Abstract
Most of our present knowledge about the impacts of solar UVB radiation on terrestrial ecosystems comes from studies with plants. Recently, the effects of UVB on the growth and survival of consumer species have begun to receive attention, but very little is known about UVB impacts on animal behavior. Here we report that manipulations of the flux of solar UVB received by field-grown soybean crops had large and consistent effects on the density of the thrips (Caliothrips phaseoli, Thysanoptera: Thripidae) populations that invaded the canopies, as well as on the amount of leaf damage caused by the insects. Solar UVB strongly reduced thrips herbivory. Thrips not only preferred leaves from plants that were not exposed to solar UVB over leaves from UVB-exposed plants in laboratory and field choice experiments, but they also appeared to directly sense and avoid exposure to solar UVB. Additional choice experiments showed that soybean leaf consumption by the late-season soybean worm Anticarsia gemmatalis (Lepidoptera: Noctuidae) was much less intense in leaves with even slight symptoms of an early thrips attack than in undamaged leaves. These experiments suggest that phytophagous insects can present direct and indirect behavioral responses to solar UVB. The indirect responses are mediated by changes in the plant host that are induced by UVB and, possibly, by other insects whose behavior is affected by UVB.
Keywords: insect behavior, plant–insect interactions, global change, herbivory, photobiology
Depletion of stratospheric ozone (1) is a cause of concern because the biological impacts of an increase in solar UVB (290–320 nm) are unknown. Most studies to date on ecological effects of solar UVB have been carried out on plants (2–4); however, there is growing awareness in the UVB research community (2–7), as well as among those studying other aspects of global environmental change (8), about the limitations of impact predictions that result from up-scaling information obtained in studies of a single organism or trophic level. The concentration of the research effort on plants is in part a consequence of the assumption that the effects of UVB on ecosystem functioning are largely mediated by its effects on the primary producers (6).
Recent studies on animal consumers have focused on those effects of UVB on consumer growth and survival that are mediated by changes in host chemistry (9–11) and on direct damaging effects of UVB. In the latter regard, it has been shown that certain animal species (particular instars), such as juvenile forms of some aquatic organisms, are not well protected from UV radiation and are damaged by prolonged exposure to present-day levels of UVA (320–400 nm) and UVB (4, 12, 13) radiation. Damaging effects of acute exposures on zooplanktonic organisms have also been documented in studies carried out in Antarctic waters under ozone-hole episodes (14). Interestingly, one of the few studies that included more than one trophic level has shown that direct damaging effects of solar UV radiation on phytophagous insect larvae can counterbalance the negative impact of UV on algal photosynthesis and result in increased biomass accumulation of the primary producers in experimental freshwater ecosystems (5).
Almost no research has been carried out on the effects of UVB on animal behavior. There is some evidence from field studies for indirect, plant-mediated effects of UVB on insect feeding choices (15), but the generality and underlying mechanisms of these effects have not been established. The possibility of direct behavioral responses of animals to solar UVB has received little attention. Controlled-environment studies with protozoans (16) have suggested that some species may detect and avoid exposure to UVB radiation; however, because light sources that emitted predominantly in the UVB region were used in those experiments, it was impossible to separate potentially specific effects of UVB from simple responses induced by changes in total irradiance. Some animals are believed to use UVA and “human-visible” (i.e., λ ≥ 400 nm) light as a cue to avoid exposure to harmful UVB radiation (see ref. 5). Others, such as the housefly, are attracted to both UVA and UVB sources in indoor experiments, but the response to lamps that emit predominantly in the UVB is weaker than the response to UVA lamps. Roberts et al. (16) concluded that houseflies do not have a genuine spectral preference within the UV region; the reduced behavioral response in the UVB is simply a consequence of the decline in photoreceptor response (as evaluated with electroretinograms) toward the UVB region. Thus, although UV photoreceptors whose sensitivity extends down to wavelengths as short as 320 nm have been identified in the visual systems of several species of animals (ref. 18; reviewed in ref. 19), animals are generally thought to be unable to resolve UVB from other wavelengths in natural sunlight.
We used a simple agroecosystem, in which soybeans were the main primary producers and a few species of insects the consumers, to investigate the impacts of solar UVB on plant–consumer interactions. Our results suggest that (i) apart from inducing changes in the plant hosts that strongly affect their attractiveness to thrips (Caliothrips phaseoli), solar UVB radiation triggers direct behavioral responses in the insects and (ii) thrips induce changes in their plant hosts that, in turn, feed forward to other species of phytophagous insects.
