Abstract
Systematic toxicity testing, using conventional toxicology methodologies, of single chemicals and chemical mixtures is highly impractical because of the immense numbers of chemicals and chemical mixtures involved and the limited scientific resources. Therefore, the development of unconventional, efficient, and predictive toxicology methods is imperative. Using carcinogenicity as an end point, we present approaches for developing predictive tools for toxicologic evaluation of chemicals and chemical mixtures relevant to environmental contamination. Central to the approaches presented is the integration of physiologically based pharmacokinetic/pharmacodynamic (PBPK/PD) and quantitative structure--activity relationship (QSAR) modeling with focused mechanistically based experimental toxicology. In this development, molecular and cellular biomarkers critical to the carcinogenesis process are evaluated quantitatively between different chemicals and/or chemical mixtures. Examples presented include the integration of PBPK/PD and QSAR modeling with a time-course medium-term liver foci assay, molecular biology and cell proliferation studies. Fourier transform infrared spectroscopic analyses of DNA changes, and cancer modeling to assess and attempt to predict the carcinogenicity of the series of 12 chlorobenzene isomers. Also presented is an ongoing effort to develop and apply a similar approach to chemical mixtures using in vitro cell culture (Syrian hamster embryo cell transformation assay and human keratinocytes) methodologies and in vivo studies. The promise and pitfalls of these developments are elaborated. When successfully applied, these approaches may greatly reduce animal usage, personnel, resources, and time required to evaluate the carcinogenicity of chemicals and chemical mixtures.
Full text
PDF



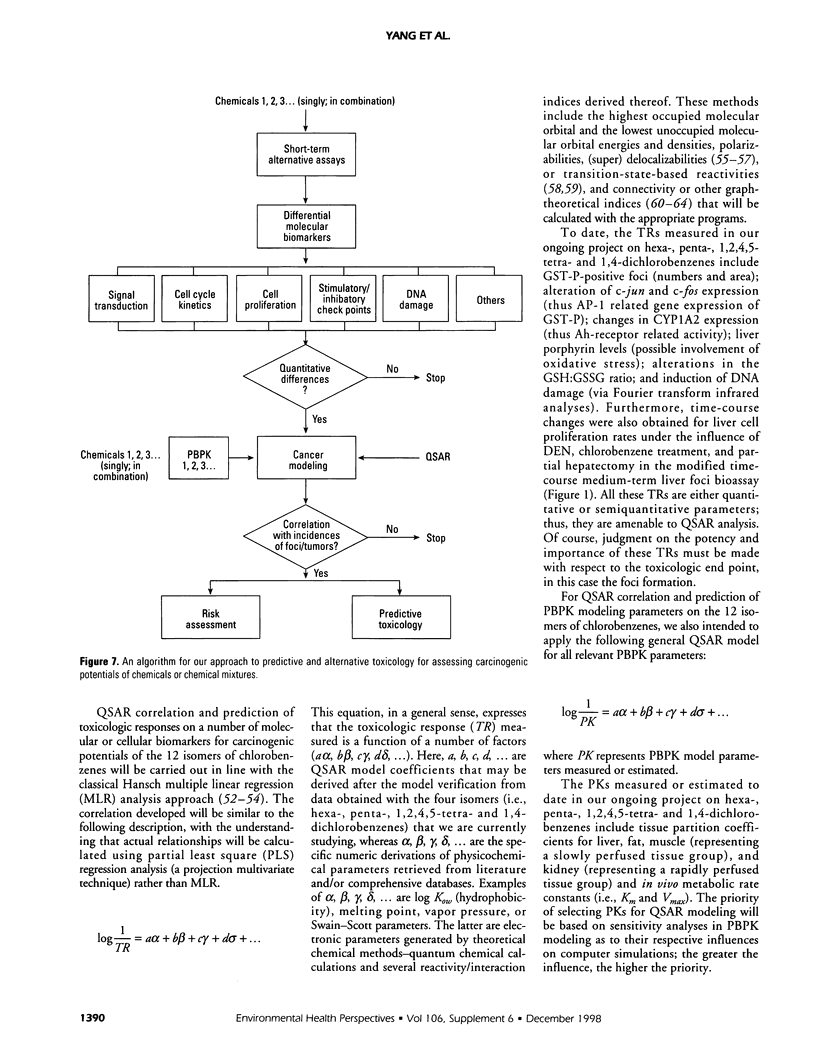



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baden H. P., Kubilus J., Kvedar J. C., Steinberg M. L., Wolman S. R. Isolation and characterization of a spontaneously arising long-lived line of human keratinocytes (NM 1). In Vitro Cell Dev Biol. 1987 Mar;23(3):205–213. doi: 10.1007/BF02623581. [DOI] [PubMed] [Google Scholar]
- Barton H. A., Creech J. R., Godin C. S., Randall G. M., Seckel C. S. Chloroethylene mixtures: pharmacokinetic modeling and in vitro metabolism of vinyl chloride, trichloroethylene, and trans-1,2-dichloroethylene in rat. Toxicol Appl Pharmacol. 1995 Feb;130(2):237–247. doi: 10.1006/taap.1995.1029. [DOI] [PubMed] [Google Scholar]
