Abstract
Hybridomas secreting specific monoclonal antibodies (MAb) to all members of the genus Leptospira (clone LF9) and those that are specific only to the pathogenic species (clones LD5 and LE1) were produced. MAb LF9, which was immunoglobulin G1 (IgG1), reacted to a 38-kDa component of the sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated whole-cell lysates of all Leptospira spp., while MAb LD5 and MAb LE1, which were IgG1 and IgG2a, respectively, reacted to the 35- to 36-kDa components of all serogroups of the pathogenic species of Leptospira. The MAb LD5 was used in a dot blot-enzyme-linked immunosorbent assay (dot-ELISA) for detecting Leptospira antigen in urine samples serially collected from two groups of patients diagnosed with leptospirosis, i.e., 36 clinically diagnosed patients and 25 Leptospira culture confirmed patients. Their serum samples were tested serologically by IgM Dipstick assay, indirect immunofluorescence assay (IFA), and/or microscopic agglutination test (MAT). Urine samples of 26 patients diagnosed with other illnesses and 120 healthy individuals served as controls. For the first group of patients, who had been ill for an average of 3.4 days before hospitalization, the IgM Dipstick test, IFA, and MAT were positive for 69.4, 70.0, and 85.7% of patients, while the Leptospira antigenuria tested by the MAb-based dot-ELISA was positive for 75.0, 88.9, 97.2, 97.2, and 100% of patients on days 1, 2, 3, 7, and 14 of hospitalization, respectively. All but 1 of 11 patients whose serum samples collected on the first day of hospitalization were IgM seronegative, were positive by urine antigen test on day 1. This is strong evidence that detection of antigen in urine can provide diagnostic information that could be useful in directing early therapeutic intervention. The MAT was positive in 10 of 12 patients (83.3%) of the 25 culture-positive Leptospira patients who had been ill for an average of 5.04 days before hospitalization, and the Leptospira antigen was found in 64.0, 84.0, 96.0, 100, 100, 100, and 100% of the patients' urine samples collected on days 1, 2, 3, 4, 5, 6, and 7 of hospitalization, respectively. Leptospira antigenuria was found in 3 of the 26 patients diagnosed with other illnesses and 1 of the 120 healthy controls. The reasons for this positivity are discussed. The detection of antigen in urine by the monoclonal antibody-based dot-ELISA has high potential for rapid, sensitive, and specific diagnosis of leptospirosis at a low cost.
Leptospirosis is an important infectious disease of humans and animals worldwide. It is caused by a pathogenic species of spirochetes in the genus Leptospira which are not readily distinguishable from the free-living nonpathogenic species on the basis of morphology and biochemical characteristics. In temperate zones, this zoonotic disease of humans is usually associated with occupational or recreational exposures, such as meat inspections, veterinary work, abattoir work, farming, or wild water sport activities. However, in tropical or subtropical regions, the spread of this pathogen is wider. The infections occur either from direct exposures to infected animals or their products or indirectly through contact with water or soil contaminated with urine of a Leptospira shedder (10). Symptoms and severity of leptospirosis vary greatly from mild, flu-like illness to fatal (ictero)hemorrhagic forms with severe involvement of vital organs such as the liver, lung, and kidney (11). Early and accurate diagnosis of leptospirosis is important for proper and prompt treatment, which is life-saving for patients with severe illness. However, diagnosis based on the clinical picture of this disease is inaccurate, especially in the tropical regions where other similar acute febrile illnesses are common. Leptospirosis may be confused with malaria, viral hepatitis, influenza, dengue fever, rickettsial infections, typhoid fever, melioidosis, and others (12).
Laboratory diagnosis of leptospirosis is based primarily on either isolation of the pathogen from the specimen or demonstration of a rise in serum antibodies (37). The former is laborious and expensive and may not be successful (low sensitivity). Leptospires require delicate and complex culture media; i.e., the media must contain several growth-promoting substances and selected antimicrobial agents for suppressing contaminants, e.g., fungi or other saprophytes. The organism has a relatively long doubling time (6 to 8 h or more). All of the aforementioned points make leptospire culture too slow for early diagnosis. Direct demonstration of leptospires in preparations from specimens by dark-field microscopy, direct immunofluorescence, and immunoperoxidase staining have been hampered by the lack of specificity due to nonspecific background. Several serological assays have been developed for detecting the rise of serum antibodies. Among them, the microscopic agglutination test (MAT) is the reference method with which the other developing techniques have to be compared for evaluating their diagnostic sensitivity, specificity, and accuracy. However, the MAT encounters several drawbacks which limit its wide use in the developing parts of the world where leptospirosis occurs more extensively. These include the requirements of maintaining a broad range of Leptospira serovars for live antigen preparations, a panel of serotyping antisera, standard antiserum, microscope, and technical expertise. Diagnosis of leptospirosis by the MAT often requires paired serum samples, which delays the diagnosis. Moreover, false-negative results were frequently reported when the causative leptospire serovar was not included in the panel of typing organisms (30). Several other alternatives of antibody detection assays have subsequently been developed for early diagnosis of leptospirosis. These include the hemolytic test (8), the slide agglutination test (14), the indirect hemagglutination assay (19, 28), the indirect immunofluorescence test (32), the microcapsule agglutination test (2), the indirect enzyme-linked immunosorbent assay (ELISA) for immunoglobulin M (IgM) antibodies (1, 22), the dot-ELISA for IgM (23, 26, 36), the LEPTO Stick (15), and the lateral flow assay (27). While these methods are much simpler than the MAT and many of them are currently available commercially, they still need a lag period after the infection before the antibodies become detectable, and once incited, the antibodies stay for a long time, even after the pathogenic organisms have been eliminated. IgM antibodies were detected by dot-ELISA in patients up to the 6th month, decreasing to 57% by the 10th month, and persisting in some patients beyond the 12th month (26). This implies that the antibody detection assay is less sensitive during the very early period of infection, as IgM antibodies were not detected in many patients during the first few days of the acute phase of illness (36) and also implies that such assays cannot be used for monitoring the efficacy of the treatment. As for the MAT, false-negative results may be obtained due to the absence of homologous antigens to the patient's causative organisms in the antigenic preparations used in the assays.
DNA-DNA hybridization and PCR have been used for Leptospira detection in a variety of samples from both humans and animals (3, 17, 20, 21, 29, 35). While both techniques are sensitive and specific and have high potential for early diagnosis of leptospirosis, they are laborious and expensive and the DNA probe is time consuming; thus, they are inappropriate for routine work, especially in the developing parts of the world where high endemicity of leptospirosis has been established.
In this report, hybridomas secreting monoclonal antibodies (MAbs) specific to all members of the genus Leptospira and others specific only to the pathogenic species were produced. One of the latter was used as a detection reagent for diagnosis of human leptospirosis.