MATERIALS AND METHODS
Plant Culture.
All experiments were carried out during the summer and autumn of 1995–1996 and 1996–1997 at the experimental fields of IFEVA in Buenos Aires (34°35′ S, 58°29′ W). In all experiments, soybean was grown in field plots (1.2 × 1.2 m; plant density = 60 per m2) that were covered with plastic filters designed to exclude various levels of solar UVB. In the 1995–1996 growing season, the plants were contained in 10-liter pots. There were two planting dates: 5 December, 1995 with four genotypes in each plot: CNS, Essex, Lincoln, and Williams (combined population), and 12 February 1996, using the line PI227687. In the 1996–1997 season, the seeds were planted in rows 12 cm apart; four commercial soybean varieties were planted in all plots (each in a different row): A5308, Williams, Charata-76, and Dekalb 458 (sowing date: 28 February 1997). The plots were watered periodically to maintain the soil near field capacity and were kept free of weeds. The autumn of 1997 was exceptionally warm and allowed continued growth of the crops until mid-May.
UV Manipulation Techniques.
All plots were covered before crop seedling emergence with plastic film that transmitted more than 88% of the photosynthetically active radiation (400–700 nm) and attenuated different regions of the UV band. The UVB-transparent control (SUN) plots were covered with either 0.02-mm thick polyethylene (Rolopac, Buenos Aires) or Aclar (0.04 mm thick; Allied Signal Plastics, Morristown, NJ) films, which transmit more than 80% throughout the UVB and UVA bands. The −UVB plots were covered with Mylar-D film (0.1 mm thick; DuPont), which blocks essentially all radiation below 310 nm (see spectrum in ref. 15). Intermediate UVB irradiances were obtained by superimposing Mylar strips on sheets of the UV-transparent Rolopac or Aclar films (see ref. 15). The relative UVB irradiances under the various filters were measured at midday with a cosine-corrected UVB detector (SUD/240/W) attached to a IL-1700 research radiometer (International Light, Newburyport, MA). The spectral response of the detector head is centered at 290 nm (half-bandwidth = 20 nm) and its noontime signal is reduced by more than 95% when covered with a Mylar filter, indicating that the detector is virtually blind in the UVA region. In both seasons there were four true replicates (blocks) of each UVB treatment. Each individual field plot was surrounded by an almost continuous soybean canopy, which greatly reduced the contribution of sidelight, and the filters were kept at a short distance (≈5 cm) from the upper-canopy leaves. Consequently, UVB attenuation at the center of the plots was very effective (≈98% in the −UVB plots compared with the SUN plots; see Fig. 1); all leaves used for field and laboratory bioassays were collected from plants located at the center of the plots. The plastic filters might have had some influence on the canopy microclimate compared with a no-filter situation. However, this should not affect the comparisons between the UVB treatments used in our experiments, as previous studies have shown no differences between UVB exclusion treatments in leaf or soil temperature (15).
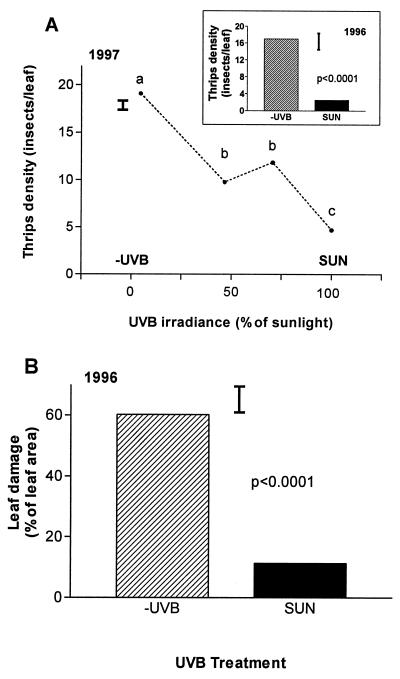
Figure 1.
Effects of solar UVB on thrips density (A) and crop damage (B). Insect counts and damage assessments were carried out at the beginning of flowering (1996: line PI227687; 1997: all cultivars combined) as explained in Materials and Methods. Different letters indicate significant differences (P < 0.005) between treatment means; thin bars indicate SEs; the overall UVB effect in 1997 was significant at P < 0.0001, n = 4 independent plots per treatment.
