Abstract
Periodontitis is a common chronic oral infection caused by gram-negative bacteria, including Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontitis evokes inflammatory host response locally in the periodontium but also systemically. The systemic humoral antibody response against oral pathogens can conveniently be measured by an immunoassay. The aim of the study was to measure serum immunoglobulin G class antibodies against A. actinomycetemcomitans and P. gingivalis by an enzyme-linked immunosorbent assay (ELISA) in which mixtures of several serotypes of the pathogens were used as antigens to avoid biasing of the results in favor of a particular strain. For A. actinomycetemcomitans the antigen consisted of six strains representing serotypes a, b, c, d, and e and one nonserotypeable strain. In the P. gingivalis ELISA, antigens representing serotypes a, b, and c were used. Serum samples from 90 subjects, including 35 samples from patients with diagnosed periodontitis, 10 samples from periodontally healthy controls, and 45 samples from randomly selected apparently healthy volunteers (referred to as “healthy subjects”), were tested. For both pathogens the antibody levels (means ± standard deviations) of the patients—expressed as area under the dilution curve—were significantly higher than those for healthy controls or healthy subjects, with values for A. actinomycetemcomitans and P. gingivalis, respectively, as follows: patients, 22.60 ± 9.94 mm2 and 26.72 ± 11.13 mm2; healthy controls, 9.99 ± 3.92 mm2 and 6.90 ± 3.38 mm2; and healthy subjects, 16.85 ± 6.67 mm2 and 8.51 ± 4.23 mm2. The serotype mixture ELISA is suitable for measuring antibodies against periodontal pathogens in large epidemiological studies in order to evaluate the role of periodontitis as a risk factor for other diseases.
Periodontal diseases are characterized by inflammatory changes in the periodontium caused by bacterial infections. Inflammation may lead to destruction of the tooth-supporting tissues and eventually to tooth loss. Most of the periodontal tissue destruction is due to indirect activity of bacteria, including activation of the defense mechanisms of the host (17). Out of more than 500 cultivable bacterial species isolated from the gingival crevices of humans (24), only 3 gram-negative species—including Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis—are presently considered periodontal pathogens and primary etiological agents in the destructive forms of the periodontal diseases (40).
The finding that periodontitis is a plausible risk factor for cardiovascular diseases (2), stroke (41), rheumatoid arthritis (26), and premature birth (27) adds a new perspective to the importance of oral health. The increased bacterial burden in the inflamed periodontal pockets often leads to the presence of oral bacteria and their components in the systemic circulation (5, 15). From the bacterial components involved in the inflammatory process, the most widely studied are lipopolysaccharides, heat shock proteins, and fimbria. The continued systemic exposure to bacteria and, for example, lipopolysaccharide, results in a release of proinflammatory mediators, which may be a significant factor in the pathogenesis of atherosclerosis and stroke (12, 19, 31). Periodontitis is accompanied by the systemic antibody response against periodontal pathogens, which can conveniently be measured by an immunoassay. In order to evaluate periodontitis as a potential risk factor for other diseases, levels of antibody against periodontal pathogens in serum are useful markers for periodontal infection as in many other common viral and bacterial infections (9, 10, 33).
Both periodontal pathogen species investigated here are both genetically and serologically heterogeneous; A. actinomycetemcomitans has at least 50 genotypes (32), and P. gingivalis has 78 genotypes (21, 37). Up to now, five A. actinomycetemcomitans serotypes (a, b, c, d, and e) have been designated (34, 42). However, 3 to 8% of A. actinomycetemcomitans isolates still remain nonserotypeable (13, 29, 32). Most patients with oral A. actinomycetemcomitans infections harbor only one serotype (34, 38, 42), and multiple serotypes are found in less than 10% of subjects (35). Contrary to those harboring A. actinomycetemcomitans, patients harboring P. gingivalis may be seropositive for more than one serotype (4, 7). P. gingivalis strains can be divided into several serogroups based on their protein antigen expression (14, 28) or into six serotypes designated K1 to K6 based on their capsular structures (18). No single serotype, clone, or group of clones of P. gingivalis has been shown to cause periodontitis in humans or experimental animals (11). For both pathogens, these observations support the idea of pooling strains representing different serotypes for use as target antigens in the enzyme-linked immunosorbent assay (ELISA) to avoid biasing of the antibody results in favor of a particular strain (T. Vilkuna, K. Mattila, M. Vesanen, B. Dogan, and S. Asikainen, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., p. 252, 2000).
The aim of the study was to measure serum immunoglobulin G (IgG) class antibody responses against A. actinomycetemcomitans and P. gingivalis by an ELISA in which mixtures of several serotypes of the pathogens were used as antigens. The immunoassay was designed to be used as a serological marker of periodontitis in large epidemiological studies in which no clinical or radiographic information about the periodontal status of the subjects is available.