MATERIALS AND METHODS
Antigen preparation.
The antigens used in this study are listed in Table 1.Sonicates of young cultures (7 to 14 days old in Ellinghausen-McCullough-Johnson-Harris [EMJH] medium) of various serovars of both pathogenic and nonpathogenic Leptospira were prepared. Individual cultures were centrifuged at 10,000 × g at 4°C for 30 min and washed with distilled water three times by centrifugation, and the washed bacteria were then subjected to sonication at 20 kHz for 5 min three to four times. The dry weights of the individual sonicates were determined and adjusted to the desired concentrations when used. Whole-cell lysates of other bacteria were prepared as previously described (5).
TABLE 1.
List of organisms of which antigens were used in this study
| Organism type | Name | Specific characteristic | Serovar (strain) |
|---|---|---|---|
| Bacterium | Leptospira | Pathogenic | autumnalis (Aki-yami A) |
| bangkok | |||
| bataviae | |||
| bratislava | |||
| bullum | |||
| canicola | |||
| cynopteri (3522C) | |||
| djasiman | |||
| grippotyphosa | |||
| hebdomadis | |||
| icterohaemorrhagiae | |||
| javanica | |||
| pomona | |||
| pyrogenes | |||
| saigon | |||
| wolffi | |||
| Leptospira | Saprophytic | andamana (CH11) | |
| patoc (Patoc 1) | |||
| patoc (P 136) | |||
| patoc (P 138) | |||
| Escherichia coli | O 126 | ||
| O 86 | |||
| Enteroinvasive | EI 54:32 | ||
| Enterotoxigenic | SLT-1 (CG-94-2017) | ||
| ST (CAM 10-10) | |||
| LT (CJ 1417-1) | |||
| SP 1+ (2389-1) | |||
| SP 1+ (2436-1) | |||
| CJ 612-6 | |||
| Enterohemorrhagic | O 11 ac:H 40 (V 826-2) | ||
| O 157:H7 (V 837-1) | |||
| W 4671-1 | |||
| W 412-1 | |||
| W 225-1-2 | |||
| Diffusely adherent | WC 407-1-4 | ||
| Aggregative | W 177-1-1 | ||
| K-12 | |||
| Shigella flexneri | 1a | ||
| Shigella dysenteriae | Type 1 | ||
| Salmonella enterica | Typhi | ||
| Salmonella enterica | Typhimurium | ||
| Salmonella enterica | Paratyphi A | ||
| Salmonella enterica | Paratyphi B | ||
| Salmonella enterica | C 1 | ||
| Salmonella enterica | E | ||
| Salmonella enterica | Panama D | ||
| Salmonella enterica | Blockley | ||
| Klebsiella pneumoniae | |||
| Proteus vulgaris | |||
| Staphylococcus aureus | Coagulase positive | ||
| Staphylococcus sp. | Coagulase negative | ||
| Streptococcus pneumoniae | |||
| Streptococcus sp. | Group A | ||
| Streptococcus sp. | Group B | ||
| Burkholderia pseudomallei | |||
| Pseudomonas aeruginosa | |||
| Rickettsia | Orientia tsutsugamuchi | ||
| Fungus | Candida albicans | ||
| Virus | Cytomegalovirus | ||
| Japanese encephalitis virus | |||
| Dengue virus | Type 1 | ||
| Dengue virus | Type 2 | ||
| Dengue virus | Type 3 | ||
| Dengue virus | Type 4 | ||
| Rubella virus | |||
| Influenza virus | A (H3 N2) | ||
| Influenza virus | A (H1 N1) | ||
| Influenza virus | B |
Specimens.
Two sets of clinical specimens were used in this study. The first set was from 36 presumptively diagnosed leptospirosis patients admitted to Khon Kaen Provincial Hospital (about 390 km northeast of Bangkok, Thailand), during July to November 2000. The patients had been ill for 1 day to 8 days (average, 3.4 days). World Health Organization (WHO) criteria (12) were used for the diagnosis of leptospirosis. Serum samples collected on the day of hospital arrival (day 1) were first tested for IgM anti-Leptospira antibodies by IgM Dipstick (Organon, Brussels, Belgium). Samples positive for the IgM were subjected to indirect immunofluorescence assay (IFA), and the IFA-positive specimens were further tested by the MAT at the Regional Medical Sciences Center, Muang District, Khon Kaen Province, Thailand, against a panel of Leptospira serovars recommended by the WHO (12). On day 5 or 6 of hospitalization other serum samples were taken from the patients who tested IgM Dipstick negative on day 1, and these samples were tested by the Dipstick assay. Positive samples were subjected to IFA and the MAT as for the first serum samples. Urine samples were collected from patients on day 1 before treatment and on days 2, 3, 7, and 14 of hospitalization (see Table 3). They were kept frozen at −20°C and subsequently were sent to a laboratory in Bangkok where antigen detection was performed by dot blot-ELISA (dot-ELISA) by a different scientist. The second set of specimens were urine samples collected serially from 25 culture-positive leptospirosis patients admitted to Ubon Rachathanee Provincial Hospital (about 600 km northeast of Bangkok) and Maharat Regional Hospital, Muang District, Nakhon Ratchaseema Province (about 260 km northeast of Bangkok), during October to November 2000. These 25 patients consisted of 15 blood culture-positive, 1 blood and cerebrospinal fluid (CSF) culture-positive, 1 CSF culture-positive, 2 blood- and urine-positive, and 6 urine culture-positive patients. The patients were ill for 1 day to 14 days (range, 5.04 days) before hospital arrival. Urine samples were collected from all patients on the first day (day 1) before treatment. Follow-up daily urine samples were available from some patients up to day 7 (see Table 4). Serum samples of these patients were tested for antibodies by the MAT. Urine samples of 26 patients—which included 10 patients with advanced cholangiocarcinoma associated with liver fluke (Opisthorchis viverrini) infection, 8 patients with clinically diagnosed melioidosis, 3 patients with parasite-confirmed falciparum malaria (Plasmodium falciparum in blood smears), 1 patient with scrub typhus (antibody positive by IFA), and 4 patients with liver fluke infection (O. viverrini eggs were found in stools)—were collected as patient controls. Urine samples of apparently healthy inhabitants of the same geographical area as the patients, i.e., northeastern Thailand, where leptospirosis has long established its endemicity, served as controls. Written informed consent was obtained from the individuals or their legal representatives before sample collection.
TABLE 3.