- Basak S. C., Bertelsen S., Grunwald G. D. Use of graph theoretic parameters in risk assessment of chemicals. Toxicol Lett. 1995 Sep;79(1-3):239–250. doi: 10.1016/0378-4274(95)03375-u. [DOI] [PubMed] [Google Scholar]
- Basak S. C., Grunwald G. D. Predicting mutagenicity of chemicals using topological and quantum chemical parameters: a similarity based study. Chemosphere. 1995 Jul;31(1):2529–2546. doi: 10.1016/0045-6535(95)00122-o. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJongh J., Verhaar H. J., Hermens J. L. A quantitative property-property relationship (QPPR) approach to estimate in vitro tissue-blood partition coefficients of organic chemicals in rats and humans. Arch Toxicol. 1997;72(1):17–25. doi: 10.1007/s002040050463. [DOI] [PubMed] [Google Scholar]
- Debnath A. K., Debnath G., Shusterman A. J., Hansch C. A QSAR investigation of the role of hydrophobicity in regulating mutagenicity in the Ames test: 1. Mutagenicity of aromatic and heteroaromatic amines in Salmonella typhimurium TA98 and TA100. Environ Mol Mutagen. 1992;19(1):37–52. doi: 10.1002/em.2850190107. [DOI] [PubMed] [Google Scholar]
- Debnath A. K., Hansch C. Structure-activity relationship of genotoxic polycyclic aromatic nitro compounds: further evidence for the importance of hydrophobicity and molecular orbital energies in genetic toxicity. Environ Mol Mutagen. 1992;20(2):140–144. doi: 10.1002/em.2850200210. [DOI] [PubMed] [Google Scholar]
- Dlugosz A. A., Glick A. B., Tennenbaum T., Weinberg W. C., Yuspa S. H. Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol. 1995;254:3–20. doi: 10.1016/0076-6879(95)54003-2. [DOI] [PubMed] [Google Scholar]
- Hansch C., Hoekman D., Leo A., Zhang L., Li P. The expanding role of quantitative structure-activity relationships (QSAR) in toxicology. Toxicol Lett. 1995 Sep;79(1-3):45–53. doi: 10.1016/0378-4274(95)03356-p. [DOI] [PubMed] [Google Scholar]
- Hansch C. Structure-activity relationships of chemical mutagens and carcinogens. Sci Total Environ. 1991 Dec;109-110:17–29. doi: 10.1016/0048-9697(91)90167-d. [DOI] [PubMed] [Google Scholar]
- Huff J., Haseman J., Rall D. Scientific concepts, value, and significance of chemical carcinogenesis studies. Annu Rev Pharmacol Toxicol. 1991;31:621–652. doi: 10.1146/annurev.pa.31.040191.003201. [DOI] [PubMed] [Google Scholar]