MATERIALS AND METHODS
Study subjects.
Serum samples from 90 subjects were included in the study. Out of these, 35 samples were from patients (18 males and 17 females; mean age ± standard deviation [SD], 43.6 ± 6.1 years) with diagnosed periodontitis (referred to simply as “patients”), indicating clinical and/or radiographic periodontal attachment loss at more than six teeth. Ten samples were from controls (two males and eight females; mean age ± SD, 40.5 ± 12.4 years) with clinically healthy periodontal tissues (referred to as “healthy controls”) with no periodontal attachment loss. The third group comprised 45 samples from randomly selected apparently healthy volunteers (referred to herein as “healthy subjects”) who worked at a research institute in Helsinki, Finland (12 males and 33 females; mean age ± SD, 42.9 ± 9.9 years). From 31 patients, another serum sample was taken after periodontal treatment approximately 3 months after the first sampling (mean ± SD, 3.2 ± 1.4 months).
Bacterial sampling and PCR detection.
Subgingival bacterial samples were collected with a curette from the deepest and most inflamed periodontal pockets of the patients with periodontitis and from all approximal sites of teeth in the healthy controls. The samples were stored in VMGA III transport medium (22) at −70°C to be used as PCR templates. DNA was isolated according to the supplier's instructions using Chelex 100 resin (Bio-Rad, Helsinki, Finland), and A. actinomycetemcomitans and P. gingivalis were detected by PCR as reported earlier (1, 20). In every series of PCR, chromosomal DNA extracted (23) from A. actinomycetemcomitans (ATCC 43718) and P. gingivalis (W50) strains served as positive controls and water served as the negative control.
ELISA assay.
Serum IgG antibodies against A. actinomycetemcomitans and P. gingivalis were determined by an ELISA essentially as described earlier (6). As antigens we used mixtures of six strains of A. actinomycetemcomitans and three strains of P. gingivalis. The strains were ATCC 29523, ATCC 43718, ATCC 33384, IDH 781, IDH 1705, and C59A for A. actinomycetemcomitans, representing serotypes a, b, c, d, and e and one nonserotypeable (x) strain, and ATCC 33277, W50, and OMGS 434 for P. gingivalis, representing serotypes a, b, and c, respectively. A. actinomycetemcomitans strains were grown on supplemented Brucella agar plates (containing 5% horse blood, hemin [5 μg/ml], vitamin K1 [100 mg/ml], and Brucella agar) and incubated in an atmosphere of 5% CO2 at 37°C for 3 days. The cultures were transferred into Todd-Hewitt broth (3% TH, 1% yeast extract), where they were further grown for 2 days (1 day in 5 ml and 1 day in 200 ml) under the conditions mentioned below. To confirm the purity of the cultures by colony morphology and Gram staining, a sample from each bottle was further cultivated on a Brucella agar plate. After removing the broth by centrifugation at 5,500 × g at room temperature for 15 min, the bacteria were washed with phosphate-buffered saline (PBS) (10 mM phosphate [pH 7.4], 150 mM NaCl). P. gingivalis strains were cultured on supplemented Brucella agar plates anaerobically for 5 to 6 days, after which the purity of the cultures was checked by colony morphology and Gram staining.
Both strains used as antigens in the ELISA were fixed in 0.5% formalin-PBS overnight at 4°C and washed three times with PBS. The density of the bacterial suspensions in the antigen buffer (PBS, 0.5% bovine serum albumin, 0.05% Tween 20) was adjusted to give an absorbance of 0.15 at 580 nm. For serotype mixture ELISA, equal volumes of the six A. actinomycetemcomitans or three P. gingivalis strains were mixed and used to coat microtiter plates (Cliniplate Uni; Labsystems, Helsinki, Finland). For serotype-specific ELISAs, the plates were coated with one strain in a similar manner. The unspecific binding was blocked by 5% bovine serum albumin in PBS at room temperature for 30 min. Four dilutions of the serum samples in duplicate were added on the plate and incubated for 2 h at room temperature. The dilutions used were 1/500, 1/1,500, 1/4,500, and 1/13,500 for A. actinomycetemcomitans and 1/100, 1/400, 1/1,600, and 1/6,400 for P. gingivalis. The bound antibodies were visualized using horseradish peroxidase-coupled goat anti-human IgG (Sigma) diluted 1/20,000 and measured spectrophotometrically at 492 nm. Unspecific binding was monitored by blank wells which contained no sample, and four dilutions of a “low” (pool of core blood) and a “high” (a high-level responding) control serum in duplicate were measured on each plate.
