Abstract
The ant(4′)-IIb gene of Pseudomonas aeruginosa BM4492, which encodes an aminoglycoside 4′-O-adenylyltransferase, was identified as a coding sequence of 756 bp corresponding to a protein with a calculated mass of 27,219 Da. Analysis of the deduced sequence indicated that the protein was related to aminoglycoside 4′-O-adenylyltransferases IIa and Ia found in P. aeruginosa and gram-positive bacteria, respectively. The enzyme conferred resistance to amikacin and tobramycin but not to dibekacin, gentamicin, or netilmicin. The ant(4′)-IIb gene had a chromosomal location in five of six clinical isolates of P. aeruginosa tested and was plasmid borne in the remaining strain. The ant(4′)-IIb gene was detected by PCR in some clinical strains of P. aeruginosa from the same hospital but not in members of other bacterial genera.
Aminoglycosides, in particular, amikacin, are antibiotics of major importance in the treatment of infections due to Pseudomonas aeruginosa. Since the introduction of these antibiotics in clinical practice, numerous strains of P. aeruginosa have developed resistance to this class of drugs. Although efflux has recently been shown to be involved in resistance to aminoglycosides in this bacterial species (11, 13, 18), the main resistance mechanism remains enzymatic modification of the drugs (16). In P. aeruginosa resistance to amikacin is due to production of either 6′-N-acetyltransferase type I [AAC(6′)-I], 3′-O-phosphotransferase type VI [APH(3′)-VI], or 4′-O-nucleotidyltransferase type II [ANT(4′)-II] (6, 10). In gram-negative bacteria, AAC(6′)-I enzymes are common, whereas APH(3′)-VI and ANT(4′)-II remain rare (16). The ANT(4′)-II enzyme is mediated in P. aeruginosa by the ca. 450-kb plasmid pMG77, and in Escherichia coli and Klebsiella pneumoniae it is mediated by plasmids of the IncM incompatibility group (4). The modifying enzyme confers resistance to amikacin, isepamicin, tobramycin, and other aminoglycosides with a 4′-hydroxyl group but not to dibekacin (4). In contrast, the ANT(4′)-I enzyme of gram-positive microorganisms has been shown to modify both the 4′- and 4"-hydroxyl groups, and therefore also confers resistance to dibekacin (7, 9, 12). We have studied P. aeruginosa strains isolated in Bulgaria which were resistant to amikacin and susceptible to netilmicin and which did not harbor the ant(4′)-IIa gene (15). We have characterized the determinant involved in this resistance, ant(4′)-IIb, and studied its dissemination in P. aeruginosa clinical isolates.
(An initial report of this work was presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy [S. Sabtcheva et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-166, 2001].).
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Seven epidemiologically unrelated clinical strains of P. aeruginosa isolated between 1992 and 1998 at the National Oncology Center in Sofia, Bulgaria, were selected for this study (Table 1). Five isolates (isolates BM4492, BM4529, BM4530, BM4532, and BM4533) that exhibited the unusual phenotype of resistance to amikacin and susceptibility to netilmicin were detected in routine laboratory testing with a Vitek apparatus (bioMérieux). The two remaining strains were BM4531, which was susceptible to amikacin, gentamicin, netilmicin, and tobramycin, and BM4534, which was resistant to these aminoglycosides. E. coli JM83 was used as a recipient for cloning of the ant(4′)-IIb gene into the pUC18 vector. E. coli BL21(DE3)pLysS was used with the pET23a(+) expression vector (Novagen, Madison, Wis.), and P. aeruginosa PAO38 (5) was used for transformation and conjugation assays. The strains were grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) at 37°C.
TABLE 1.