Results of IgM Dipstick assay, IFA, MAT, and dot-ELISA performed on specimens of clinically diagnosed leptospirosis patients (first set)a
| Patient no. | Length of fever before hospital arrival (day) | IgM resultb
|
IFA result | MAT titer(s) (serovar[s]) | Result of antigen detection in urine by dot-ELISA performed on sample collected at day:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 1 | 2 | 3 | 7 | 14 | ||||
| 1 | 3 | − | + | + | 1:100 (copenhageni and saigon) | + | − | 0 | 0 | 0 |
| 2 | 3 | − | + | + | 1:400 (bratislava) | + | + | 0 | 0 | + |
| 3 | 3 | − | + | + | 1:100 (sejroe) | + | + | 0 | + | 0 |
| 4 | 6 | − | + | + | 1:100 (bangkok, copenhageni, and saigon) | + | + | 0 | 0 | 0 |
| 5 | 3 | − | + | + | 1:200 (sejroe) | + | 0 | 0 | 0 | − |
| 6 | 3 | + | 0 | + | 1:100 (bratislava) | − | + | + | 0 | 0 |
| 7 | 4 | + | 0 | + | 1:100 (bangkok and sejroe), 1:200 (bratislava and icterohaemorrhagiae), 1:400 (rachmati) | + | + | 0 | 0 | + |
| 8 | 7 | + | 0 | + | 1:100 (bratislava), 1:200 (saigon) | + | + | + | 0 | 0 |
| 9 | 8 | + | 0 | + | 1:100 (bangkok and sejroe), 1:200 (bratislava) | + | + | 0 | 0 | 0 |
| 10 | 4 | + | 0 | + | 1:100 (bangkok and sejroe), 1:200 (bratislava) | + | − | 0 | 0 | 0 |
| 11 | 4 | + | 0 | + | 1:200 (saigon), 1:400 (pyrogenes) | − | − | + | + | + |
| 12 | 4 | + | 0 | + | 1:100 (pyrogenes and sejroe), 1:200 (autumnalis [Akiyami A] and bratislava) | + | − | 0 | 0 | − |
| 13 | 3 | + | 0 | + | 1:100 (bangkok and copenhageni), 1:200 (sejroe), 1:400 (rachmati), 1:800 (saigon) | + | + | 0 | 0 | 0 |
| 14 | 3 | + | 0 | + | 1:100 (saigon), 1:200 (autumnalis [Akiyami A]), 1:400 (copenhageni), 1:800 (bangkok and bratislava) | + | − | 0 | + | 0 |
| 15 | 2 | + | 0 | + | 1:100 (bangkok, sejroe, and rachmati), 1:200 (bratislava) | − | + | 0 | − | 0 |
| 16 | 5 | + | 0 | + | 1:200 (saigon) | + | + | + | + | + |
| 17 | 3 | + | 0 | + | 1:100 (wolffi) | + | + | 0 | 0 | 0 |
| 18 | 3 | + | 0 | + | 1:100 (wolffi), 1:800 (bratislava) | − | + | + | 0 | 0 |
| 19 | 1 | + | 0 | + | − | + | + | 0 | 0 | + |
| 20 | 4 | + | 0 | + | − | + | + | 0 | 0 | 0 |
| 21 | 3 | + | 0 | + | − | − | − | − | 0 | + |
| 22 | 4 | + | 0 | − | 0 | + | + | 0 | 0 | 0 |
| 23 | 4 | + | 0 | − | 0 | + | − | 0 | − | 0 |
| 24 | 3 | + | 0 | − | 0 | − | + | + | + | + |
| 25 | 2 | + | 0 | − | 0 | + | + | + | + | 0 |
| 26 | 2 | + | 0 | − | 0 | + | + | 0 | 0 | 0 |
| 27 | 3 | + | 0 | − | 0 | − | + | + | + | + |
| 28 | 2 | + | 0 | − | 0 | − | 0 | + | 0 | 0 |
| 29 | 2 | + | 0 | − | 0 | + | + | 0 | 0 | 0 |
| 30 | 3 | + | 0 | − | 0 | + | + | 0 | 0 | 0 |
| 31 | 2 | − | − | 0 | 0 | + | − | 0 | 0 | − |
| 32 | 3 | − | − | 0 | 0 | + | + | 0 | − | 0 |
| 33 | 5 | − | − | 0 | 0 | + | + | + | 0 | 0 |
| 34 | 3 | − | − | 0 | 0 | + | 0 | 0 | 0 | 0 |
| 35 | 3 | − | − | 0 | 0 | − | 0 | + | − | − |
| 36 | 2 | − | − | 0 | 0 | + | − | − | 0 | − |
| No. positive/total no. tested | 25/36 | 5/11 | 21/30 | 18/21 | 27/36 | 23/32 | 11/13 | 7/11 | 8/12 | |
| % Positivity | 69.4 | 45.5 | 70.0 | 85.7 | 75.0 | 71.9 | 84.6 | 63.6 | 66.7 | |
| Cumulative positivity [no. (%)] | 25 (69.4) | 30 (83.3) | 21 (70.0) | 18 (85.7) | 27 (75) | 32 (88.9) | 35 (97.2) | 35 (97.2) | 36 (100) | |
+, positive; −, negative; 0, no sample.
1st, serum sample collected on the first day of hospitalization; 2nd, serum samples collected on day 5 or 6 of hospitalization.
TABLE 4.