The IgG levels are expressed as the areas (square millimeters) under the dose-response curves (AUCs) of the test and reference sera as suggested earlier by Sedgwick et al. (36). The areas were calculated by the midpoint estimate procedure for approximating the integral function by the following working formula:
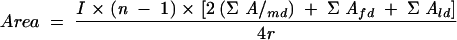 |
where A is the net absorbance at different dilutions (md, middle dilution; fd, first dilution; ld, last dilution), n is the number of dilutions, I is the orbit ray interval used on the graph paper (here, 10 mm), and r is the number of replicates.
Statistics.
The statistical significance for the differences between the means of separate groups was determined using a two-tailed Student's t test. A P value of <0.05 was considered significant. The correlation analysis was performed by using Excel Software (Windows 97).
RESULTS
ELISA quality control.
In the ELISA the interassay coefficients of variation were 4.1 and 8.1, and the intra-assay reproducibilities (SD) between duplicate assays were 0.20 and 0.15 for A. actinomycetemcomitans and P. gingivalis, respectively. As a low control in each assay we used a serum pool from cord blood, whose IgG results represented the zero level in serum for the assay, 3.40 ± 0.11 mm2 for A. actinomycetemcomitans and 3.32 ± 0.52 mm2 for P. gingivalis. The detection limits for A. actinomycetemcomitans and P. gingivalis were 0.53 and 0.65 mm2, respectively.
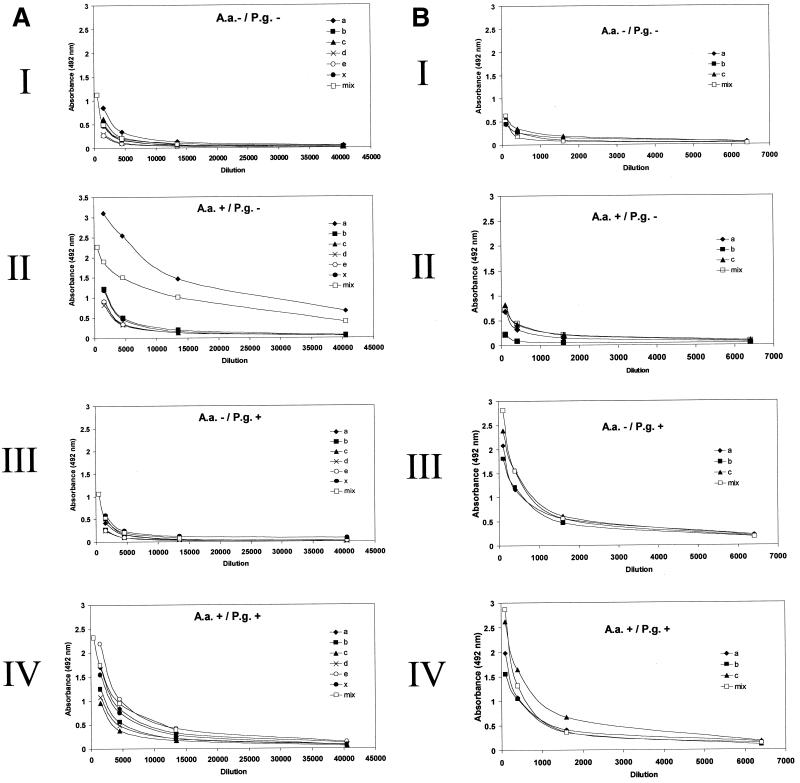
To analyze how individual serum IgG levels differ in serotype-specific and serotype mixture ELISAs, serum samples from 10 subjects were evaluated by both types of assays. The 10 samples were chosen to represent different results in the PCR detection of the two pathogens from the subgingival samples. As an example from these analyses, Fig. 1 shows the dilution curves of serum samples obtained from four subjects whose results in the PCR detection were as follows: negative and negative (panel I), positive and negative (panel II), negative and positive (panel III), and positive and positive (panel IV) for A. actinomycetemcomitans and P. gingivalis, respectively. In both A. actinomycetemcomitans (Fig. 1A) and P. gingivalis (Fig. 1B) ELISAs, there was a good correlation between the two ELISA types for all 10 subjects (r = 0.91 and r = 0.98 [P < 0.001], respectively). In the A. actinomycetemcomitans ELISA, the AUCs measured on a serotype mixture plate differed ±0.95 mm2 from the average of the AUCs measured on serotype-specific plates. When the titer against one serotype was clearly dominant, like that against A. actinomycetemcomitans serotype a in Fig. 1A, panel II (AUC, 14.73 mm2), the mixture result (AUC, 12.54 mm2) lay between the titers of low-responding serotypes (average AUC, 2.71 mm2) and the high-responding serotype. In the P. gingivalis ELISA, there were no significant differences between the levels of IgG against the three distinct serotypes, and the AUCs on the serotype mixture plates differed only ±0.87 mm2 from the mean AUCs of serotype-specific plates (Fig. 1B). The figure (Fig. 1) also shows that there was no cross-reaction between the A. actinomycetemcomitans and P. gingivalis assays.