MICs of various aminoglycosides for P. aeruginosa and E. coli strains with and without the ant(4′)-IIb gene
| Strain | MIC (μg/ml)a
|
||||
|---|---|---|---|---|---|
| Amik- acin | Dibeka- cin | Gentam- icin | Netilmi- cin | Tobramy- cin | |
| P. aeruginosa BM4492 | >256 | >256 | >256 | 4 | >256 |
| P. aeruginosa BM4529 | >256 | 0.5 | 0.5 | 0.25 | >256 |
| P. aeruginosa BM4530 | >256 | 0.5 | 0.5 | 0.25 | >256 |
| P. aeruginosa BM4531 | 1 | 0.5 | 0.5 | 0.25 | 0.25 |
| P. aeruginosa BM4532 | 128 | 0.5 | 0.5 | 0.25 | >256 |
| P. aeruginosa BM4533 | >256 | >256 | >256 | 4 | >256 |
| P. aeruginosa BM4534 | >256 | >256 | >256 | >256 | >256 |
| E. coli JM83 | 0.5 | 0.25 | 0.25 | 0.25 | 0.25 |
| E. coli JM83(pAT795) | 32 | 0.25 | 0.25 | 0.25 | 16 |
MICs were determined on MH agar after 24 h of incubation at 37°C.
Susceptibility testing.
Antibiotic susceptibility was tested by disk diffusion on Mueller-Hinton (MH) agar (Bio-Rad, Marnes-la-Coquette, France). The MICs were determined on MH agar containing serial twofold dilutions of aminoglycosides with inocula of ca. 104 CFU per spot after incubation for 18 h at 37°C. The activities of 2′- and 6′-N-ethylnetilmicin were studied by diffusion on MH agar at 37°C with disks containing 100 μg of antibiotic.
Assay for aminoglycoside-modifying enzymes.
The activities of aminoglycoside-modifying enzymes in bacterial extracts were detected by the phosphocellulose paper-binding technique with [U-14C]ATP as a cofactor (3). The reaction was allowed to proceed for 30 min at 30°C.
DNA preparation and transformation.
Isolation of total DNA was done as described previously (14), and small- and large-scale preparations of plasmid DNA were made as described previously (14). Amplification of DNA was performed in a 2400 thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) with Pfu DNA polymerase (Stratagene, La Jolla, Calif.), as recommended by the manufacturer. PCR elongation times and temperatures were adjusted according to the expected sizes of the PCR products and the nucleotide sequences of the primers, respectively. The ant(4′)-IIb 3′-end truncated gene was amplified with specific oligodeoxynucleotides O1 and O2 (Table 2) with BM4492 DNA as a template. After digestion of the 644-bp PCR product with NdeI and EcoRI, the fragment was cloned into similarly digested pET23a(+) DNA. The resulting recombinant plasmid was introduced by transformation into E. coli BL21(DE3)pLysS competent cells that were plated on BHI agar containing ampicillin (50 μg/ml) and isopropyl-β-d-thiogalactopyranoside (0.6 mM). The amplification products obtained with primers O1 and O2 and primers R1 and R2 were used as specific probes for the ant(4′)-IIb gene and the rrs gene, respectively (Table 2). These fragments were purified by using the QIAquick PCR purification kit (Qiagen, Inc., Chatsworth, Calif.) and radiolabeled with [α-32P]dCTP by use of the nick-translation kit from Bethesda Research Laboratories Inc. (Gaithersburg, Md.), as described previously (14).
TABLE 2.
Primers used for PCR
| Gene | Primer name | Sequence (5′ → 3′)a | Positions | GenBank accession no. |
|---|---|---|---|---|
| ant(4′)-IIb | O1 | GAGAACCCATATGCAACATACTATCGCC | 1059-1068 | AY114142 |
| O2 | TAGAATTCTAGCGCGCACTTCGCTCTTC | 1683-1667 | ||
| rrs | R1 | CAACAGAATAAGCACCGGCT | 485-504 | X06684 |
| R2 | CACGATTACTAGCGATTCCG | 1350-1331 |
NdeI and EcoRI restriction sites are underlined.