In the short-term UV-supplementation experiments, we irradiated portions of the field plots with light from UVB bulbs placed 50 cm above the canopy. UVB-313 bulbs (Q-Panel, Cleveland) were covered with either a polyethylene film (transparent to UVC, UVB, and UVA), a cellulose diacetate filter (transparent to UVB and UVA), or a Mylar filter (transparent to UVA). The relative energy output of the unfiltered UVB-313 lamps in the UVC (<290 nm), UVB, and UVA spectral bands is 1%, 80%, and 19%, respectively. The absolute irradiance provided by the lamps at canopy height measured with the UVB radiometer (λmax = 290 nm) was 1.5 10−9 W/cm2, which represented a 10-fold increase over the normal noontime value under clear-sky conditions in May. Measurements taken with the sensor pointing downward showed that the UVB component in the diffuse light received at the abaxial surface of upper-canopy leaves increased 4.25 ± 0.33 times (P < 0.0001) as a result of UVB supplementation with the lamps. For treatment with UVA (see Fig. 3C), we used UVA TL 40W/05 bulbs (Philips). The relative spectral output of the unfiltered TL/05 lamp in the UVC, UVB, and UVA spectral bands is 0.5%, 2.5%, and 97%, respectively.
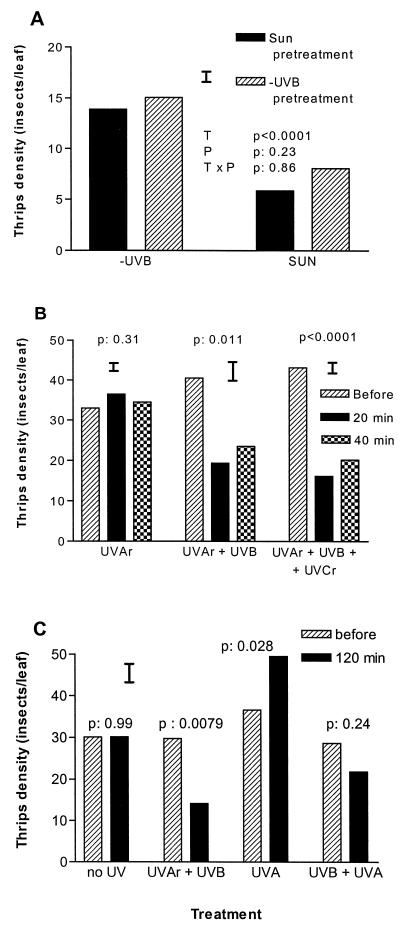
Figure 3.
Rapid responses of thrips to natural and supplemental UVB from experiments carried out with the 1997 crops (all cultivars combined). (A) Soybean crops grown either with (SUN pretreatment) or without solar UVB (−UVB pretreatment) had their filters changed at flowering, when the plots were covered with either UVB-transparent or UVB-opaque films (SUN and −UVB treatments, respectively). The filter change was performed at 1000 h on 6 May 1997; the bars indicate the average number of insects counted in each treatment (T)/pretreatment (P) combination 8 h later (the thin bar indicates the SE; n = 4 plots per T × P; 10 randomly selected upper canopy leaves per plot). (B) Rapid effects of UVB irradiation on thrips densities. To create the different treatments, UVB lamps were covered with either Mylar, cellulose diacetate, or polyethylene films (see Materials and Methods). The Mylar filter blocks UVC and UVB radiation but lets through the small amount of UVA emitted by the lamps (UVAr); the cellulose diacetate filter blocks the UVC but lets through most of the UVB and UVAr emitted by the lamps (UVAr + UVB), whereas the polyethylene film is also transparent to the small amount of UVC (UVCr) produced by the UVB lamp (UVAr + UVB + UVCr). The bars show the average thrips density (6 repeated measurements for each treatment) before and at the indicated times after turning the UVB lamps on; the P values shown indicate the significance of the irradiation effect; the thin bars indicate the SEs calculated from the ANOVA. The experiment was replicated on 3 different days with similar results; the results shown are from an experiment carried out on 8 May 1997. (C) Effects of UVB and UVA irradiation. The different treatments were created as follows: no UV = 1 UVB313 + 1 TL/05 lamps covered with a 2-mm thick polycarbonate sheet (Lexan, General Electric, Mt Vernon, IN); UVAr + UVB = 1 UVB313 lamp wrapped with a diacetate filter; UVA = 1 UVB313 + 1 TL/05 lamps wrapped with a Mylar film; UVA + UVB = 1 UVB313 lamp + 1 TL/05 lamps wrapped with a diacetate film. The bars show the average thrips density (16 randomly selected leaves per treatment; thin bar indicates SE) before and 120 minutes after turning the lamps on (experiment carried out on 15 May 1997).