FIG. 1.
Comparison of serum IgG levels against A. actinomycetemcomitans and P. gingivalis measured by serotype-specific and serotype mixture ELISA. Four subjects (I, II, III, and IV) were chosen according to presence (+) or absence (−) of subgingival A. actinomycetemcomitans (A.a.) and P. gingivalis (P.g.) by PCR detection, and their serum samples were analyzed by ELISA. (A) A. actinomycetemcomitans serotypes a (ATCC 29523), b (ATCC 43718), c (ATCC 33384), d (IDH 781), e (IDH 1705), and × (non-serotypeable) or their mixture (mix) was used as antigen. (B) P. gingivalis serotypes a (ATCC 33277), b (W50), and c (OMGS 434) or their mixture (mix) was used as antigen.
PCR results.
DNA was extracted from the subgingival bacterial samples of patients before treatment (n = 35) and healthy controls (n = 10) and amplified by PCR to detect A. actinomycetemcomitans and P. gingivalis by using species-specific primers. Among the patients with periodontitis, 15 (42.9%) were positive for A. actinomycetemcomitans and 28 (80.0%) were positive for P. gingivalis. Ten patients (28.6%) displayed both pathogens, and two (5.7%) exhibited neither of them. All periodontally healthy controls were negative for both species.
Serotype-mixture-ELISA results.
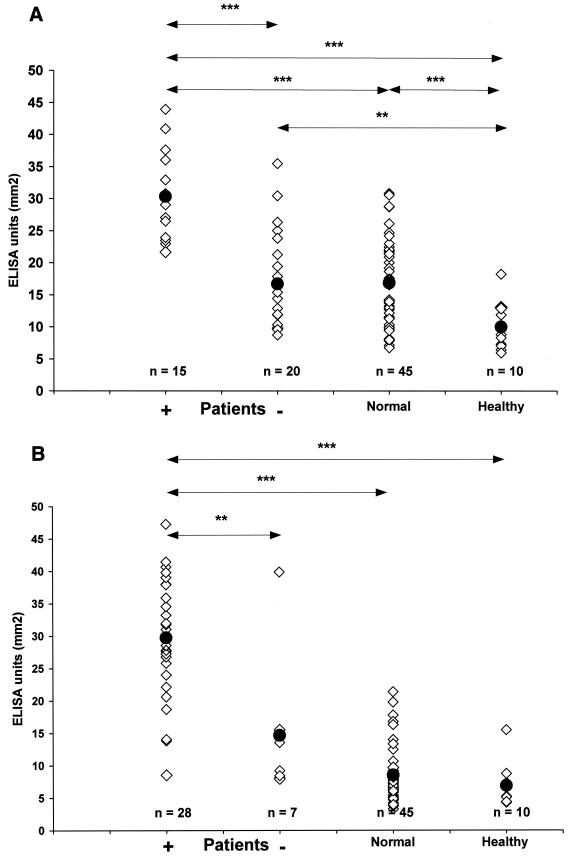
The IgG level (mean ± SD) of all patients with periodontitis (n = 35) against A. actinomycetemcomitans was 22.60 ± 9.94 mm2 (Fig. 2A). The corresponding values of PCR-positive and PCR-negative patients were 30.47 ± 6.74 mm2 and 16.69 ± 7.94 mm2, respectively. The mean AUCs of healthy controls and normal subjects were 9.99 ± 3.92 mm2 and 16.85 ± 6.67 mm2, respectively. The A. actinomycetemcomitans antibody levels of PCR-positive patients were significantly higher than those of PCR-negative patients (P < 0.001), healthy controls (P < 0.001), and healthy subjects (P < 0.001). The antibody results of the PCR-negative patients did not differ significantly from those of the healthy subjects (P > 0.05), but they were significantly higher than the results of the healthy controls (P < 0.01). When the titers of all patients were analyzed as one group, the results were significantly higher than those of healthy controls (P < 0.001) or healthy subjects (P < 0.01).
FIG. 2.
A. actinomycetemcomitans and P. gingivalis IgG results in different subject groups. The IgG levels, expressed as AUCs, against the two periodontal pathogens were measured by an ELISA in which mixtures of six A. actinomycetemcomitans strains (A) and three P. gingivalis strains (B) were used as antigens. The patients (n = 35) were divided in two groups according to the subgingival presence or absence of the pathogen by PCR detection. The statistical significance of the difference in the results between the groups is depicted (∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05). The black circles represent the mean of antibody levels within the group. Normal, healthy subjects; healthy, healthy controls.