Results of MAT and dot-ELISA performed on specimens of culture-positive leptospirosis patients (second set)a
| Patient no. | Length of fever before hospital arrival (day) | MAT titer(s) (serovar[s])b
|
Culture-positive result
|
Result of dot-ELISA performed on sample collected at day:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | Specimen(s)c | Day(s)d | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| 1 | 6 | 1:200 (bratislava) | 1:400 (bangkok and ballico), 1:800 (new), 1:6,400 (bratislava) | Bl | 1 | + | + | + | 0 | 0 | 0 | 0 |
| 2 | 12 | − | 0 | Bl | 1 | − | + | − | − | 0 | 0 | 0 |
| 3 | 6 | 0 | 0 | Bl | 3 | − | + | + | + | + | 0 | 0 |
| 4 | 4 | − | 0 | Bl | 1 | + | + | + | 0 | 0 | 0 | 0 |
| 5 | 14 | − | − | Bl | 1 | + | + | − | + | + | + | + |
| 6 | 2 | − | 0 | Bl | 4 | + | − | + | + | − | + | + |
| 7 | 5 | − | 1:1,600 (bratislava) | Bl | 1 | + | − | + | + | + | 0 | 0 |
| 8 | 3 | − | 0 | Bl | 1 | + | + | − | − | − | + | 0 |
| 9 | 4 | − | 1:1,600 (pyrogenes) | Bl | 1 | + | + | + | + | 0 | 0 | 0 |
| 10 | 1 | − | 0 | Bl | 1 | + | − | 0 | 0 | 0 | 0 | 0 |
| 11 | 4 | − | 0 | Bl | 1 | + | + | 0 | 0 | 0 | 0 | 0 |
| 12 | 2 | − | 0 | Bl | 1 | − | + | + | 0 | 0 | 0 | 0 |
| 13 | 5 | − | 0 | Bl | 1 | − | − | − | + | + | + | + |
| 14 | 3 | − | 0 | Bl | 1 | − | 0 | + | + | 0 | 0 | 0 |
| 15 | 2 | − | 0 | Bl | 1 | − | 0 | + | + | + | + | + |
| 16 | 3 | 1:200 (bratislava) | 1:1,600 (bratislava) | Bl, CSF | 1 | − | − | + | + | 0 | 0 | 0 |
| 17 | 4 | − | 1:400 (pyrogenes) | CSF | 1 | + | + | + | + | + | + | 0 |
| 18 | 8 | 1:200 (pyrogenes) | 1:400 (pyrogenes) | Bl, urine | 5, 13 | + | − | − | + | + | + | 0 |
| 19 | 7 | − | 0 | Bl, urine | 1, 1 | + | + | + | + | + | + | + |
| 20 | 4 | 1:200 (hebdomadis), 1:400 (hyos), 1:1,600 (bratislava) | 1:3,200 (bratislava) | Urine | 7 | − | + | + | − | − | 0 | + |
| 21 | 3 | − | 0 | Urine | 5 | + | + | + | + | + | 0 | 0 |
| 22 | 6 | − | 1:1,600 (bratislava) | Urine | 3 | + | + | + | + | + | + | + |
| 23 | 7 | − | 1:100 (saigon), 1:200 (ballico and copenhageni), 1:400 (new and rachmati), 1:800 (autumnalis [Akiyami A]), 1:1,600 (bratislava) | Urine | 1, 10, 11 | + | + | + | + | + | 0 | + |
| 24 | 7 | − | − | Urine | 2, 6, 7 | − | + | + | + | + | + | + |
| 25 | 4 | 0 | 1:1,600 (bratislava) | Urine | 3 | + | + | + | 0 | 0 | 0 | 0 |
| No. positive/total no. tested | 4/23 | 10/12 | 25/25 | 16/25 | 17/23 | 18/23 | 16/19 | 12/15 | 10/10 | 9/9 | ||
| % Positivity | 17.4 | 83.3 | 100 | 64.0 | 73.9 | 78.2 | 84.2 | 80.0 | 100.0 | 100.0 | ||
| Cumulative positivity [no. (%)] | 4 (17.4) | 10 (83.3) | 25 (100) | 16 (64) | 21 (84) | 24 (96) | 25 (100) | 25 (100) | 25 (100) | 25 (100) | ||
+, positive result; −, negative result; 0, sample not available.
1st, first serum sample; 2nd, second serum sample.
Abbreviations: Bl, blood; CSF, cerebrospinal fluid.
Number of days after admission.
Leptospira culture.
Leptospira culture of blood, CSF, and/or urine was performed using semisolid EMJH medium (16). Blood samples were taken from individual patients using a 21-gauge needle, and 1 drop of the freshly drawn specimen was placed into the tube containing the EMJH medium while the remaining blood was allowed to clot and the serum was collected. One drop of CSF obtained from lumbar puncture was placed in the EMJH medium. Samples of midstream urine (at least 50 ml) were collected from the patients. However, for the patients who could not urinate, a catheter was inserted into the urethra and the urine was drained into the collected tube. An aliquot of 10 ml of each urine sample was centrifuged immediately after the collection, at 3,000 × g at room temperature for 30 min; most supernatant was discarded, and 1 drop of fluid containing sediment at the bottom of the tube was placed into the EMJH medium. The tube was incubated at 30°C. Subcultures were performed appropriately thereafter. Checking for the presence of Leptospira was done by placing a small drop of the culture onto a glass slide and observing by dark-field microscopy at a magnification of ∼×200. The cultures were maintained for at least 4 months before the negatives were discarded. The Leptospira isolates were maintained in the laboratory for further study.
Hybridoma production.
Young adult BALB/c mice (6 to 7 weeks old) were kindly supplied by the Armed Forces Research Institute of Medical Sciences (AFRIMS), U.S. Component, Bangkok, Thailand. For the hybridoma production, four mice were immunized. Before immunization, the mice were bled individually via the retro-orbital plexus, and the sera were collected, pooled, and used as a pool of negative control serum. After bleeding, each mouse was injected intraperitoneally with 0.2 ml of a mixture of equal volume of 50 μg of the L. interrogans serovar icterohaemorrhagiae sonicate (500 μg/ml in normal saline solution) and Freund's complete adjuvant. The mice were reimmunized four more times at 2-week intervals using the same immunogen and same route but with Freund's incomplete adjuvant. The second, third, fourth, and fifth booster doses were 50, 50, 100, and 120 μg, respectively. Seven days after the fifth immunization, the mice were bled and their sera were assessed for titers of antibody against the homologous antigen by an indirect ELISA, described below. The immune mouse showing the highest titer was used as a spleen cell donor in hybridoma production while the others were bled and their sera were pooled and used as a pool of positive control serum (PS). Three days before the cell fusion, the immune mouse was given an intravenous injection of 50 μg of the immunogen in 0.2 ml of normal saline solution.
Three days after the intravenous booster, the immune mouse was bled and the serum was subsequently used as an immune serum (IS). The animal was then sacrificed by cervical dislocation. Spleen cells were fused with P3x-63-Ag8.653 myeloma cells by using polyethylene glycol 4000 as a fusogen at spleen cells/myeloma cell ratio of about 10:1 for production of hybridomas as previously described (5). Culture fluids were collected and screened for antibodies against the homologous antigen. Cells from the antibody-positive wells were subjected to cloning by the limiting dilution method using spleen cells of the nonimmune BALB/c mouse as feeder cells. Culture fluids from these clones (hybridomas) were retested against the homologous antigen and also against the heterologous antigens (Table 1) for cross-reactivity by indirect ELISA. Antigenic specificities of the MAbs secreted by individual clones were determined by Western blotting (WB) against the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-separated homologous antigen and heterologous antigens prepared from other Leptospira serovars.
ELISAs.