Transformation of E. coli JM83 was performed as described previously (14) with selection on BHI agar containing ampicillin at 100 μg/ml and tobramycin at 5 μg/ml. Transformation (2) and conjugation experiments with BM4535, a spontaneous mutant of P. aeruginosa PAO38 resistant to imipenem, as a recipient and BM4492 or BM4534 as donors were performed with selection on BHI agar containing imipenem at 8 μg/ml and tobramycin at 32 μg/ml.
DNA sequence determination and analysis.
The ca. 2-kb PstI insert in pAT795 containing the ant(4′)-IIb gene was sequenced by using synthetic oligodeoxynucleotides and a CEQ 2000 DNA analysis system automatic sequencer (Beckman Coulter, Palo Alto, Calif.). Nucleotide and deduced amino acid sequences were analyzed with the GCG sequence analysis software package (version 10.1; Genetics Computer Group, Madison, Wis.). BLAST program searches were performed by using the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov). Multiple-sequence alignment was performed with the ClustalX program available at the website http://genome.jouy.inra.fr/.
Enzymes and chemicals.
T4 DNA ligase was from Amersham (Buckinghamshire, England), and RNase A (bovine pancreas) was from Calbiochem-Behring (La Jolla, Calif.). [α-32P]dCTP and [U-14C]ATP were obtained from the Radiochemical Centre (Amersham, England). The following antibiotics were provided by the indicated laboratories: amikacin, ampicillin, and kanamycin B, Bristol-Myers Squibb (Princeton, N.J.); imipenem, Merck Sharp & Dohme (West Point, Pa.); tobramycin, Eli Lilly & Co. (Indianapolis, Ind.); and gentamicin, gentamicins C1a, C1, and C2, isepamicin, netilmicin, 2′-N-ethylnetilmicin, and 6′-N-ethylnetilmicin, Schering-Plough Research Institute (Kenilworth, N.J.).
Nucleotide sequence accession number.
The nucleotide sequence of ant(4′)-IIb has been deposited in the GenBank data library under accession number AY114142.
RESULTS AND DISCUSSION
Aminoglycoside resistance in P. aeruginosa isolates.
The MICs of aminoglycosides for P. aeruginosa BM4492, BM4529, BM4530, BM4532, and BM4533 indicated that the strains were resistant to amikacin and tobramycin but remained susceptible to netilmicin (Table 1). Strains BM4492 and BM4533 were, in addition, resistant to dibekacin and gentamicin. These resistance phenotypes suggest the presence in the strains of an aminoglycoside 4′-O-adenylyltransferase, associated in BM4492 and BM4533 with an aminoglycoside 2"-O-adenylyltransferase. BM4534 was resistant to the aminoglycosides designated in Table 1; this resistance was due in part to an aac(6′)-Ib gene. All attempts to transfer tobramycin resistance from BM4492 and BM4534 to P. aeruginosa BM4535 by conjugation or transformation were unsuccessful.
Cloning and expression of the ant(4′)-IIb gene.
Total DNA from BM4492 and pUC18 DNA was digested with PstI, mixed, ligated, and introduced by transformation into E. coli JM83. Transformants selected on medium containing ampicillin plus tobramycin were screened for their plasmid contents by agarose gel electrophoresis of crude bacterial lysates. The smallest recombinant plasmid, pAT795, contained a 2-kb PstI fragment that conferred resistance to tobramycin and amikacin but not to gentamicin, dibekacin, or netilmicin (Table 1).
Extracts of E. coli JM83(pAT795) were shown to contain aminoglycoside adenylyltransferase activity. The substrate profile of the enzyme was consistent with an ANT(4′)-II activity, since amikacin and tobramycin were modified but gentamicin (containing a mixture of gentamicin C1a, C1, and C2 components) and dibekacin, which have a hydroxyl group at the 4" position but not at the 4′ position, were not modified (data not shown).
Sequence analysis of the ant(4′)-IIb gene and of the deduced protein.