Insect Surveys and Feeding Experiments.
The plants at our field site were colonized by natural populations of thrips, which were particularly abundant in the abaxial surface of leaves in the upper third of the canopy. The insects scraped the leaf surface and eventually induced chlorosis in the affected areas. Insect counts were made at the beginning of flowering (1996: end of March, genotype PI227687; 1997: end of April, all four cultivars combined) on the youngest fully expanded leaf (15–30 randomly selected plants per plot; four independent blocks per treatment). Leaf damage was assessed in late March 1996 (genotype PI227687) by estimating the fractional area that was damaged by thrips in the youngest fully expanded leaf (11th node; 16 plants per plot; 4 plots per treatment). The thrips lesions could be visually identified as areas in which the leaf surface appeared scraped and the inner tissues were slightly chlorotic.
The effect of solar UVB on plant tissue attractiveness to thrips was tested in field and laboratory “choice” experiments. In both cases, the response variable was the number of insects that landed on leaves with contrasting UVB history at various times from the beginning of the experiment. In the field, trifoliate leaves collected from soybean crops grown with (SUN) or without (−UVB) solar UVB (1997 sowing; all cultivars combined) were placed at the center of a soybean canopy that was covered with a −UVB filter. The petioles of the detached leaves were kept continuously under water in a glass jar during the course of the experiment. There were six leaves in each jar (three from the SUN pretreatment and three from the −UVB pretreatment) with a total of 12 independent replicate jars distributed within the soybean canopy. The experiment was carried out on 7 and 8 May 1997. In the laboratory, young fully expanded leaves from −UVB and SUN plants (Genotype PI227687) were offered to locally collected thrips in 35 × 60 × 15 (height)-cm plastic boxes (40 freshly collected adults and three leaves of each treatment per box). The experiment was carried out in three opportunities during April 1996 (the data were pooled for analysis), and there were 15 independent choice boxes.
To test for antiherbivore responses induced by thrips, fully expanded leaves from SUN plants (1995–1996 sowing) with or without visual symptoms of thrips damage were presented in a laboratory choice experiment to larvae of Anticarsia gemmatalis (soybean worm), which is an important late-season soybean pest that feeds on aerial parts of the plant and can cause severe yield losses in commercial crops. The experiments were carried out during March and April of 1996 by using leaves from the combined population and the line PI227687. In all “damaged” leaves, the affected areas (scraped leaf surface; slightly chlorotic mesophyll) covered less than 15% of the lamina and were evident only on careful inspection of the leaves. The larvae were 15 days old at the beginning of the experiment and had been fed on a standard artificial diet (20). The larvae and the leaves were placed inside 35 × 60 × 15 (height)-cm plastic boxes (5 larvae per box); the amount of tissue consumed was estimated from leaf area measurements (LI-3000, Li-Cor, Lincoln, NE) taken before and 24 h after the beginning of the feeding experiment. The initial leaf area was approximately 100 cm2 per box for each treatment (thrips damage level); leaves of both treatments were offered in the same box. To prevent leaf desiccation, the petioles were wrapped in cotton saturated with water; temperature varied between 25 and 30°C. There were 10 replicate boxes.
Data Analysis.
All data were analyzed by using the SAS Version 6.12 statistical package (SAS Institute, Cary, NC). The standard errors reported in the figures were calculated from the error-mean-square values (S2) of the relevant ANOVA tables as SE = (S2/n − 1)1/2.
RESULTS AND DISCUSSION
Effects of Solar UVB on Herbivory.