An indirect ELISA was used for determining antibody titers of sera from immunized mice and for detecting antibodies in the cell culture fluids in screening for positive hybrids. The technique was also used for determining specificity versus cross-reactivity of the MAbs. The microtiter plates were coated with appropriate antigens (10 μg of antigen per ml of carbonate-bicarbonate buffer [pH 9.6]) listed in Table 1. Sonicate of L. interrogans serovar icterohaemorrhagiae was also included in the test, which served as a positive control. The antigen-sensitized plates were incubated at 37°C in a humid chamber for 2 h and at 4°C overnight. The unbound antigens were washed off with phosphate-buffered saline (PBS) containing 0.5% Tween 20 (PBST). The unoccupied sites on the plates were blocked with 1% bovine serum albumin (BSA) in PBS and incubated at 37°C for 1 h. The excess BSA was washed off, and 100 μl of antibody preparation (serially diluted individual mouse immune sera, negative control serum, IS, positive control serum, diluted or undiluted cell culture fluid) and fresh culture medium or culture fluid of myeloma cells, which served as a negative control or blank, were added to the appropriate wells. The antigen-antibody reaction was allowed to take place for 1 h at 37°C. After washing thoroughly with PBST, 100 μl of a 1:1,000 dilution of rabbit anti-mouse Ig-horseradish peroxidase (Dakopatts, Glostrup, Denmark) in PBS containing 0.2% BSA and 0.2% gelatin was added to each well and incubated as described above for 1 h. The unbound conjugate was removed by washing with PBST. The enzyme substrate was added to all wells (100 μl per well). The reaction was allowed to take place in the dark for 30 min and was then stopped by adding 50 μl of 1 N NaOH per well. The optical density of each well was measured at 492 nm with an ELISA reader (Multiscan EX; Labsystems, Helsinki, Finland). The ELISA titer of the antibody preparation was the highest dilution of the antibody giving an optical density of ≥0.05. One indirect ELISA unit was defined as the smallest amount of the antibody which gave a positive indirect ELISA reaction.
MAb-based dot-ELISA was used for cross-reactivity testing of the MAbs secreted from the hybrid cells and for detecting antigen of Leptospira in urine samples of patients. For cross-reactivity testing, either 3-μl aliquots of individual antigens listed in Table 1 (1 mg/ml) which had been boiled for 30 min were manually and duplicately dotted onto two nitrocellulose (NC) strips (NC membrane; Bio-Rad, Richmond, Calif.) or 300-μl aliquots containing the same concentration of individual antigens were dotted on the NC using a slot blot device (Pierce). The positive and negative controls were boiled sonicate of Leptospira serovar icterohaemorrhagiae and PBS, respectively. For the antigen detection assay with urine, individual samples (3.0 ml) were centrifuged at 12,000 × g for 10 min at room temperature; 2.5 ml of the supernatant was discarded, the remaining portions were boiled for 30 min, and 200-μl aliquots of boiled urine samples were dotted individually and duplicately onto two NC strips using the slot blot device. The positive and negative controls, which were 200-μl aliquots of normal urine with and without 3 μg of sonicate of Leptospira serovar icterohaemorrhagiae, respectively, were treated similarly and included on both NC strips. The NC strips were air dried, blocked by placing into 5% skim milk in PBS (pH 7.4), incubated at room temperature for 10 min, and washed with three changes of 0.01 M PBS (pH 7.4). One strip (test strip) was then incubated with appropriate MAbs (at the concentration of 80 indirect ELISA units/ml in the experiments for detecting the Leptospira antigen in the patients' specimens; the culture fluid of the hybridoma was used undiluted in the cross-reactivity checking of the MAbs) for 20 min on a rotator. Another NC strip (control strip) was placed in a spent culture medium of P3x-63-Ag8.653 myeloma cells. After the incubation, both NC strips were washed with PBS (pH 7.4) as described above and then incubated for 20 min with biotinylated rabbit anti-mouse Igs (1:2,000; Dakopatts). After 20 min, the strips were washed with PBS (pH 7.4) three times and then incubated with alkaline phosphatase-conjugated streptavidin (1:2,000; Dakopatts). After 20 min, the strips were washed with PBS (pH 7.4) three times and once with 0.15 M Tris (pH 9.6), immersed in a substrate solution for color development for 5 min, washed with distilled water, and air dried. The results were read visually by comparing the color which appeared at the duplicative spots where individual urine samples were dotted onto the test and control strips. Positive reaction (presence of cross-reacting antibodies to Leptospira antigen in the cross-reactivity testing or presence of Leptospira antigen for urine antigen detection assay) appeared as blue or purplish-blue spots on the test strip distinguishable from the same samples on the control strip (negative), which appeared as spots of other colors (nonspecific) or as clear areas. The positive and negative controls on the test strip should reveal appropriate colors, i.e., blue or purplish blue and original normal urine-colored or clear areas, respectively. Both positive and negative controls on the control strip should reveal the normal urine color.
SDS-PAGE and WB analysis.
SDS-PAGE was carried out in a vertical slab gel apparatus (Bio-Rad) according to the method of Laemmli (18). A 4% stacking gel and 12.5% acrylamide separating gel were used in the procedure. WB was performed by transblotting the SDS-PAGE-separated sonicates of Leptospira spp. from the gel to an NC membrane (33). The unoccupied sites on the NC membrane were blocked by soaking the membrane in a blocking buffer (3% BSA, 5% gelatin in PBS [pH 7.4]) at room temperature with gentle rocking for 1 h. After washing thoroughly, the NC membrane was treated with antibody preparation (at an appropriate dilution of IS or of the selected MAbs or undiluted culture supernatant of the hybridoma) at room temperature for 1 h. After washing thoroughly, the membrane was put in a solution of rabbit anti-mouse Ig-horseradish peroxidase conjugate (Dakopatts) at a dilution of 1:1,000 in PBS (pH 7.4) containing 1% BSA and 1% gelatin for 30 min at room temperature. It was washed with 1/15 M phosphate buffer (pH 7.6) before being placed in a substrate solution for 5 min, washed with distilled water, and air dried.
RESULTS
A total of 3.9 × 108 spleen cells were obtained from a selected immunized mouse which had an indirect ELISA titer of antibody against the homologous antigen of 1:12,800; the cells were mixed with 3.6 × 107 myeloma cells in the cell fusion procedure. The culture fluids from 790 wells which revealed growing cells were tested for antibodies against the homologous antigen, i.e., sonicate of L. interrogans serovar icterohaemorrhagiae, and the fluids from 48 wells (4.7%) were positive. The positive culture fluids were then subjected to WB analysis against the SDS-PAGE-separated homologous antigen. Cells from the wells which had culture fluids showing different patterns in the WB analysis against the homologous antigen were selected for subsequent cloning by the limiting dilution method, and 41 monoclones (hybridomas) were obtained. Culture fluids of these hybridomas were tested against the heterologous antigens listed in Table 1 by indirect ELISA and dot-ELISA and only three clones showed specificity to the antigens prepared from Leptospira organisms. These three clones were designated 15C4G11, 13E2B9, and 2D9G8. They were then recloned by limiting dilution in order to assure the monoclonality of all clones. Four hybridoma clones (namely, clones LB4, LD5, LD6, and LD8) were finally obtained from the parental clone 15C4G11, and one clone each (namely, clones LF9 and LE1), was derived from the parental clones 13E2B9 and 2D9G8, respectively. The MAb isotypes were IgG1 for the clones LB4, LD5, LD6, LD8, and LF9 and IgG2a for the clone LE1. The antibody titers presented in the spent culture media when the monoclones had grown to the stationary phase are shown also in Table 2. The antibody titers were constant; several subcultures of these clones always yielded the same titers.