In the P. gingivalis ELISA, the PCR-positive (29.73 ± 9.02 mm2) and PCR-negative (14.67 ± 11.50 mm2) patients had significantly different serum IgG levels (P < 0.01). The IgG level of all patients (mean ± SD) was 26.72 ± 11.13 mm2, and those of healthy subjects and healthy controls were 8.51 ± 4.23 mm2 and 6.90 mm2 ± 3.38, respectively (Fig. 2B). The antibody result of the PCR-positive patients differed significantly from the results of the healthy subjects (P < 0.001) and the healthy controls (P < 0.001), but the AUCs of P. gingivalis PCR-negative patients did not differ from those of the two other groups (P = 0.21 and P = 0.13, respectively). When analyzed as one group, the patients had higher antibody levels than healthy subjects (P < 0.001) and healthy controls (P < 0.001), which in turn did not differ significantly from each other (P = 0.21).
From the serum antibody results, a score for the combined response to both pathogens was calculated by summarizing the individual A. actinomycetemcomitans and P. gingivalis AUCs. Thus, the AUC scores (means ± SDs) for the patients, the normal subjects, and the healthy subjects are 49.30 ± 12.46, 25.36 ± 8.57, and 17.91 ± 8.64 mm2, respectively. All the differences between the groups remained statistically significant: for patients versus healthy controls, P < 0.001; for patients versus healthy subjects, P < 0.001; and for healthy subjects versus healthy controls, P < 0.05. With a mean AUC threshold of the value for healthy controls ± 1.5× SD, the sensitivity of the assay for finding periodontitis is 71%, and the specificity is 90%.
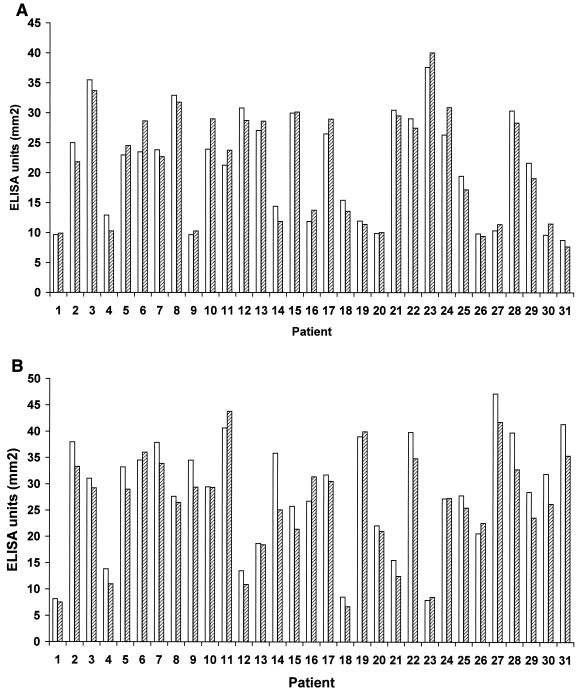
From 31 patients, a posttreatment follow-up serum sample was taken on average 3 months after the first sampling, and the titers of antibody against A. actinomycetemcomitans and P. gingivalis were measured again (Fig. 3). Within this period, the antibody levels remained essentially unchanged; correlation coefficients between the first and second samples were 0.968 and 0.953 for A. actinomycetemcomitans and P. gingivalis, respectively. The decrease in the antibody levels (mean ± SD) between the first and the second serum sample was 1.73 ± 0.78 mm2 (8.87% ± 5.08%) for A. actinomycetemcomitans (n = 16) and 3.59 ± 2.45 mm2 (12.78% ± 7.12%) for P. gingivalis (n = 24). There was no significant difference between the initially PCR-positive and PCR-negative groups: for A. actinomycetemcomitans 42% of the PCR-positive and 58% of the PCR-negative patients had a lower level in the follow-up sample. For P. gingivalis the corresponding figures were 77 and 80%, respectively.
FIG. 3.
Individual serum IgG levels against A. actinomycetemcomitans and P. gingivalis before and after periodontal treatment. From 31 patients with periodontitis, follow-up samples were collected approximately 3 months after the first sampling, and their levels of IgG against A. actinomycetemcomitans (A) and P. gingivalis (B) were measured using a serotype mixture ELISA. The white bars and striped bars represent the level (AUC) before and after treatment, respectively.
DISCUSSION
The aim of our study was to set up a rapid and economical laboratory method to screen for subjects with periodontitis to be used in studies where systemic effects of periodontal infection are investigated. In a large number of epidemiological studies and materials, assumptions on periodontal status are usually unavailable because clinical or radiographic data on the periodontal status of the subjects are absent. The role of dental plaque bacteria in the etiology of periodontitis is widely accepted (40). It is also well-established that periodontitis, as a chronic infection, is accompanied by systemic host responses, such as elevation of levels of antibody against periodontal pathogens in serum (8, 16, 25). In the absence of clinical data, serology has commonly been used in epidemiological studies to reveal various present or past bacterial and viral infections (9, 10, 33), but only rarely as regards periodontal infections (30).
