFU recovered from the noses of animals instilled with wild-type MBT 5040 in the same experiment.
To further explore the relationship between lysostaphin resistance and nasal colonization, treatment of S. aureus strain MRSA 12/12 nasal colonization with a single dose of 0.5% lysostaphin cream was also examined. MRSA 12/12 colonized the cotton rat nares (Table 3), and a single treatment of 0.5% lysostaphin cream also eradicated nasal colonization by this strain. Nasal supernatant from treated animals was plated on both TSA-NaCl plus nafcillin and TSA-NaCl alone because, when MRSA becomes lysostaphin resistant, it reverts to a methicillin sensitive phenotype (9). Forty-three colonies that grew on TSA without the antibiotic were examined for identity, and none were found to be S. aureus by the Staphyloslide latex test, i.e., no lysostaphin-resistant S. aureus MRSA 12/12 was recovered from the noses of S. aureus-instilled animals treated with a single dose of 0.5% lysostaphin cream or from the nose of any animal treated with lysostaphin cream regardless of which strain of S. aureus was instilled (Table 3).
The antistaphylococcal activity of intranasal lysostaphin instilled in cream was retained for at least 24 h postinstillation.
To determine whether the cotton rat nasal colonization model could discriminate between the in vivo activity of antibacterials that showed significant antistaphylococcal activity in vitro, experiments with naive cotton rats were performed. As shown in Table 5, the antistaphylococcal activity of lysostaphin formulated in a petrolatum-based cream was retained intranasally for at least 24 h postinstillation. When cotton rats were instilled with 0.5% lysostaphin in cream and the noses were surgically removed 4 or 24 h postinstillation, there was sufficient residual lysostaphin activity to eliminate 103 CFU of S. aureus ex vivo. The 0.125% lysostaphin cream reduced the S. aureus by 87% 4 h postinstillation, while the placebo cream resulted in only a small reduction, 4 or 24 h postinstillation, in the number of exogenously added S. aureus CFU recovered from the excised noses.
TABLE 5.
Retention of the antistaphylococcal activity of various creams in cotton rat noses over time
| Treatment, timea (h) | % Reduction in CFUb |
|---|---|
| Control (no cream) | 0 |
| Placebo cream, 4 | 30 |
| Placebo cream, 24 | 20 |
| 0.5% lysostaphin, 4 (∼150 μg) | 100 |
| 0.5% lysostaphin, 24 (∼150 μg) | 100 |
| 0.125% lysostaphin, 4 (∼37.5 μg) | 87 |
| 5% nisin, 2 (∼1,500 μg) | 35 |
Time after instillation of cream when nose was excised and incubated with 103 CFU of exogenous S. aureus.
Determined in relationship to the control sample. Mean of three samples.
Neutralization of residual lysostaphin activity.
Since sufficient antistaphylococcal activity of lysostaphin formulated in cream to eliminate 103 S. aureus CFU was retained for at least 24 h postinstillation (Table 5) and since 10 μg of lysostaphin (∼7% of the original dose of lysostaphin instilled intranasally) will eliminate 105 S. aureus CFU within 20 min (0 CFU recovered after treatment with lysostaphin in buffer versus 105 CFU recovered after treatment with buffer only [control] [averages of three samples]), it was necessary to identify a neutralizing substance for lysostaphin which would effectively eliminate lysostaphin activity in excised noses but not impair the viability or growth of residual S. aureus in the nose. A number of potential neutralizers were tested, including 0.5 M EDTA buffer, pH 3.6, 10 mg of trypsin/ml, various protease inhibitors, and excess quantities of heat-killed S. aureus; none of these significantly inhibited lysostaphin activity in vitro (data not shown). Proteinase K at 10 mg/ml, however, rapidly neutralizes lysostaphin but does not affect the viability of S. aureus (105 CFU recovered after treatment with proteinase K alone or lysostaphin and proteinase K [average of three samples]). In a follow-up experiment, 0.5% lysostaphin cream was instilled in naive cotton rat noses in an experiment similar to those in Table 5. Three hours postinstillation the animals were sacrificed and the excised noses were placed in 500 μl of PBS-Tween with or without proteinase K at 10 mg/ml. S. aureus strain MBT 5040 (105 CFU) was immediately added to the excised noses, which were vortexed and incubated for 30 min. A volume of the supernatant was plated for enumeration. The residual lysostaphin in sample noses at 3 h, when placed in PBS-Tween without proteinase K, greatly reduced the viable S. aureus in the samples (52 CFU recovered versus 105 CFU recovered for the control [averages of two samples]). The presence of 10 mg of proteinase K/ml neutralized all residual lysostaphin in the noses at 3 h, i.e., there was no reduction in the number of S. aureus CFU recovered from samples when the noses were placed in PBS-Tween containing 10 mg of proteinase K/ml (105 CFU recovered [average of two samples]).