TABLE 2.
Specific hybridomas, their secreted immunoglobulin isotypes, and the indirect ELISA titers of their culture supernatants at the stationary phase of growth
| Parental hybridoma clone | Monoclone | Ig isotype
|
Reciprocal indirect ELISA titer | |
|---|---|---|---|---|
| Heavy chain | Light chain | |||
| 15C4G11 | LD5 | γ1 | Kappa | 1,024 |
| LD6 | γ1 | Kappa | 1,024 | |
| LD8 | γ1 | Kappa | 1,024 | |
| LB4 | γ1 | Kappa | 256 | |
| 13E2B9 | LF9 | γ1 | Kappa | 512 |
| 2D9G8 | LE1 | γ2a | Kappa | 32 |
The MAbs of the six clones reacted to the sonicates of all pathogenic Leptospira serovars listed in Table 1 by both indirect ELISA and dot-ELISA. However, only the MAbs secreted by clone LF9 (MAb LF9) reacted also to the sonicates of the nonpathogenic Leptospira. Figure 1 illustrates the dot-ELISA results of MAbs to boiled sonicates of various Leptospira serovars.
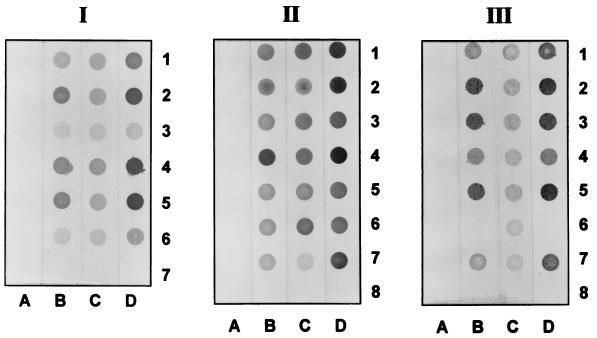
FIG. 1.
Dot-ELISA results of MAb LD5, MAb LF9, and MAb LE1 against boiled sonicates of various Leptospira serovars. Rows 1 to 6 of block I were blotted with antigens of serovars pomona, bratislava, bataviae, canicola, pyrogenes, and icterohaemorrhagiae (positive control), respectively; row 7 was blotted with PBS (negative control). Rows 1 to 7 of block II were blotted with antigens of serovars cynopteri, andamana, bangkok, grippotyphosa, hebdomadis, javanica, and icterohaemorrhagiae (positive control), respectively; row 8 was blotted with PBS (negative control). Rows 1 to 7 of block III were blotted with antigens of serovars autumnalis, bullum, djasiman, saigon, wolffi, patoc, and icterohaemorrhagiae (positive control); row 8 was blotted with PBS (negative control). Strips A were reacted with spent culture medium of P3x-63-Ag8.653 myeloma cells; strips B to D were reacted with MAb LD5, MAb LF9, and MAb LE1, respectively.
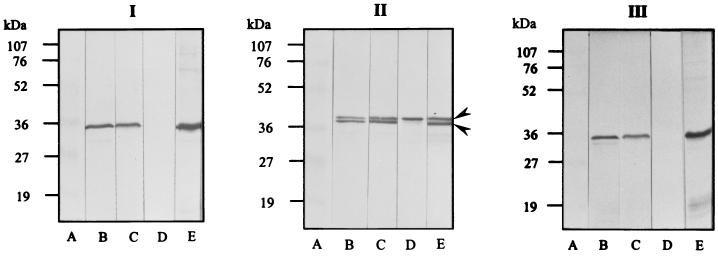
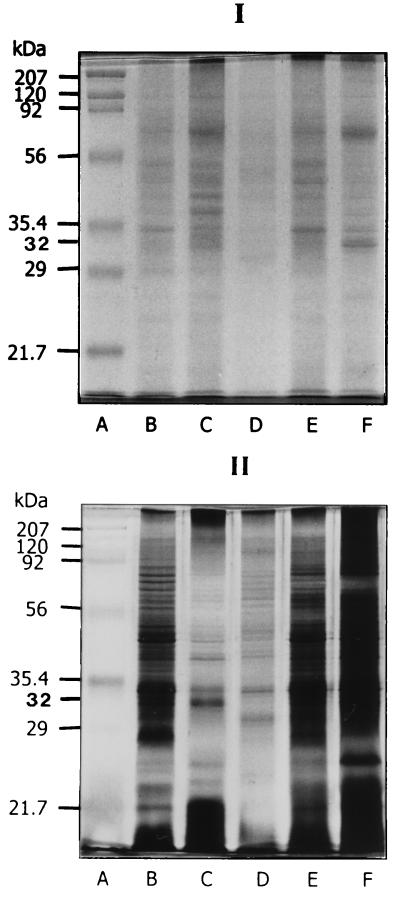
WB analysis revealed that the MAb LF9 reacted to the antigen located at about 38 kDa of all Leptospira serovars tested (both pathogenic and nonpathogenic organisms). However, the WB pattern of all pathogenic Leptospira SDS-PAGE-separated sonicates with the MAb LF9 appeared as a doublet of upper and low bands (Fig. 2, block II, lanes B and E), while the WB pattern of nonpathogenic strains—i.e., L. biflexa serovar patoc strains Patoc 1, P 136, and P 138—with the MAbs showed only the upper band (Fig. 2, block II, lane D). The SDS-PAGE-separated sonicate of the serovar andamana (CH11), although classified as nonpathogenic Leptospira, acquired patterns similar to those of pathogenic strains after reaction with MAb LF9 (Fig. 2, block II, lane C). The MAbs from clones LB4, LD5, LD6, and LD8 did not react to antigens of the nonpathogenic Leptospira serovar patoc strains Patoc 1, P 136, and P 138 (Fig. 2, block I, lane D) but reacted to the SDS-PAGE-separated sonicates of all pathogenic Leptospira strains and CH11 at the location about 35 to 36 kDa (Fig. 2, block I, lanes B, C, and E). The MAbs from clone LE1 gave positive WB reactions to the antigens located at about 35 to 36 kDa of pathogenic Leptospira and nonpathogenic serovar andamana (Fig. 2, block III, lanes B and C) but also produced a smear with the antigen at about 23 kDa and lower with some members of pathogenic Leptospira (Fig. 2, block III, lane E). The antigenic components specific to all of these MAbs appeared in the SDS-PAGE-separated sonicates stained for proteins with Coomassie brilliant blue stain and could be visualized also when stained with silver stain (Fig. 3). The MAbs secreted by the clone LD5 (MAb LD5) were used as an antigen detection reagent in the dot-ELISA performed on all urine specimens of the patients and controls. The MAb LD5 was chosen because the spent medium of the clone LD5 yielded the highest indirect ELISA titers and the cells grew fast. Also, we did not use the MAb LF9, which reacted with all Leptospira species, including pathogenic and saprophytic because of the possibility of false-positive reactions with specimens or any reagents or glassware contaminated with nonpathogenic Leptospira in the tap water or other attributes.