In immunological analyses the most essential point is the choice of antigen. For both A. actinomycetemcomitans and P. gingivalis, several serotypes have now been designated (4, 42), and the individual antibody response depends on the amount and virulence of the infecting strains. There is still some disagreement on the localization and immunodominant nature of the antigen for both these species, which justifies the use of whole cells as antigens in the assay. For A. actinomycetemcomitans, it was earlier shown in periodontal patients that the antibody response is highest against the autologous strain and the reference strain representing the same serotype as the autologous strain (39; Vilkuna et al., Abstr. 100th Gen. Meet. Am. Soc. Microbiol.). Although the finding may differ between different species, the number of false negatives undoubtedly increases when only one strain has been chosen as a source of antigen. Like this study demonstrates, the serotype mixture ELISA distinguishes as positive also the subjects whose antibody response against only one serotype is clearly dominant.
To calculate the results obtained by the ELISA, we recruited an earlier-devised method, which is currently only seldom used (36). Although serial dilutions are laborious, the AUC gives a result, which is linearly proportional to the antibody concentration (36). In this way the results are as reliable as possible without a proper affinity-purified standard antibody. By exploring dilution curves, one also gets an idea about the affinity of the antibodies measured, which vary individually. By calculating the AUCs, even small differences in the antibody levels are found, because the scale ranges between 0.65 and about 55 mm2.
With our limited number of samples, both A. actinomycetemcomitans and P. gingivalis ELISA clearly distinguished the PCR-positive patients for the species from the PCR-negative ones, as well as from the healthy subjects and the healthy controls. If this kind of test were used as a marker of periodontitis in general, the simplest way would be to summarize the antibody results against these two pathogens individually. As our results show, the score for combined response, thus calculated, makes clear a statistically significant difference between the three groups tested regardless of which each of the pathogens was recovered in the oral cavity. Also, the sensitivity and specificity data for finding periodontitis (71 and 90%, respectively), based on the cut-off point of the mean + 1.5× SD, are well within the values of other comparable tests (3, 30). A. actinomycetemcomitans and P. gingivalis are the two most common periodontal pathogens, but a similar assay could be developed to measure antibody responses also against other dental plaque bacteria.
Based on our results, we consider the serotype mixture ELISA to be a suitable tool to assess the periodontal status from serum samples in epidemiological studies. The assay is particularly valuable in the study of the association between periodontal infections and systemic health.
Acknowledgments
This work was supported by the Academy of Finland (grants 45921, 44626, 48725, 77613, and 75953), the Paulo Foundation, and the Finnish Dental Association.
REFERENCES
- 1.Ashimoto, A., C. Chen, I. Bakker, and J. Slots. 1996. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol. Immunol. 11:266-273. [DOI] [PubMed] [Google Scholar]
- 2.Beck, J., R. Garcia, G. Heiss, P. S. Vokonas, and S. Offenbacher. 1996. Periodontal disease and cardiovascular disease. J. Periodontol. 67:1123-1137. [DOI] [PubMed]
- 3.Breslin, N. P., J. M. Lee, M. J. Buckley, E. Balbirnie, D. Rice, and C. A. O'Morain. 2000. Validation of serological tests for Helicobacter pylori infection in an Irish population. Ir. J. Med. Sci. 169:190-194. [DOI] [PubMed] [Google Scholar]
- 4.Califano, J. V., R. E. Schifferle, J. C. Gunsolley, A. M. Best, H. A. Schenkein, and J. G. Tew. 1999. Antibody reactive with Porphyromonas gingivalis serotypes K1-6 in adult and generalized early-onset periodontitis. J. Periodontol. 70:730-735. [DOI] [PubMed] [Google Scholar]
- 5.Ebersole, J. L. 1990. Systemic humoral immune responses in periodontal disease. Crit. Rev. Oral Biol. Med. 1:283-331. [DOI] [PubMed] [Google Scholar]