- Ito N., Imaida K., Hasegawa R., Tsuda H. Rapid bioassay methods for carcinogens and modifiers of hepatocarcinogenesis. Crit Rev Toxicol. 1989;19(4):385–415. doi: 10.3109/10408448909029328. [DOI] [PubMed] [Google Scholar]
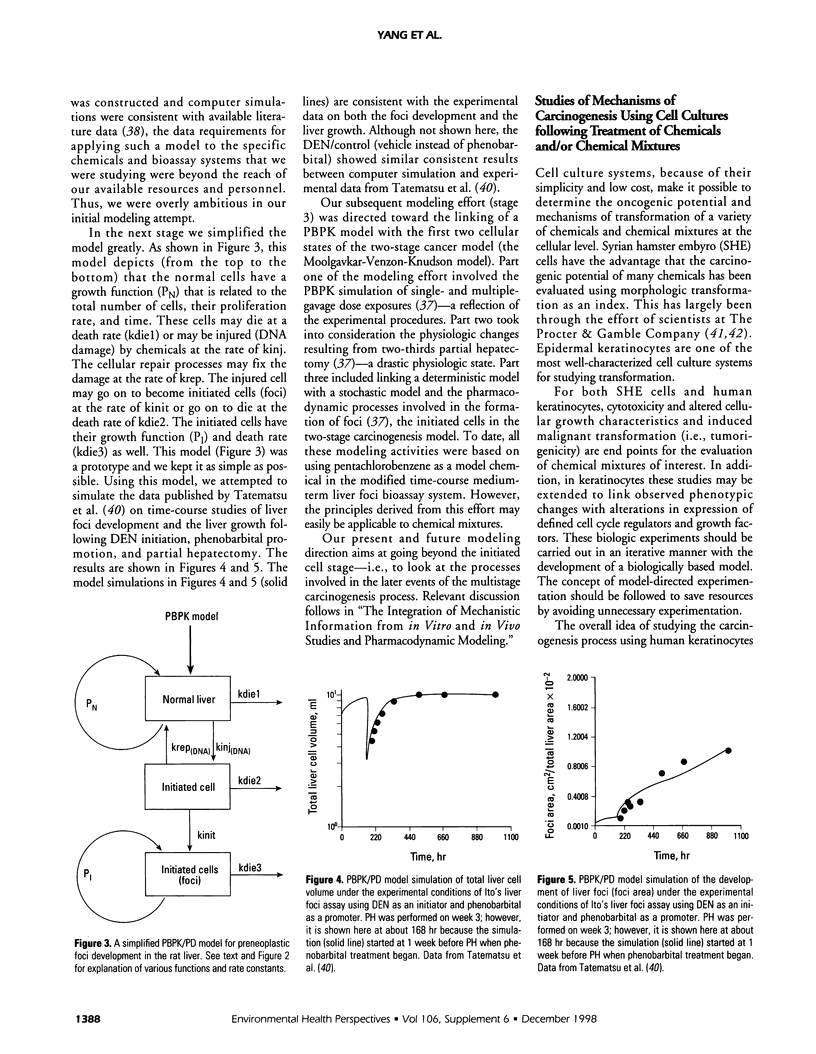
- Ito N., Tatematsu M., Hasegawa R., Tsuda H. Medium-term bioassay system for detection of carcinogens and modifiers of hepatocarcinogenesis utilizing the GST-P positive liver cell focus as an endpoint marker. Toxicol Pathol. 1989;17(4 Pt 1):630–641. doi: 10.1177/0192623389017004108. [DOI] [PubMed] [Google Scholar]
- Kerckaert G. A., Isfort R. J., Carr G. J., Aardema M. J., LeBoeuf R. A. A comprehensive protocol for conducting the Syrian hamster embryo cell transformation assay at pH 6.70. Mutat Res. 1996 Sep 21;356(1):65–84. doi: 10.1016/0027-5107(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Kerckaert G. A., Isfort R. J., Carr G. J., Aardema M. J., LeBoeuf R. A. A comprehensive protocol for conducting the Syrian hamster embryo cell transformation assay at pH 6.70. Mutat Res. 1996 Sep 21;356(1):65–84. doi: 10.1016/0027-5107(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Pelekis M., Krishnan K. Assessing the relevance of rodent data on chemical interactions for health risk assessment purposes: a case study with dichloromethane-toluene mixture. Regul Toxicol Pharmacol. 1997 Feb;25(1):79–86. doi: 10.1006/rtph.1996.1075. [DOI] [PubMed] [Google Scholar]
- Purcell K. J., Cason G. H., Gargas M. L., Andersen M. E., Travis C. C. In vivo metabolic interactions of benzene and toluene. Toxicol Lett. 1990 Jul;52(2):141–152. doi: 10.1016/0378-4274(90)90148-f. [DOI] [PubMed] [Google Scholar]
- Purdy R. The utility of computed superdelocalizability for predicting the LC50 values of epoxides to guppies. Sci Total Environ. 1991 Dec;109-110:553–556. doi: 10.1016/0048-9697(91)90208-v. [DOI] [PubMed] [Google Scholar]
- Quann R. J. Modeling the chemistry of complex petroleum mixtures. Environ Health Perspect. 1998 Dec;106 (Suppl 6):1441–1448. doi: 10.1289/ehp.98106s61441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Jay G., Arnstein P., Price F. M., Sanford K. K., Aaronson S. A. Neoplastic transformation of human epidermal keratinocytes by AD12-SV40 and Kirsten sarcoma viruses. Science. 1985 Mar 8;227(4691):1250–1252. doi: 10.1126/science.2579430. [DOI] [PubMed] [Google Scholar]
- Roomi M. W., Ho R. K., Sarma D. S., Farber E. A common biochemical pattern in preneoplastic hepatocyte nodules generated in four different models in the rat. Cancer Res. 1985 Feb;45(2):564–571. [PubMed] [Google Scholar]