FIG. 2.
WB patterns of MAb LD5, MAb LF9, and MAb LE1 against SDS-PAGE-separated sonicates of Leptospira spp. (blocks I, II, and III, respectively). Lanes B, C, D, and E contain serovars icterohaemorrhagiae, andamana, patoc, and djasiman, respectively. Lanes A contain standard molecular mass markers (sizes indicated at left).
FIG. 3.
SDS-PAGE-separated sonicates of various Leptospira serovars (icterohaemorrhagiae, andamana, patoc, djasiman, and grippotyphosa) stained with Coomassie brilliant blue stain (block I) and silver stain (block II). Lanes A contain molecular mass standards (Broad Range Standards; Bio-Rad). Lanes B, C, D, E, and F contain sonicates of serovars icterohaemorrhagiae, andamana, patoc, djasiman, and grippotyphosa, respectively. The numbers at the left of each block are molecular masses.
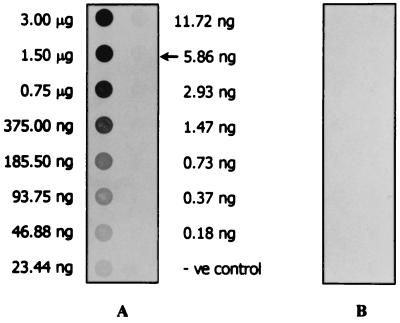
The smallest amount of L. interrogans serovar icterohaemorrhagiae sonicate in normal urine which could be detected by the MAb LD5 dot-ELISA was 5.86 ng (Fig. 4). The MAb LD5 dot-ELISA was performed on specimens obtained from the two sets of patients in comparison with the clinical features (WHO criteria), serological test(s) (IgM Dipstick assay, IFA, and/or MAT), and/or Leptospira culture. For the first set of patients, IgM Dipstick assay was positive for 25 of 36 (69.4%) of the day 1 serum samples. IFAs were positive for 16 of the 25 IgM Dipstick-positive samples (64.0%). IgM Dipstick assays performed on other serum samples taken at day 5 or 6 of the day 1 IgM-negative patients were positive for 5 of 11 samples (45.5%), which made the cumulative positivity of the IgM 30 of 36 patients (83.3%). The five serum samples were all IFA positive (100%), which made the cumulative IFA positivity 21 of 30 patients (70%). The MAT was positive for 18 of the 21 IgM-positive, IFA-positive serum samples (85.7%). The results of these serological tests of the 36 clinically diagnosed leptospirosis patients are given in Table 3. MAb LD5 dot-ELISA performed on urine samples collected on day 1 was positive in 27 of 36 (75.0%) patients. Among the 27 urine samples collected on day 1 which were antigen positive were urine samples of 10 of 11 patients whose day 1 serum samples were IgM seronegative (patients 1 to 5 and patients 31 to 36). Cumulative positivities of Leptospira antigenuria were 32 of 36 (88.9%), 35 of 36 (97.2%), 35 of 36 (97.2%), and 36 of 36 (100%) on days 2, 3, 7, and 14, respectively (Table 3).
FIG. 4.
MAb LD5 dot-ELISA results with various concentrations of boiled sonicate of Leptospira serovar icterohaemorrhagiae. Strip A was reacted with MAb LD5 (test strip). Strip B was reacted with spent culture medium of P3x-63.Ag8.653 myeloma cells (control strip). The lowest concentration of the sonicate which could be detected by the MAb dot-ELISA was 5.86 ng (arrow). −ve control, negative control.
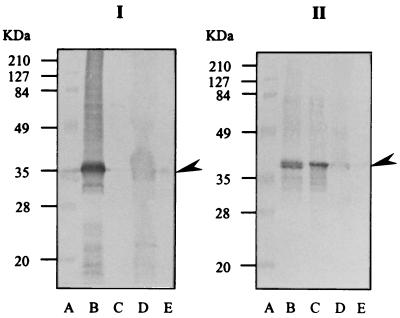
In order to confirm that the urine samples of the six patients (patients 31 to 36) (Table 3) who tested antibody negative but positive for Leptospira antigenuria by dot-ELISA really contained the Leptospira epitopes, all antigen-positive urine samples of these patients were individually and extensively dialyzed against distilled water at 4°C and lyophilized. The dried samples were restored in the appropriate buffer and subjected to SDS-PAGE and WB analysis against MAb LD5 and MAb LF9 using the whole-cell sonicate of L. interrogans serovar icterohaemorrhagiae and L. biflexa serovar patoc as controls. However, the immune complexes formed by the SDS-PAGE-separated Leptospira antigens in the concentrated urine samples and the MAb LD5 or MAb LF9 were reacted with anti-mouse Ig-biotin followed by streptavidin-alkaline phosphatase conjugate and substrate, respectively, instead of anti-mouse Ig-alkaline phosphatase and substrate. WB results revealed that the dot-ELISA-positive samples contained Leptospira epitopes which could be recognized by MAb LD5 at the location of about 35 to 36 kDa and by the MAb LF9 at the location of about 38 kDa. Representatives of the results are shown in Fig. 5.
FIG. 5.
WB patterns of SDS-PAGE-separated sonicates of L. interrogans serovars icterohaemorrhagiae and patoc and dialyzed urine samples I and II against MAb LD5 (block I) and MAb LF9 (block II), respectively. The immune complexes formed between the SDS-PAGE-separated antigens and antibodies were first reacted with anti-mouse Igs labeled with biotin and then with streptavidin-conjugated enzyme and substrate, respectively, in order to increase sensitivity of the assay. Lanes B, C, D, and E are serovars icterohaemorrhagiae and patoc and urine samples I and II, respectively. Lane A contains standard molecular mass markers (sizes indicated at left). The presence of 35- to 36-kDa (block I) and 38-kDa (block II) antigens in urine samples of patients with Leptospira culture-negative, antibody-negative, MAb dot-ELISA-positive results are indicated (arrowheads).
Of the 25 Leptospira culture-positive patients (second set), serum samples of 12 patients were tested by the MAT and 10 (83.3%) were positive. MAb LD5 dot-ELISA performed on urine samples of the 25 patients collected on day 1 was positive for 16 (64.0%) patients, while the MAT of serum samples collected on the same day was positive for 4 of 23 patients (17.4%). Cumulative positivities of Leptospira antigenuria at days 2, 3, and 4 were 21 of 25 (84.0%), 24 of 25 (96.0%), and 25 of 25 (100%), respectively (Table 4).