In both seasons, filtering out solar UVB resulted in a 3- to 5-fold increase in the density of the thrips populations that invaded our field crops (−UVB vs. SUN plots; Fig. 1A). This was a specific effect of UVB exclusion, because (i) the filters had virtually the same transmittance above 320 nm, (ii) filtering out the UVB component of sunlight changed total photon flux density between 280 and 700 nm by less than 1%, and (iii) the crop microenvironment (e.g., soil and canopy temperature) was not differentially affected by UVB treatments (see ref. 15). There was a clear dose-response relationship between UVB levels and thrips density (Fig. 1A), and the greater abundance of thrips in the −UVB treatment resulted in significantly higher leaf damage (Fig. 1B). This UVB effect on insect abundance and herbivory is much larger than any previously reported effect of solar UVB on plant growth and gross morphology (refs. 2, 3, and 15, and refs. therein). Indeed, the soybean crops used in our experiments displayed pigmentation and morphological responses to solar UVB that were only modest in comparison with the effects of UVB exclusion on their herbivores. Thus, in 1995–1996 we found that solar UVB inhibited stem elongation and promoted the accumulation of methanol-soluble phenolics in some of the cultivars; although these effects were statistically significant, they always involved changes of less than ±30% of the −UVB mean value. In the 1996–1997 crops, which were planted in late summer (with lower ambient UVB levels), we did not detect significant effects of UVB exclusion on stem elongation, leaf area expansion, and total phenolics in any of the cultivars tested (data not shown from measurements taken between 15 and 32 days after sowing).
Plant-Mediated Effects of Solar UVB on Insect Behavior.
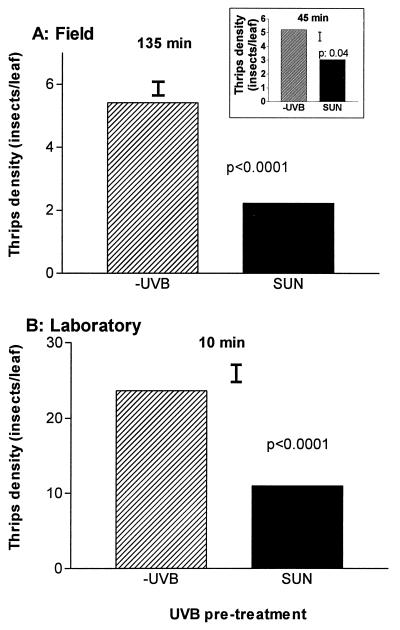
To test the possibility that the effects of solar UVB on herbivory were mediated by behavioral responses to UVB-induced changes in the plant host (cf. ref. 15), we carried out two preference tests. In one of them, we collected leaves from soybean plants grown in the SUN and −UVB treatments and placed them within a soybean crop that was covered with a −UVB film and presented a dense thrips infestation. Within a few minutes the leaves were invaded by thrips, and the insects clearly preferred leaves from plants grown in the −UVB treatment over leaves of the SUN plants (Fig. 2A). In complementary experiments carried out in the laboratory, we offered soybean leaves from −UVB and SUN plants to captive specimens of thrips. Insect counts (Fig. 2B) again demonstrated that leaves not exposed to solar UVB were preferred over leaves from SUN plants. Previous laboratory experiments have shown that changes in the plant host induced by UVB can affect the performance (growth and survival) of insect herbivores (refs. 9–11, and literature cited in ref. 7). All of these experiments were “no-choice” assays—i.e., the insects were fed with leaves that were either treated or not treated with UVB. Our data clearly show that exposure to solar UVB causes persistent changes in the leaves that are recognized by thrips and used in their host-selection decisions.
Figure 2.
Effects of solar UVB on plant tissue attractiveness to thrips. (A) Trifoliate leaves collected from soybean crops grown with (SUN) or without solar UVB (−UVB) (1997 sowing; all cultivars combined) were placed at the center of a soybean canopy that was covered with a −UVB film, as explained in Materials and Methods. The bars indicate the average number of insects found feeding on leaves from −UVB and SUN plants at the indicated times after the transfer. Thin bars indicate SEs; n = 12 independent replicates (each with three trifoliate leaves per UVB pre-treatment). (B) Young fully expanded leaves from −UVB and SUN plants (1995–1996 sowing, genotype PI227687) were offered to captive thrips in a laboratory choice experiment carried out in closed bioassay boxes (40 thrips per box; see Materials and Methods). The bars indicate the average number of insects that had landed on leaves from −UVB and SUN plants 10 minutes after the beginning of the experiment (the thin bar indicates SE; n = 15 independent boxes; insect counts after 2 h gave similar results).
Rapid Effects of Solar UVB on Insect Behavior.