Comparison of the sequence of the insert in pAT795 with sequences in the GenBank data library revealed a 794-bp open reading frame (ORF) homologous to the ant(4′)-IIa gene (72% identity over 786 nucleotides). A typical ribosome binding site, AGGAGA (positions 1050 to 1055), was located five nucleotides upstream from a GTG putative initiation codon. Promoter sequences were not readily apparent from the analysis of the region upstream from ant(4′)-IIb. The gene was identified as a coding sequence of 756 bp corresponding to a protein with a calculated mass of 27,219 Da. Alignment of ANT(4′)-IIb with ANT(4′)-IIa indicated that 156 of the first 205 amino acids were conserved (76% identity). The similarity was interrupted at Arg205, whereas homology at the nucleotide level was conserved throughout the genes (Fig. 1). This was due to an additional guanosine at position 1677 of the deposited sequence that led to a frameshift mutation. This result suggests that the C-terminal portions of ANT(4′) enzymes may not be required for activity. In order to test this hypothesis, a PCR fragment encoding a protein that corresponds to ANT(4′)-IIa but in which the 43 C-terminal amino acids are deleted was cloned in the expression vector pET23a(+). The recombinant plasmid did not confer aminoglycoside resistance to E. coli BL21. These data suggest that the carboxy terminus is required for enzyme activity but that the amino acid sequence can be altered.
FIG. 1.
Alignment of the deduced amino acid sequences of ANT(4′). Sequences are from P. aeruginosa BM4492 [ANT(4′)-IIb], P. aeruginosa [ANT(4′)-IIa] (GenBank accession number M98270), Staphylococcus aureus [ANT(4′)-Ia] (GenBank accession number P05057), and Bacillus subtilis [ANT(4′)-Ib] (GenBank accession number P05057). Alignment was performed with the ClustalX program. Identical amino acids are indicated by an asterisk; conservative amino acid substitutions (indicated by dots) correspond to the following exchange groups: A, G, P, S, and T; H, K, and R; F, W, and Y; D, E, N, and Q; I, L, M, and V; and C.
Distribution of the ant(4′) gene in P. aeruginosa.
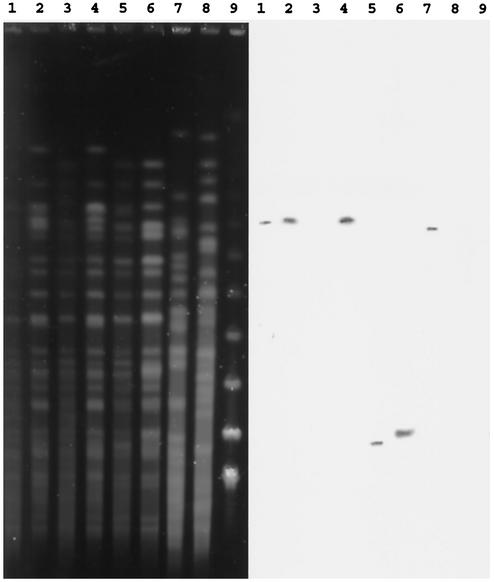
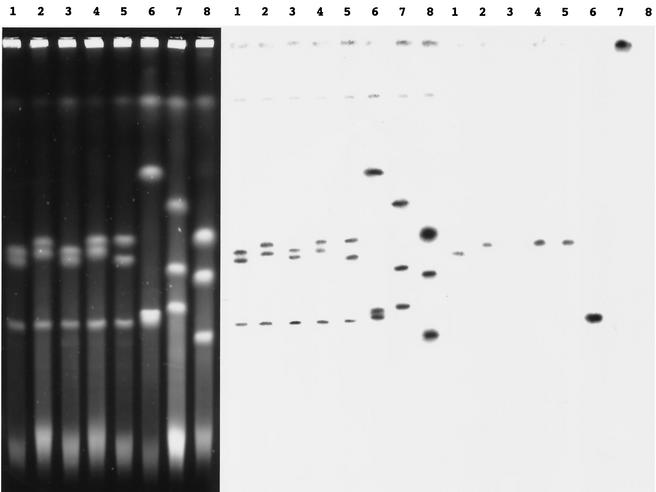
Seven clinical isolates including an aminoglycoside-susceptible strain were analyzed by pulsed-field gel electrophoresis (PFGE) after digestion of total DNA by SpeI. All strains tested except BM4530 and BM4531 could be distinguished by this technique (Fig. 2). Common bands indicated that the clinical isolates were related, whereas the PFGE profile of control strain PAO38 was clearly distinct. The SpeI fragments which hybridized with the ant(4′)-IIb probe were larger than 320 kb in strains BM4529, BM4530, BM4532, and BM4534 and ca. 80 and 90 kb in strains BM4533 and BM4492, respectively (Fig. 2). These results confirm that the strains did not represent a single clone. To study the genetic basis of the ant(4′)-IIb gene, total DNA of the same