To confirm that the nasal clearance consistently seen with a single dose of 0.5% lysostaphin cream (Table 2) occurs in the nose and not in buffer after excision of the nose by lysostaphin carryover, two groups of cotton rats nasally colonized with MBT 5040 were treated with a single application of 0.5% lysostaphin cream. Four hours posttreatment, the animals were sacrificed and the noses were excised. One group of noses was vortexed in PBS-Tween while the other group of noses was vortexed in PBS-Tween containing 10 mg of proteinase K/ml. Upon plating the supernatants from these excised noses, it was determined that all treated cotton rats were clear of S. aureus at 4 h posttreatment in the presence or absence of proteinase K (four of four and five of five, respectively) (Table 6). This finding demonstrated that lysostaphin eradication of S. aureus colonization in cotton rat noses occurred within 4 h in the nose and not ex vivo by lysostaphin carryover following excision of the nose.
TABLE 6.
Lysostaphin eradicates nasal colonization by S. aureus MBT 5040 in vivo within 4 h of treatment and not ex vivo due to antibiotic carryover
| Treatment of rat nosea | No. of rats with nasal colonization/no. tested | Mean CFU recovered/nose |
|---|---|---|
| Control (buffer only) | 4/4 | 1,065 |
| 0.5% lysostaphin in buffer | 0/4 | 0 |
| 0.5% lysostaphin + proteinase K | 0/5 | 0 |
Treated noses were excised 4 h posttreatment.
Nisin cream did not reduce S. aureus nasal colonization.
The lantibiotic peptide nisin has potent in vitro antistaphylococcal activity both in buffer (23) and in cream (data not shown). Nisin at 50 μg/ml (∼3% of the dose instilled intranasally) was bactericidal for 105 S. aureus CFU within 1 h. To determine whether nisin would be as effective as lysostaphin in vivo, cotton rats nasally colonized with S. aureus MBT 5040 were treated with 0.5 or 5% nisin cream once a day for 3 days. S. aureus nasal colonization was not reduced in any of these cotton rats, even though the identical cream preparations of nisin had potent antistaphylococcal activity in vitro (data not shown). Five of five animals treated with either 0.5 or 5% (actual doses, ∼150 and 1,500 μg, respectively) nisin cream were still colonized after three treatments; mean levels of colonization were 5,003 and 4,310 CFU/nose, respectively, compared to 4,264 CFU/nose in control animals. The ineffectiveness of nisin cream for clearance of S. aureus nasal carriage was supported by the observation that naive cotton rat noses instilled with 5% nisin cream (actual dose, ∼1,500 μg) retained little antistaphylococcal activity ex vivo, even at 2 h postinstillation, while, as mentioned above, those noses instilled with lysostaphin cream retained potent antistaphylococcal activity for 24 h postinstillation (Table 5). There was also no residual antistaphylococcal activity in cotton rat noses instilled with 5% nisin cream 4 or 24 h prior to sacrifice (data not shown).