Animals of many taxa, including invertebrates (18, 21), fishes (22), birds (23, 24), and rodents (25) present visual sensitivity to UV radiation. However, photoreceptor sensitivity curves always have maxima in the UVA region (above 320 nm) (17–19, 21–23, 25), and it would seem unlikely, therefore, that these photoreceptors may detect variations in natural UVB levels. This is particularly true because, in natural sunlight, the spectral irradiance is at least 10 times greater in the UVA than in the UVB region; therefore, detection of solar UVB would require a high photoreceptor specificity. To investigate rapid behavioral responses of thrips to solar UVB, we performed a filter switch experiment. Filters were removed from the field plots, which were divided in two; each half was covered either with the same original film (i.e., SUN→SUN or −UVB→−UVB) or with a film that created the opposite UVB condition (i.e., SUN→−UVB or −UVB→SUN). Surprisingly, switching the filters induced a rapid migration of the insects within the plots and, a few hours after the switch, the greatest thrips densities were found in the plot halves covered by the −UVB film (Fig. 3A). Rapid changes in host quality (such as those triggered by mechanical damage in some species; ref. 26) could, at least in principle, explain this response to the filter switch; however, we consider this possibility unlikely for two reasons. First, insect surveys carried out 4 h after the switch already showed a highly significant treatment effect (P = 0.005; data not shown). Second, even if the decline in tissue quality caused by the −UVB → SUN switch was very rapid, it seems unlikely that the opposite change (SUN → −UVB) could have caused an almost instantaneous increase in leaf attractiveness. In fact, our own data (Fig. 2A) show that the antiherbivore response induced by solar UVB in soybeans is effective at deterring thrips for at least a few hours after the end of the UVB exposure, which is consistent with observations in other systems showing that antiherbivore defenses can be sustained for long periods after induction (for a recent review, see ref. 27).
To further investigate the apparently direct response of the insects to UVB, we irradiated portions of a field plot with light from UVB bulbs that were covered with either a polyethylene film (transparent to UVC, UVB, and UVA), a cellulose diacetate filter (transparent to UVB and UVA), and a Mylar filter (transparent to UVA). After a few minutes of irradiation, we counted thrips in the leaves located under the lamps. Insect density was not changed by illumination with residual UVA from the UVB-313 lamps (Fig. 3B, UVAr); in contrast, illumination with UVB or UVB + residual UVC prompted ≈50% of the thrips to migrate toward other locations of the crop (Fig. 3B, UVAr + UVB and UVAr + UVB + UVCr). These results strongly suggest that thrips can directly detect and react behaviorally to natural (Fig. 3A) and augmented UVB (Fig. 3B), even in a background of very strong visible and UVA radiation. Complementary experiments using UVB and UVA lamps (λmax = 340 nm) again demonstrated a clear avoidance behavior induced by UVB (Fig. 3C, UVAr + UVB), and suggested that thrips, like several other insects (see refs. 17, 21, and 28), are somewhat attracted by UVA (Fig. 3C, UVA). In other words, the behavioral response induced by UVA was exactly the opposite of the response induced by UVB, demonstrating that perception and avoidance of UVB by thrips is not cued by UVA (see ref. 5). Our experiments cannot rule out the possibility that at least part of the behavioral effects of UVB in our system are mediated by the activation of photosensitizer compounds in the leaves coupled with the production of oxygen free radicals at leaf surface, which could be detected by the insects when they approach the boundary layer (see, e.g., ref. 29). However, if such a mechanism takes place in soybean leaves, the UV-absorbing compounds involved should have a fairly unusual specificity for UVB to explain the contrasting effects of UVB and UVA wavelengths on the insects (Fig. 3C). Regardless of the sensory mechanism, the observations of deleterious effects of solar UV exposure in other animal species (12, 13), suggest that the UVB-avoidance behavior that we have documented for thrips is likely to have fitness implications for the insects.
Insect-Mediated Effects on the Behavior of Other Insects.