strains was digested by I-CeuI, an intron-encoded endonuclease specific for rRNA genes, or SpeI (Fig. 2 and 3). Analysis of the genome sequence of strain PAO1 (17) indicates that it contains four copies of the rRNA operon in I-CeuI fragments of ca. 4,000, 950, 775, and 475 kb (Fig. 3). Four copies of the rRNA operon were detected in all strains; and in BM4529, BM4530, BM4532, BM4533, and BM4492, the I-CeuI fragments cohybridized with the rrs (8) and ant(4′)-IIb probes, indicating that the aminoglycoside resistance determinant was located in the chromosome (Fig. 3). By contrast, for strain BM4534, the ant(4′)-IIb probe gave a strong hybridization signal with the DNA that remained in the well but did not hybridize with the four fragments resolved in the gel (Fig. 3). These observations indicate that the resistance determinant is not part of the chromosome. The ant(4′)-IIb probe, but not the rrs probe (data not shown), hybridized with a ca. 320-kb SpeI fragment, consistent with the fact that the resistance gene in BM4534 was carried by a plasmid with a size of a minimum of 320 kb (Fig. 2). The presence of the ant(4′)-IIb gene at various genomic loci in P. aeruginosa suggests that the gene could be part of a mobile element. Sequence analysis of the neighboring environment of the gene in BM4492 indicated that it was not part of an integron. However, the 990 bp at the 5′ end of the 1,989-bp PstI fragment was 91% identical to a region of the genomic island of Salmonella sp. strain DT104 (GenBank accession number AF071555) associated with multidrug resistance (1). These regions are part of an ORF proposed to specify a protein related to putative transposases coded for by ORF341E from Salmonella enterica serovar Typhimurium and ORF513 from E. coli (GenBank accession numbers AJ310778 and L06418, respectively). In BM4492, a 9-bp motif consisting of CCCGATCTG was directly repeated (positions 889 to 907 and positions 908 to 916), and a stop codon at position 672 led to a truncated, probably inactive protein. This genetic organization suggests that the ant(4′)-IIb gene could have been part of an active genetic element that was later stabilized following a nonsense mutation. This proposal is consistent with the exclusive distribution of ant(4′)-IIb in P. aeruginosa, despite an extensive search for the gene in other gram-negative bacteria such as members of the family Enterobacteriaceae and strains of Acinetobacter from the same hospital.
FIG. 2.
PFGE of total SpeI-digested DNA of P. aeruginosa strains BM4529 (lane 1), BM4350 (lane 2), BM4531 (lane 3), BM4532 (lane 4), BM4533 (lane 5), BM4492 (lane 6), BM4534 (lane 7), and PAO38 (lane 8); concatemers of bacteriophage lambda were used as a molecular size standard (lane 9). Left, analysis of DNA by agarose gel electrophoresis; right, the resulting fragments were transferred to a Nytran membrane and hybridized to an ant(4′)-IIb probe labeled with 32P in vitro.
FIG. 3.