DISCUSSION
The ability to rapidly clear S. aureus nasal colonization may have a dramatic impact on the prevention of life-threatening S. aureus infections and on the spread of drug-resistant S. aureus in hospitals and in the community. Testing antistaphylococcal therapeutics for this application requires a suitable animal model. The mouse nasal colonization model (25) did not provide consistent and reproducible levels of colonization, which led us to explore other animal models. The cotton rat (Sigmodon hispidus) is a well-documented model for infection by a number of human respiratory pathogens (41), suggesting that this animal's respiratory tract is similar to that of humans. In this study we found that the cotton rat provides a reliable and robust animal model to study S. aureus nasal colonization. High-level colonization persisted for up to 6 weeks and does not seem to adversely affect the animals, i.e., there was no evidence of associated pneumonia or cutaneous infection. Additionally, as is the case for humans, eradication of nasal colonization in this animal model required multiple applications of mupirocin over 3 days in sharp contrast to lysostaphin, which eradicated nasal colonization in a single application (Table 3).
The true strength of the cotton rat nasal colonization model was highlighted by its discriminatory ability, in that eradication of nasal colonization could be induced by some, but not all, antistaphylococcal drugs. Nisin, a lantibiotic with potent and rapid antistaphylococcal activity (23), formulated in a petrolatum-based cream, retained comparable antistaphylococcal activity in vitro (data not shown). However, 5% nisin in cream (actual dose, ∼1,500 μg) had no effect on S. aureus nasal colonization in the cotton rat model. Further investigation determined that no antistaphylococcal activity was detectable in cotton rat noses within 2 h of instillation of 5% nisin cream (Table 5). S. aureus colonizing the nares does, however, remain susceptible to nisin (data not shown). These data suggest that nisin is either rapidly inactivated or absorbed in the cotton rat nares.
Bactroban Nasal (2% mupirocin ointment), a topical antimicrobial that competitively inhibits bacterial isoleucyl-tRNA synthesis and thus interferes with protein synthesis (35), is the current standard of care for elimination of S. aureus nasal colonization (15, 27, 31, 46). S. aureus has repeatedly demonstrated its resourcefulness for developing resistance to antibiotics, and, indeed, mupirocin resistance has emerged (8, 11, 13, 29, 30, 50). This resistance is associated with prolonged topical and intranasal use (3, 24). It has been suggested that intranasal application of mupirocin ointment leads to low concentrations of the antibiotic in the pharynx that may contribute to selection of mupirocin resistance (50). Low- to intermediate-level mupirocin resistance (MIC of 8 to 256 μg/ml) occurs when there are mutations around the Rossman fold of the isoleucyl-tRNA synthetase that may prevent the competitive binding of mupirocin to the target enzyme (2). High-level mupirocin resistance (MIC of >512 μg/ml) occurs through the acquisition of a conjugative plasmid (20, 34) that carries the mupA gene, which encodes a novel isoleucyl-tRNA synthetase that is not affected by mupirocin.
Lysostaphin is an alternative to mupirocin for eradication of S. aureus nasal colonization. A single dose of lysostaphin (formulated at 0.5%, 5 mg/ml, in a petrolatum-based cream) eradicates MSSA, MRSA, and mupirocin-resistant S. aureus from the cotton rat nares, some within 4 h of application, while three doses of mupirocin ointment over 3 days are required for eradication of MRSA in the same model (Table 4). The potency of the lysostaphin formulation was also persistent in the nares and maintained its antistaphylococcal activity for at least 24 h after instillation (Table 5), suggesting that lysostaphin cream may also prevent subsequent S. aureus nasal colonization. Lysostaphin cream could therefore be instilled in the nares of an uncolonized patient upon admission to a health care setting and protect the patient from nasal colonization for at least 24 h.
Given the persistence of intranasal lysostaphin formulated in cream, however, there was the possibility that S. aureus colonizing the cotton rat nares was not eliminated by lysostaphin in the nose but was instead killed ex vivo by residual lysostaphin found in the nares and then released into buffer at the time of sacrifice. Experiments using proteinase K (see Results; Table 6) to neutralize residual lysostaphin found in the cotton rat noses at the time of sacrifice demonstrated that 0.5% lysostaphin cream eradicated S. aureus nasal colonization within 4 h after application in the noses of living animals and not in the buffer ex vivo.