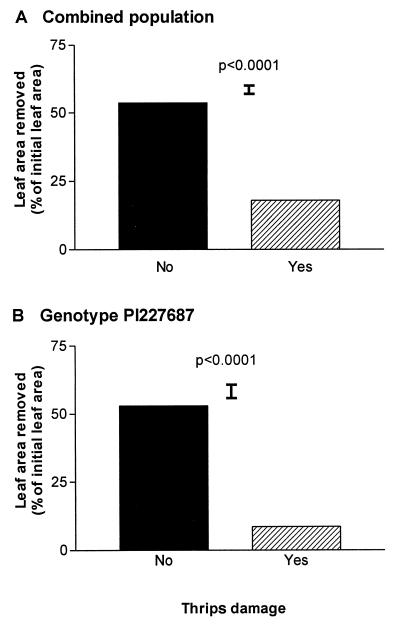
Dense thrips infestations can inflict serious damage to plants but, in our experiments, the effects of the thrips appeared to influence other consumers as well, like the soybean worm A. gemmatalis. Field and controlled-environment experiments in our laboratory have demonstrated that Anticarsia caterpillars, like thrips, consistently prefer soybean leaves grown under −UVB filters over leaves grown under full sunlight (unpublished results). Interestingly, however, in controlled-environment preference experiments, we found that caterpillars consistently avoided soybean leaves with even slight symptoms of previous thrips attack (Fig. 4). Therefore, thrips damage, which is promoted by low UVB, also appears to induce antiherbivore responses in soybeans, an effect that may have parallels with the defense responses induced by insect attack and mechanical damage in other systems (see literature in refs. 27 and 30; see also refs. 31–34). It is important to point out, however, that because our experiments (Fig. 4) made use of leaves that were naturally damaged by thrips, the possibility exists that the caterpillar preferences were determined by leaf traits that were inversely correlated with the severity of the thrips attack rather than by a product of the attack itself.
Figure 4.
Relationship between leaf damage induced by thrips and A. gemmatalis feeding choices. Fully expanded leaves from SUN plants with or without visual symptoms of thrips damage were presented in a choice experiment to Anticarsia larvae in the laboratory. (A) Fully expanded leaves were picked from various nodes from plants of the combined population. There were 30 replicate boxes. (B) Leaves were picked from the fourth node of PI227687 plants. There were 10 replicate boxes; thin bars indicate SE.
CONCLUSIONS
The magnitude of the effects of solar UVB on insect density in our field studies was very significant (Fig. 1), especially considering that in some of the soybean crops used for these experiments we failed to detect any effects of solar UVB on crop gross morphology, UV-absorbing pigments, or biomass accumulation. Therefore, without negating the prevailing idea that plant responses to solar UVB are important for predicting ecosystem responses to a rise in UVB irradiances, our studies suggest that the possibility of direct effects of UVB on consumer behavior (Fig. 3) deserves close examination. They also demonstrate that even in conditions in which plant growth responses to UVB are not detected, other as-yet-unidentified responses do take place and are sensed by phytophagous insects as part of their landing and host-selection clues (Fig. 2). Variations in the behavior of grazing insects can have important effects on the dynamics of terrestrial food webs (ref. 35, and references therein). The effects of UVB on insect behavior that we have documented are likely to feed back on the primary producers and, more intriguingly, feed forward to other species of consumers (Fig. 4). Exploring these networks of interactions in other systems will provide important clues on the ecological roles of solar UVB.
Acknowledgments
We thank Martyn Caldwell, Steve Flint, Martín Oesterheld, and two anonymous reviewers for thoughtful comments on the manuscript. This work was supported by Grants PID1/394 (Ministerio de Educación, Secretaría de Ciencia y Tecnología) and AG-023 (Universidad de Buenos Aires) and also in part through the Interagency Program on Terrestrial Ecology and Global Change (TECO) by the National Science Foundation under Grant IBN9524144.
ABBREVIATIONS
- −UVB
UVB exclusion treatment
- SUN
UVB-transparent control
References
- 1.World Meteorological Organization. Report no. 37: Scientific Assessment of Ozone Depletion. Geneva: W.M.O.; 1995. [Google Scholar]
- 2.Caldwell M M, Teramura A H, Tevini M, Bornman J F, Björn L O, Kulandaivelu G. Ambio. 1995;24:166–173. [Google Scholar]
- 3.Rozema J, Van de Staaij J, Björn L O, Caldwell M. Trends Ecol Evol. 1997;12:22–28. doi: 10.1016/s0169-5347(96)10062-8. [DOI] [PubMed] [Google Scholar]