- Sabljić A. Chemical topology and ecotoxicology. Sci Total Environ. 1991 Dec;109-110:197–220. doi: 10.1016/0048-9697(91)90178-h. [DOI] [PubMed] [Google Scholar]
- Sabljić A., Protić M. Molecular connectivity: a novel method for prediction of bioconcentration factor of hazardous chemicals. Chem Biol Interact. 1982 Dec;42(3):301–310. doi: 10.1016/0009-2797(82)90074-6. [DOI] [PubMed] [Google Scholar]
- Sato A., Endoh K., Kaneko T., Johanson G. Effects of consumption of ethanol on the biological monitoring of exposure to organic solvent vapours: a simulation study with trichloroethylene. Br J Ind Med. 1991 Aug;48(8):548–556. doi: 10.1136/oem.48.8.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif R., Charest-Tardif G., Brodeur J., Krishnan K. Physiologically based pharmacokinetic modeling of a ternary mixture of alkyl benzenes in rats and humans. Toxicol Appl Pharmacol. 1997 May;144(1):120–134. doi: 10.1006/taap.1996.8096. [DOI] [PubMed] [Google Scholar]
- Tardif R., Laparé S., Charest-Tardif G., Brodeur J., Krishnan K. Physiologically-based pharmacokinetic modeling of a mixture of toluene and xylene in humans. Risk Anal. 1995 Jun;15(3):335–342. doi: 10.1111/j.1539-6924.1995.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Tardif R., Laparé S., Krishnan K., Brodeur J. Physiologically based modeling of the toxicokinetic interaction between toluene and m-xylene in the rat. Toxicol Appl Pharmacol. 1993 Jun;120(2):266–273. doi: 10.1006/taap.1993.1111. [DOI] [PubMed] [Google Scholar]
- Tatematsu M., Aoki T., Kagawa M., Mera Y., Ito N. Reciprocal relationship between development of glutathione S-transferase positive liver foci and proliferation of surrounding hepatocytes in rats. Carcinogenesis. 1988 Feb;9(2):221–225. doi: 10.1093/carcin/9.2.221. [DOI] [PubMed] [Google Scholar]
- Tatematsu M., Mera Y., Ito N., Satoh K., Sato K. Relative merits of immunohistochemical demonstrations of placental, A, B and C forms of glutathione S-transferase and histochemical demonstration of gamma-glutamyl transferase as markers of altered foci during liver carcinogenesis in rats. Carcinogenesis. 1985 Nov;6(11):1621–1626. doi: 10.1093/carcin/6.11.1621. [DOI] [PubMed] [Google Scholar]
- Thakore K. N., Gargas M. L., Andersen M. E., Mehendale H. M. PB-PK derived metabolic constants, hepatotoxicity, and lethality of BrCCl3 in rats pretreated with chlordecone, phenobarbital, or mirex. Toxicol Appl Pharmacol. 1991 Jul;109(3):514–528. doi: 10.1016/0041-008x(91)90014-6. [DOI] [PubMed] [Google Scholar]