Lysostaphin-resistant S. aureus has been isolated both in vitro and in vivo (9, 10), and the most common mechanism of resistance to lysostaphin involves mutations that affect femA or femB, the genes responsible for addition of the second and third or fourth and fifth glycines to the pentaglycine cross bridge, respectively. These mutations result in monoglycine or triglycine cross bridges and render the S. aureus more resistant to lysostaphin (16, 47). A second less likely mechanism could involve acquisition of the gene for the lysostaphin immunity factor (lif), which is found on the pACK1 plasmid, which also encodes lysostaphin in S. simulans biovar staphylolyticus (48). This factor is also called the endopeptidase resistance gene (epr) (14) and produces the substitution of serines for glycines in the pentaglycine cross bridges when introduced into S. aureus on a shuttle vector. These serine substitutions render the recipient strain more resistant to lysostaphin. It has recently been shown that either of these mutations in S. aureus is incompatible with resistance to β-lactam antibiotics (9), even if the strain is initially an MRSA. Thus, MRSA that acquires resistance to lysostaphin becomes susceptible to β-lactams.
Surprisingly, lysostaphin-resistant S. aureus has never been isolated from the nares of colonized cotton rats treated with lysostaphin, despite the fact that a number of different S. aureus strains (Table 1) were used in these experiments. A single treatment of 0.5% lysostaphin cream eradicated nasal colonization by all of these strains and did not lead to the emergence of lysostaphin resistance from any of them. Conditions where selection of lysostaphin resistance in the animal model might be expected were also examined. These conditions included several experiments performed with MBT 5040 at suberadication concentrations (0.125%) of lysostaphin and with careful screening of any surviving colonies for identity and resistance to lysostaphin. No lysostaphin resistance was found even under these conditions. There are several possible explanations for this observation. The first may be found in the results of the experiment in which a lysostaphin-resistant variant of MBT 5040 (MBT 5040 LysoR), isolated in vitro, did not colonize the cotton rat nares well. Only one of five animals became nasally colonized with low numbers of lysostaphin-resistant MBT 5040 (4% of the colonization level seen for wild-type MBT 5040). This suggests that the mutations that render MBT 5040, and perhaps other S. aureus strains, lysostaphin resistant adversely affect their capacity to colonize the nares. Second, colonization data over time (Fig. 1) suggest that S. aureus colonizing the cotton rat nares is not rapidly dividing, and thus the opportunity for selection of lysostaphin resistance is low. Third, the petrolatum cream base may have some low-level antistaphylococcal activity of its own (Table 2), and this cream base may exert negative pressure on lysostaphin-resistant S. aureus. These observations suggest that the probability of lysostaphin-resistant clones of S. aureus emerging following the intranasal use of lysostaphin may be low.
In summary, the cotton rat nasal colonization model has proven to be a valuable tool for study of nasal colonization by S. aureus, and using this model we have demonstrated that lysostaphin in a cream base may be a superior alternative to mupirocin ointment for the eradication of S. aureus nasal colonization. Clinical trials using this product have begun, with the anticipation that lysostaphin cream for rapid eradication of S. aureus nasal colonization will be a valuable tool for both prevention of nosocomial S. aureus infections and control of community spread of multidrug-resistant S. aureus.
Acknowledgments
We thank Gordon Archer and Martin Ottolini for providing various S. aureus strains, John de la Harpe for helpful comments and suggestions, and Caroline Kusuma, Julio Canas, and Elizabeth Mendez for excellent technical assistance.