- 6.Ebersole, J. L., D. E. Frey, M. A. Taubman, and D. J. Smith. 1980. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J. Periodontal Res. 15:621-632. [DOI] [PubMed] [Google Scholar]
- 7.Ebersole, J. I., and M. J. Steffen. 1995. Human antibody responses to outer envelope antigens of Porphyromonas gingivalis serotypes. J. Periodontal Res. 30:1-14. [DOI] [PubMed] [Google Scholar]
- 8.Ebersole, J. L., M. A. Taubman, D. J. Smith, D. E. Frey, A. D. Haffajee, and S. S. Socransky. 1984. The relationship of antibody response categories to clinical parameters of periodontal disease. J. Periodontal Res. 19:609-613. [DOI] [PubMed] [Google Scholar]
- 9.Ekman, M. R., M. Leinonen, H. Syrjälä, E. Linnanmäki, P. Kujala, and P. Saikku. 1993. Evaluation of serological methods in the diagnosis of Chlamydia pneumoniae during an epidemic in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 12:756-760. [DOI] [PubMed] [Google Scholar]
- 10.Elkins, C., K. Yi, B. Olsen, C. Thomas, K. Thomas, and S. Morse. 2000. Development of a serological test for Haemophilus ducreyi for seroprevalence studies. J. Clin. Microbiol. 38:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan, Q., T. J. Sims, T. Nakagawa, and R. C. Page. 2000. Antigenic cross-reactivity among Porphyromonas gingivalis serotypes. Oral Microbiol. Immunol. 15:158-165. [DOI] [PubMed] [Google Scholar]
- 12.Funk, J. L., K. R. Feingold, A. H. Moser, and C. Grunfeld. 1993. Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis 98:67-82. [DOI] [PubMed] [Google Scholar]
- 13.Gmür, R., H. McNabb, T. J. van Steenbergen, P. Baehni, A. Mombelli, A. J. van Winkelhoff, and B. Guggenheim. 1993. Seroclassification of hitherto nontypeable Actinobacillus actinomycetemcomitans strains: evidence for a new serotype e. Oral Microbiol. Immunol. 8:116-120. [DOI] [PubMed] [Google Scholar]
- 14.Hendtlass, A., S. G. Dashper, and E. C. Reynolds. 2000. Identification of an antigenic protein Pga30 from Porphyromonas gingivalis W50. Oral Microbiol. Immunol. 15:383-387. [DOI] [PubMed] [Google Scholar]
- 15.Hernichel-Gorbach, E., K. S. Kornman, S. C. Holt, F. Nichols, H. Meador, J. T. Kung, and C. A. Thomas. 1994. Host responses in patients with generalized refractory periodontitis. J. Periodontol. 65:8-16. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, I., K. Nakashima, T. Koseki, T. Nagasawa, H. Watanabe, S. Arakawa, H. Nitta, and T. Nishihara. 1997. Induction of the immune response to periodontopathic bacteria and its role in the pathogenesis of periodontitis. Periodontol. 2000 14:79-111. [DOI] [PubMed] [Google Scholar]
- 17.Kornman, K. S., R. C. Page, and M. S. Tonetti. 1997. The host response to the microbial challenge in periodontitis: assembling the players. Periodontal 2000 14:33-53. [DOI] [PubMed] [Google Scholar]
- 18.Laine, M. L., B. J. Appelmelk, and A. J. van Winkelhoff. 1996. Novel polysaccharide capsular serotypes in Porphyromonas gingivalis. J. Periodontal. Res. 31:278-284. [DOI] [PubMed] [Google Scholar]
- 19.Marcus, A. J., and D. P. Hajjar. 1993. Vascular transcellular signaling. J. Lipid Res. 34:2017-2031. [PubMed] [Google Scholar]
- 20.Mättö, J., M. Saarela, S. Alaluusua, V. Oja, H. Jousimies-Somer, and S. Asikainen. 1998. Detection of Porphyromonas gingivalis from saliva by PCR by using a simple sample-processing method. J. Clin. Microbiol. 36:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard, C., and C. Mouton. 1995. Clonal diversity of the taxon Porphyromonas gingivalis assessed by random amplified polymorphic DNA fingerprinting. Infect. Immun. 63:2522-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Möller, Å. J. R. 1966. Examination of root canals and periapical tissues of human teeth. Odontol. Tidskr. 74:1-380. [PubMed]
- 23.Moncla, B. J., P. Braham, K. Dix, S. Watanabe, and D. Schwartz. 1990. Use of synthetic oligonucleotide DNA probes for the identification of Bacteroides gingivalis. J. Clin. Microbiol. 28:324-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore, W. E., and L. V. Moore. 1994. The bacteria of periodontal diseases. Periodontol. 2000 5:66-77. [DOI] [PubMed] [Google Scholar]
- 25.Mouton, C., M. Desclauriers, H. Allard, and M. Bouchard. 1987. Serum antibodies to Bacteroides gingivalis in periodontitis: a longitudinal study. J. Periodontal Res. 22:426-430. [DOI] [PubMed] [Google Scholar]