- Thomas R. S., Gustafson D. L., Ramsdell H. S., el-Masri H. A., Benjamin S. A., Yang R. S. Enhanced regional expression of glutathione S-transferase P1-1 with colocalized AP-1 and CYP 1A2 induction in chlorobenzene-induced porphyria. Toxicol Appl Pharmacol. 1998 May;150(1):22–31. doi: 10.1006/taap.1998.8385. [DOI] [PubMed] [Google Scholar]
- Verhaar H. J., Morroni J. R., Reardon K. F., Hays S. M., Gaver D. P., Jr, Carpenter R. L., Yang R. S. A proposed approach to study the toxicology of complex mixtures of petroleum products: the integrated use of QSAR, lumping analysis and PBPK/PD modeling. Environ Health Perspect. 1997 Feb;105 (Suppl 1):179–195. doi: 10.1289/ehp.97105s1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburger E. K. History of the Bioassay Program of the National Cancer Institute. Prog Exp Tumor Res. 1983;26:187–201. doi: 10.1159/000407260. [DOI] [PubMed] [Google Scholar]
- Yang R. S. Some current approaches for studying combination toxicology in chemical mixtures. Food Chem Toxicol. 1996 Nov-Dec;34(11-12):1037–1044. doi: 10.1016/s0278-6915(97)00072-0. [DOI] [PubMed] [Google Scholar]
- Yang R. S., el-Masri H. A., Thomas R. S., Constan A. A. The use of physiologically-based pharmacokinetic/pharmacodynamic dosimetry models for chemical mixtures. Toxicol Lett. 1995 Dec;82-83:497–504. doi: 10.1016/0378-4274(95)03579-6. [DOI] [PubMed] [Google Scholar]
- el-Masri H. A., Constan A. A., Ramsdell H. S., Yang R. S. Physiologically based pharmacodynamic modeling of an interaction threshold between trichloroethylene and 1,1-dichloroethylene in Fischer 344 rats. Toxicol Appl Pharmacol. 1996 Nov;141(1):124–132. doi: 10.1006/taap.1996.0268. [DOI] [PubMed] [Google Scholar]
- el-Masri H. A., Tessari J. D., Yang R. S. Exploration of an interaction threshold for the joint toxicity of trichloroethylene and 1,1-dichloroethylene: utilization of a PBPK model. Arch Toxicol. 1996;70(9):527–539. doi: 10.1007/s002040050310. [DOI] [PubMed] [Google Scholar]
- el-Masri H. A., Thomas R. S., Sabados G. R., Phillips J. K., Constan A. A., Benjamin S. A., Andersen M. E., Mehendale H. M., Yang R. S. Physiologically based pharmacokinetic/pharmacodynamic modeling of the toxicologic interaction between carbon tetrachloride and Kepone. Arch Toxicol. 1996;70(11):704–713. doi: 10.1007/s002040050331. [DOI] [PubMed] [Google Scholar]