REFERENCES
- 1.Anonymous. 1996. National Nosocomial Infections Survey (NNIS) report, data summary from Oct. 1986-April 1996. Am. J. Infect. Control 24:380-388. [PubMed] [Google Scholar]
- 2.Antonio, M., N. McFerran, and M. J. Pallen. 2002. Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 46:438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce, J. M., G. Potter-Bynoe, C. Chenevert, and T. King. 1997. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect. Control Hosp. Epidemiol. 18:622-627. [PubMed] [Google Scholar]
- 4.Browder, H. P., W. A. Zygmunt, J. R. Young, and P. A. Travormina. 1965. Lysostaphin: enzymatic mode of action. Biochem. Biophys. Res. Commun. 19:383-389. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin-United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 6.Chang, F. Y., N. Singh, T. Gayowski, S. D. Drenning, M. M. Wagener, and I. R. Marino. 1998. Staphylococcus aureus nasal colonization and association with infections in liver transplant recipients. Transplantation 65:1169-1172. [DOI] [PubMed] [Google Scholar]
- 7.Chapoutot, C., G.-P. Pageaux, P. F. Perrigault, Z. Joomaye, P. Perney, H. Jean-Pierre, O. Jouquet, P. Blanc, and D. Larrey. 1999. Staphylococcus aureus nasal carriage in 104 cirrhotic and control patients: a prospective study. J. Hepatol. 30:249-253. [DOI] [PubMed] [Google Scholar]
- 8.Chatfield, C., W. O'Neill, R. Cooke, K. McGhee, M. Issack, M. Rahman, and W. Noble. 1994. Mupirocin-resistant Staphylococcus aureus in a specialist school population. J. Hosp. Infect. 26:273-278. [DOI] [PubMed] [Google Scholar]
- 9.Climo, M., K. Ehlert, and G. Archer. 2001. Mechanism and suppression of lysostaphin resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Climo, M. W., R. L. Patron, B. P. Goldstein, and G. L. Archer. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 42:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cookson, B. 1990. Mupirocin resistance in staphylococci. J. Antimicrob. Chemother. 25:497-503. [DOI] [PubMed] [Google Scholar]
- 12.Dajcs, J. J., E. B. Hume, J. M. Moreau, A. R. Caballero, B. M. Cannon, and R. J. O'Callaghan. 2000. Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in the rabbit. Investig. Opthalmol. Vis. Sci. 41:1432-1437. [PubMed] [Google Scholar]
- 13.Dawson, S., L. Finn, J. McCulloch, S. Kilvington, and D. Lewis. 1994. Mupirocin-resistant MRSA. J. Hosp. Infect. 28:75-78. [DOI] [PubMed] [Google Scholar]
- 14.DeHart, H., H. Heath, L. Heath, P. LeBlanc, and G. Sloan. 1995. The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus. Appl. Environ. Microbiol. 61:1475-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doebbeling, B. N., D. Breneman, H. Neu, R. Aly, B. Yangco, H. Holley, R. Marsh, M. Pfaller, J. McGowan, B. Scully, D. Reagan, and R. Wenzel. 1993. Elimination of Staphylococcus aureus nasal carriage in health care workers: analysis of six clinical trials with calcium mupirocin ointment. Clin. Infect. Dis. 17:466-474. [DOI] [PubMed] [Google Scholar]
- 16.Ehlhart, K., W. Schroder, and H. Labschinski. 1997. Specificities of FemA and FemB for different glycine residues: FemB cannot substitute for FemA in staphylococcal peptidoglycan pentaglycine side chain formation. J. Bacteriol. 179:7573-7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fierobe, L., D. Decre, C. Muller, J.-C. Lucet, J.-P. Marmuse, J. Mantz, and J.-M. Demonts. 1999. Methicillin-resistant Staphylococcus aureus as a causative agent of postoperative intra-abdominal infection: relation to nasal colonization. Clin. Infect. Dis. 29:1231-1238. [DOI] [PubMed] [Google Scholar]