- 26.Novo, E., E. Garcia-MacGregor, N. Viera, N. Chaparro, and Y. Crozzoli. 1999. Periodontitis and anti-neutrophil cytoplasmic antibodies in systemic lupus erythematosus and rheumatoid arthritis: a comparative study. J. Periodontol. 70:185-188. [DOI] [PubMed] [Google Scholar]
- 27.Offenbacher, S., V. Katz, G. Fertik, J. Collins, D. Boyd, G. Maynor, R. McKaig, and J. Beck. 1996. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 67:1103-1113. [DOI] [PubMed] [Google Scholar]
- 28.Oyaizu, K., H. Ohyama, F. Nishimura, H. Kurihara, S. Matsushita, H., Maeda, S. Kokeguchi, H. Hongyo, S. Takashiba, and Y. Murayama. 2001. Identification and characterization of B-cell epitopes of a 53-kDa outer membrane protein from Porphyromonas gingivalis. Oral Microbiol. Immunol. 16:73-78. [DOI] [PubMed] [Google Scholar]
- 29.Paju, S., M. Saarela, S. Alaluusua, P. Fives-Taylor, and S. Asikainen. 1998. Characterization of serologically nontypeable Actinobacillus actinomycetemcomitans isolates. J. Clin. Microbiol. 36:2019-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papapanou, P. N., A. M. Neiderud, J. Sandros, and G. Dahlen. 2001. Checkerboard assessments of serum antibodies to oral microbiota as surrogate markers of clinical periodontal status. J. Clin. Periodontol. 28:103-106. [DOI] [PubMed] [Google Scholar]
- 31.Pearce, W. H., I. Sweis, J. S. Yao, W. J. McCarthy, and A. E. Koch. 1992. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J. Vasc. Surg. 16:784-789. [PubMed] [Google Scholar]
- 32.Poulsen, K., E. Theilade, E. T. Lally, D. R. Demuth, and M. Kilian. 1994. Population structure of Actinobacillus actinomycetemcomitans: a framework for studies of disease-associated properties. Microbiology 140:2049-2060. [DOI] [PubMed] [Google Scholar]
- 33.Roivainen, M., G. Alfthan, P. Jousilahti, M. Kimpimäki, T. Hovi, and J. Tuomilehto. 1998. Enterovirus infections as a possible risk factor for myocardial infarction. Circulation 98:2534-2537. [DOI] [PubMed] [Google Scholar]
- 34.Saarela, M., S. Asikainen, S. Alaluusua, L. Pyhälä, C. H. Lai, and H. Jousimies-Somer. 1992. Frequency and stability of mono- or poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d or e. Oral Microbiol. Immunol. 7:277-279. [DOI] [PubMed] [Google Scholar]
- 35.Saarela, M. H., B. Dogan, S. Alaluusua, and S. Asikainen. 1999. Persistence of oral colonization by the same Actinobacillus actinomycetemcomitans strain(s). J. Periodontol. 70:504-509. [DOI] [PubMed] [Google Scholar]
- 36.Sedgwick, A. K., M. Ballow, K. Sparks, and R. C. Tilton. 1983. Rapid quantitative microenzyme-linked immunosorbent assay for tetanus antibodies. J. Clin. Microbiol. 18:104-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sims, T. J., R. W. Ali, E. S. Brockman, N. Skaug, and R. C. Page. 1999. Antigenic variation in Porphyromans gingivalis ribotypes recognized by serum immunoglobulin G of adult periodontitis patients. Oral Microbiol. Immunol. 14:73-85. [DOI] [PubMed] [Google Scholar]
- 38.Slots, J., J. J. Zambon, B. G. Rosling, H. S. Reynolds, L. A. Christersson, and R. J. Genco. 1982. Actinobacillus actinomycetemcomitans in human periodontal disease. Association, serology, leukotoxicity, and treatment. J. Periodontal Res. 17:447-448. [DOI] [PubMed] [Google Scholar]
- 39.Tinoco, E. M., S. P. Lyngstadaas, H. R. Preus, and P. Gjermo. 1997. Attachment loss and serum antibody levels against autologous and reference strains of Actinobacillus actinomycetemcomitans in untreated localized juvenile periodontitis patients. J. Clin. Periodontol. 24:937-944. [DOI] [PubMed] [Google Scholar]
- 40.World Workshop in Periodontics. 1996. Periodontal diseases: pathogenesis and microbial factors. Consensus report. Ann. Periodontol. 1:962-932. [DOI] [PubMed] [Google Scholar]
- 41.Wu, T., M. Trevisan, R. J. Genco, J. P. Dorn, K. L. Falkner, and C. T. Sempos. 2000. Periodontal disease and risk of cerebrovascular disease. Arch. Intern. Med. 160:2749-2755. [DOI] [PubMed] [Google Scholar]
- 42.Zambon, J. J., J. Slots, and R. J. Genco. 1983. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect. Immun. 41:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]