PFGE of total I-CeuI-digested DNA of P. aeruginosa strains BM4529 (lane 1), BM4350 (lane 2), BM4531 (lane 3), BM4532 (lane 4), BM4533 (lane 5), BM4492 (lane 6), BM4534 (lane 7), and PAO38 (lane 8). Left, analysis of DNA by agarose gel electrophoresis. The resulting fragments were transferred to a Nytran membrane and hybridized to rrs (middle) and ant(4′)-IIb (right) probes labeled in vitro with 32P.
Acknowledgments
This work was supported by a grant from bioMérieux and by a Bristol-Myers Squibb Unrestricted Biomedical Grant in Infectious Diseases.
REFERENCES
- 1.Briggs, C. E., and P. M. Fratamico. 1999. Molecular characterization of an antibiotic resistance gene cluster of Salmonella typhimurium DT104. Antimicrob. Agents Chemother. 43:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farinha, M. A., and A. M. Kropinski. 1990. High efficiency electroporation of Pseudomonas aeruginosa using frozen cell suspensions. FEMS Microbiol. Lett. 70:221-226. [DOI] [PubMed] [Google Scholar]
- 3.Haas, M. J., and J. E. Dowding. 1975. Aminoglycoside-modifying enzymes. Methods Enzymol. 43:611-628. [DOI] [PubMed] [Google Scholar]
- 4.Jacoby, G. A., M. J. Blaser, P. Santanam, H. Hächler, F. H. Kayser, R. S. Hare, and G. H. Miller. 1990. Appearance of amikacin and tobramycin resistance due to 4′-aminoglycoside nucleotidyltransferase [ANT(4′)-II] in gram-negative pathogens. Antimicrob. Agents Chemother. 34:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacoby, G. A., L. Sutton, L. Knobel, and P. Nammen. 1983. Properties of IncP-2 plasmids of Pseudomonas spp. Antimicrob. Agents Chemother. 24:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert, T., G. Gerbaud, and P. Courvalin. 1994. Characterization of transposon Tn1528, which confers amikacin resistance by synthesis of aminoglycoside 3′-O-phosphotransferase type VI. Antimicrob. Agents Chemother. 38:702-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Goffic, F., A. Martel, M. L. Capmau, B. Baca, P. Goebel, H. Chardon, C. J. Soussy, J. Duval, and D. H. Bouanchaud. 1976. New plasmid-mediated nucleotidylation of aminoglycoside antibiotic in Staphylococcus aureus. Antimicrob. Agents Chemother. 10:258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumura, M., Y. Katakura, T. Imanaka, and S. Aiba. 1984. Enzymatic and nucleotide sequence studies of a kanamycin-inactivating enzyme encoded by a plasmid from thermophilic bacilli in comparison with that encoded by plasmid pUB110. J. Bacteriol. 160:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller, G. H., F. J. Sabatelli, R. S. Hare, Y. Glupczynski, P. Mackey, D. Shlaes, K. Shimizu, K. J. Shaw, et al. 1997. The most frequent aminoglycoside resistance mechanisms—changes with time and geographic area: a reflection of aminoglycoside usage patterns? Clin. Infect. Dis. 24(Suppl. 1):S46-S62. [DOI] [PubMed] [Google Scholar]
- 11.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1999. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:415-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller, R. E., T. Ano, T. Imanaka, and S. Aiba. 1986. Complete nucleotide sequences of Bacillus plasmids pUB110dB, pRBH1 and its copy mutants. Mol. Gen. Genet. 202:169-171. [DOI] [PubMed] [Google Scholar]
- 13.Ramos Aires, J., T. Köhler, H. Nikaido, and P. Plésiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Shaw, K. J., H. Munayyer, P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Nucleotide sequence analysis and DNA hybridization studies of the ant(4′)-IIa gene from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 18.Westbrock-Wadman, S., D. R. Sherman, M. J. Hickey, S. N. Coulter, Y. Q. Zhu, P. Warrener, L. Y. Nguyen, R. M. Shawar, K. R. Folger, and C. K. Stover. 1999. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob. Agents Chemother. 43:2975-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]