- 18.Frebourg, N., B. Cauliez, and J.-F. Lemeland. 1999. Evidence for nasal carriage of methicillin-resistant staphylococci colonizing intravascular devices. J. Clin. Microbiol. 37:1182-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung, S., S. O'Grady, C. Kennedy, H. Deider, I. Campbell, and J. Conly. 2000. The utility of polysporin ointment in the eradication of methicillin-resistant Staphylococcus aureus colonization: a pilot study. Infect. Control Hosp. Epidemiol. 21:653-655. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert, J., C. R. Perry, and B. Slocombe. 1993. High-level mupirocin resistance in Staphylococcus aureus: evidence for two distinct isoleucyl-tRNA synthetases. Antimicrob. Agents Chemother. 37:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris, R. L., A. W. Nunnery, and H. D. Riley. 1967. Effect of lysostaphin on staphylococcal carriage in infants and children. Antimicrob. Agents Chemother. 11:110-112. [DOI] [PubMed] [Google Scholar]
- 22.Hiramatsu, K., L. Cui, M. Kuroda, and T. Ito. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 9:486-493. [DOI] [PubMed] [Google Scholar]
- 23.Hurst, A. 1981. Nisin. Adv. Appl. Microbiol. 27:85-123. [Google Scholar]
- 24.Kauffman, C. A., M. S. Terpenning, X. He, L. T. Zarins, M. A. Ramsey, and K. A. Jorgensen. 1998. Attempts to eradicate methicillin-resistant Staphylococcus aureus from a long-term-care facility with the use of mupirocin ointment. Am. J. Med. 94:371-378. [DOI] [PubMed] [Google Scholar]
- 25.Kiser, K. B., J. M. Cantey-Kiser, and J. C. Lee. 1999. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect. Immun. 67:5001-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kluytmans, J. A., J. W. Mouton, M. VandenBergh, M.-J. Manders, A. Maat, J. Wagenvoort, M. Michel, and H. Verbrugh. 1996. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 17:780-785. [DOI] [PubMed] [Google Scholar]
- 28.Lee, Y.-L., T. Cesario, A. Pax, C. Tran, A. Ghouri, and L. Thrupp. 1999. Nasal colonization by Staphylococcus aureus in active, independent community seniors. Age Ageing 28:229-232. [DOI] [PubMed] [Google Scholar]
- 29.Leski, T. A., M. Gniadkowski, A. Skoczynska, E. Stefaniuk, K. Trzcinski, and W. Hryniewicz. 1999. Outbreak of mupirocin-resistant staphylococci in a hospital in Warsaw, Poland, due to plasmid transmission and clonal spread of several strains. J. Clin. Microbiol. 37:2781-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marples, R. R., D. C. E. Speller, and B. D. Cookson. 1995. Prevalence of mupirocin resistance in Staphylococcus aureus. J. Hosp. Infect. 29:153-155. [DOI] [PubMed] [Google Scholar]
- 31.Martin, J., F. Perdreau-Remington, M. Kartalija, O. Pasi, M. Webb, J. Gerberding, H. Chambers, M. Tauber, and B. Lee. 1999. A randomized clinical trial of mupirocin in the eradication of Staphylococcus aureus nasal carriage in human immunodeficiency virus disease. J. Infect. Dis. 180:896-899. [DOI] [PubMed] [Google Scholar]
- 32.Martin, R. R., and A. White. 1967. The selective activity of lysostaphin in vivo. Lab. Clin. Med. 70:1-8. [PubMed] [Google Scholar]
- 33.Mest, D. R., D. H. Wong, K. J. Shimoda, M. E. Mulligan, and S. E. Wilson. 1994. Nasal colonization with methicillin-resistant Staphylococcus aureus on admission to surgical intensive care unit increases the risk of infection. Anesth. Analg. 78:644-650. [DOI] [PubMed] [Google Scholar]
- 34.Morton, T. M., J. L. Johnston, J. Patterson, and G. L. Archer. 1995. Characterization of a conjugative staphylococcal mupirocin-resistant plasmid. Antimicrob. Agents Chemother. 39:1272-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakama, T., O. Nureki, and S. Yokoyama. 2001. Structural basis for the recognition of isoleucyl-adenylate and an antibiotic, mupirocin, by isoleucyl-t-RNA synthase. J. Biol. Chem. 276:47387-47393. [DOI] [PubMed] [Google Scholar]