- 4.Häder D-P, Worrest R C, Kumar H D, Smith R C. Ambio. 1995;24:174–180. [Google Scholar]
- 5.Bothwell M L, Sherbot D M J, Pollock C M. Science. 1994;265:97–100. doi: 10.1126/science.265.5168.97. [DOI] [PubMed] [Google Scholar]
- 6.Scientific Committee on Problems of the Environment. Effects of Increased Ultraviolet Radiation on Biological Systems. Paris: SCOPE Secretariat; 1993. [Google Scholar]
- 7.Paul N D, Sharima Rasanayagam M, Moody S A, Hatcher P E, Ayres P G. Plant Ecol. 1997;128:296–308. [Google Scholar]
- 8.Koch G W, Mooney H A, editors. Carbon Dioxide and Terrestrial Ecosystems. San Diego: Academic; 1996. [Google Scholar]
- 9.Hatcher P E, Paul N D. Entomol Exp Appl. 1994;71:227–233. [Google Scholar]
- 10.McCloud E S, Berenbaum M R. J Chem Ecol. 1994;20:525–539. doi: 10.1007/BF02059595. [DOI] [PubMed] [Google Scholar]
- 11.Grant-Petersson J, Renwick J A A. Environ Entomol. 1996;25:135–142. [Google Scholar]
- 12.Blaustein A R, Hoffman P D, Hokit D G, Kiesecher J M, Walls S C, Hays J B. Proc Natl Acad Sci USA. 1994;91:1791–1795. doi: 10.1073/pnas.91.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson C E, Zagarese H E, Schulze P C, Hargreaves B R, Seva J. J Plankton Res. 1994;16:205–218. [Google Scholar]
- 14.Malloy K D, Holman M A, Mitchell D, Detrich H W., III Proc Natl Acad Sci USA. 1997;94:1258–1263. doi: 10.1073/pnas.94.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballaré C L, Scopel A L, Stapleton A L, Yanovsky M J. Plant Physiol. 1996;112:161–170. doi: 10.1104/pp.112.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcelo J A, Calkins J. Photochem Photobiol. 1979;29:75–83. doi: 10.1111/j.1751-1097.1979.tb07206.x. [DOI] [PubMed] [Google Scholar]
- 17.Roberts A E, Syms P R, Goodman L J. Entomol Exp Appl. 1992;64:259–268. [Google Scholar]
- 18.Smith K C, Macagno E R. J Comp Physiol A. 1990;166:597–606. doi: 10.1007/BF00240009. [DOI] [PubMed] [Google Scholar]
- 19.Tovée M J. Trends Ecol Evol. 1995;10:455–460. doi: 10.1016/s0169-5347(00)89179-x. [DOI] [PubMed] [Google Scholar]
- 20.Greene G L, Leppla N C, Dickerson W A. J Econ Entomol. 1976;69:487–488. [Google Scholar]
- 21.Stark W S, Tan K E W P. Photochem Photobiol. 1982;36:371–380. doi: 10.1111/j.1751-1097.1982.tb04389.x. [DOI] [PubMed] [Google Scholar]
- 22.Browman H I, Hawryshyn C W. J Exp Biol. 1994;193:191–207. doi: 10.1242/jeb.193.1.191. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith T H. Science. 1980;207:786–788. doi: 10.1126/science.7352290. [DOI] [PubMed] [Google Scholar]
- 24.Bennet A T D, Cuthill I C, Partridge J C, Lunau K. Proc Natl Acad Sci USA. 1997;94:8618–8621. doi: 10.1073/pnas.94.16.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs G H, Neitz J, Deegan J F, II. Nature (London) 1991;353:655–656. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- 26.Zangerl A R, Arntz A M, Berenbaum M R. Oecologia. 1997;109:433–441. doi: 10.1007/s004420050103. [DOI] [PubMed] [Google Scholar]
- 27.Karban R, Baldwin I T. Induced Responses to Herbivory. Chicago: Univ. of Chicago Press; 1997. [Google Scholar]
- 28.Antignus Y, Mor N, Joseph R B, Lapidot M, Cohen S. Pest Manage Sampling. 1996;25:919–924. [Google Scholar]
- 29.Berenbaum M R, Larson R A. Experientia. 1988;44:1030–1031. [Google Scholar]
- 30.Bernays E A, Chapman R F. Host Plant Selection by Phytophagous Insects. New York: Chapman & Hall; 1994. [Google Scholar]
- 31.Barker A M, Wratten S D, Edwards P J. Oecologia. 1995;101:251–257. doi: 10.1007/BF00317291. [DOI] [PubMed] [Google Scholar]
- 32.Wold E N, Marquis R J. Ecology. 1997;78:1356–1369. [Google Scholar]
- 33.McConn M, Creelman R A, Bell E, Mullet J E, Browse J. Proc Natl Acad Sci USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal A A. Science. 1998;279:1201–1202. doi: 10.1126/science.279.5354.1201. [DOI] [PubMed] [Google Scholar]
- 35.Beckerman A P, Uriarte M, Schmitz O J. Proc Natl Acad Sci USA. 1997;94:10735–10738. doi: 10.1073/pnas.94.20.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]