- 36.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard M7-A4, 4th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 37.Nguyen, M. H., C. Kauffman, R. Goodman, C. Squier, R. Arbeit, N. Singh, M. Wagener, and V. Yu. 1999. Nasal carriage of, and infection with Staphylococcus aureus in HIV-infected patients. Ann. Intern. Med. 130:221-225. [DOI] [PubMed] [Google Scholar]
- 38.Patron, R. L., M. W. Climo, B. P. Goldstein, and G. L. Archer. 1999. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 43:1754-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perl, T., J. Cullen, R. Wenzel, B. Zimmerman, M. Pfaller, D. Sheppard, J. Twombley, P. French, and L. Herwalt. 2002. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 24:1871-1877. [DOI] [PubMed] [Google Scholar]
- 40.Polack, J., P. D. Latta, and P. Blackburn. 1993. In vitro activity of recombinant lysostaphin-antibiotic combinations toward methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 17:265-270. [DOI] [PubMed] [Google Scholar]
- 41.Prince, G. A. 1994. The cotton rat in biomedical research. Anim. Welf. Inf. Cent. Newsl. 5.
- 42.Quickel, K. E., R. Selden, J. R. Caldwell, N. F. Nora, and W. Schaffner. 1971. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl. Microbiol. 22:446-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Recsei, P. A., A. D. Gruss, and R. P. Novick. 1987. Cloning, sequence and expression of the lysostaphin gene from Staphylococcus simulans. Proc. Natl. Acad. Sci. USA 84:1127-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindler, C. A., and V. T. Schuhardt. 1964. Lysostaphin: a new bacteriolytic agent for the staphylococci. Proc. Natl. Acad. Sci. USA 51:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, and W. R. Jarvis. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 46.Soto, N., A. Vaghjimal, A. Stahl-Avicolli, J. Protic, L. Lutwick, and E. Chapnick. 1999. Bacitracin versus mupirocin for Staphylococcus aureus nasal colonization. Infect. Control Hosp. Epidemiol. 20:351-353. [DOI] [PubMed] [Google Scholar]
- 47.Stranden, A., K. Ehlert, H. Labischinski, and B. Berger-Bachi. 1997. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 179:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thumm, G., and F. Gotz. 1997. Studies on prolysostaphin processing and characterization of the lysostaphin immunity factor (Lif) of Staphylococcus simulans biovar staphylolyticus. Mol. Microbiol. 23:1251-1265. [DOI] [PubMed] [Google Scholar]
- 49.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe, H., H. Masaki, A. Norichika, K. Watanabe, K. Oishi, S. Kobayashi, A. Sata, R. Sugita, and T. Nagatake. 2001. Low concentrations of mupirocin in the pharynx following intranasal application may contribute to mupirocin resistance in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 39:3775-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White, A., and J. Smith. 1963. Nasal reservoir as the source of extranasal staphylococci. Antimicrob. Agents Chemother. 679-683. [PubMed]
- 52.Yano, M., Y. Doki, M. Inoue, T. Tsujinaka, H. Shiozaki, and M. Monden. 2000. Preoperative intranasal mupirocin ointment significantly reduces postoperative infection with Staphylococcus aureus in patients undergoing upper gastrointestinal surgery. Surg. Today 30:16-21. [DOI] [PubMed] [Google Scholar]
- 53.Yu, V. L., A. Goetz, M. Wagener, P. B. Smith, J. D. Rihs, J. Hanchett, and J. J. Zuravleff. 1986. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. N. Engl. J. Med. 315:91-96. [DOI] [PubMed] [Google Scholar]
- 54.Zygmunt, W. A., and P. A. Tavormina. 1972. Lysostaphin: model for a specific enzymatic approach to infectious disease. Prog. Drug Res. 16:309-333. [DOI] [PubMed] [Google Scholar]