The dot-ELISA was positive for urine samples of three of eight clinically diagnosed melioidosis patients and 1 of 120 apparently healthy individuals.
DISCUSSION
Leptospirosis is considered a reemerging infectious disease, not only for the increase in its incidence during the past recent years but also for the increased severity of the illness (9, 25, 38), which frequently leads to mortality, especially in patients with delayed diagnosis and/or patients improperly treated. In the tropical areas, leptospirosis is not readily distinguishable, based on the clinical presentation and epidemiological background of the patients, from other infections which share the same geographical areas of endemicity. As such, laboratory confirmation plays an important supplementary role to the clinical findings and helps in sorting out the differential diagnosis. However, the existing methodologies for leptospirosis diagnosis, namely, Leptospira culture from the patient's clinical specimens and the standard serological test by MAT, encounter several drawbacks (12, 30, 34); thus, simple, rapid, specific, sensitive, and inexpensive alternatives have been sought. However, most of them have been designed as antibody tests, which are less sensitive at the early phase of infection or illness, or DNA techniques, such as DNA hybridization and PCR, which are laborious and expensive and may be time-consuming; thus, these are not suitable as routine diagnostic procedures for developing countries. Leptospirosis is a disseminated bacteremic infection. During the acute phase of illness, the leptospires are present in blood and many visceral organs of the patients (31, 39). Intact bacteria and their antigens are also present in the urine (13). Thus, detection of a leptospire's antigen(s) by using specific antibodies should be a sensible strategy for the diagnosis of leptospirosis. A similar approach has been used successfully for other bacteremic infections, e.g., typhoid (4, 6, 7, 24). The antigen detection assay for typhoid fever was also used in the evaluation of treatment efficacy; i.e., the antigen test became negative shortly after successful treatment (cure of patients) (24). The same may be applied for leptospirosis.
In this study, hybridomas secreting MAbs specific to antigens of the genus Leptospira (LF9) and to pathogenic species (LD5 and LE1) were produced by hybridoma technology. Splenocytes of a mouse repeatedly immunized with sonicate of L. interrogans serovar icterohaemorrhagiae were fused with non-Ig-secreting P3x-63-Ag8.653 myeloma cells. Specificities of the MAbs were tested against a broad range of pathogenic and nonpathogenic Leptospira serovars and also a panel of not only heterologous antigens of other species of bacteria but also rickettsial, fungal, and viral antigens (Table 1). MAbs of the hybridoma LF9 (MAb LF9) were reactive only to antigens prepared from Leptospira spp., both pathogenic and nonpathogenic species, but not reactive to any other heterologous antigens tested (thus, they are genus Leptospira specific). However, the WB patterns of pathogenic and nonpathogenic sonicates recognized by the MAb LF9 were different; the WB pattern of pathogenic organisms appeared as a doublet at about 38 kDa, while the WB pattern of nonpathogenic Leptospira revealed only the upper band of the doublet (Fig. 2, block II), which indicates antigenic difference between them at this particular location. The MAb LD5 reacted to the antigens of all pathogenic Leptospira serovars and only the serovar andamana CH11 strain of L. biflexa. The Leptospira antigenic components reactive to MAb LD5 are located at about 36 kDa, and the epitopes for MAb LE1 are components at about 36 kDa and 23 kDa and lower, respectively (Fig. 2, blocks I and III). All components reactive to the MAb LF9, MAb LD5, and MAb LE1 are proteins for the reasons that they are stainable by Coomassie brilliant blue dye (Fig. 3), although they could withstand boiling for at least 30 min without altering their epitope specificities (the process of dot-ELISA for detection of antigenuria). The MAbs of all hybridoma clones are γ isotype (MAb LF9 and MAb LD5 are γ1 while MAb LE1 is γ2a); this type shows a high affinity to its target antigens. Thus, all MAbs are suitable as capture molecules for their respective epitopes in the urine samples of the patients in the antigen detection assay. However, we have chosen to use the clone LD5 as a source of MAbs in the urine antigen detection.
In this study, the sensitive and simplest version of enzyme immunoassay, i.e., dot-ELISA, was used for the detection of antigen in urine. The fact that the target antigen is relatively heat stable, allows boiling of the urine specimens prior to subjecting them to the antigen test. Boiling the urine samples eliminates the nonspecific background enzymes in the urine that would otherwise render the visual reading of the enzyme-substrate color results difficult. Also, the boiling causes more release of antigens from the intact bacteria in the urine specimens and hence increases levels of free antigenic molecules in the samples. The hydrophobic nature of the target antigen (possibly lipopolysaccharide) also enhances its binding to the NC membrane; all of which increases the assay sensitivity.
The antigen detection by the MAb LD5 dot-ELISA performed on urine samples of the patients on the first day of hospital arrival revealed 75.0% sensitivity in one set of clinically diagnosed leptospirosis patients from one hospital and 64.0% sensitivity in another group of patients from other hospitals whose specimens, later, revealed Leptospira growth by culture method. The percentage of sensitivity increased progressively as seen by testing daily follow-up urine samples until 100% sensitivity was reached by day 14 and day 7 in the two groups of patients, respectively. For the first group of patients, the IgM Dipstick assay was positive for 25 of 36 (69.4%) of the serum samples collected on the same day as the first urine samples (Table 3). All but 1 of 11 patients whose day 1 serum samples were IgM seronegative were positive by urine antigen detection in day 1 samples (Table 3). This is evidence that the detection of antigen in urine could provide diagnostic information that would be useful in directing early therapeutic intervention. By the time the second serum samples of the IgM-negative patients were taken and sent for repeated IgM detection (day 5 or 6 of hospitalization), the urine antigen test positivity became as high as 97.2%, while the IgM test was only up to 83.3% positivity. IFA performed on the IgM Dipstick-positive serum samples gave only 70.0% positivity, while the MAT was positive for 85.7% of the IgM, IFA-positive samples. Besides being more laborious, the results of the MAT, which has been a standard serological test for leptospirosis diagnosis, were obtained long after the results of the antigen detection by the dot-ELISA and cannot be utilized in treatment decisions for patients in areas where leptospirosis is endemic and where other febrile illnesses caused by other pathogens are concurrently endemic. The antigen test was positive in clinically diagnosed leptospirosis patients whose serum samples were negative for antibodies (patients 31 to 36 [Table 3]). The antigen positivity by the MAb-based dot-ELISA was proven to be accurate in detecting Leptospira antigen by the finding of the respective epitopes of Leptospira in WB analysis; this confirmed that the dot-ELISA positivity has to be considered truly positive.
In another set of patients whose specimens later revealed Leptospira growth, antigenuria was detected by the MAb-based dot-ELISA. A sensitivity of 100% was reached on the 4th day of admission, while the MAT results were obtained much later and with a lower sensitivity. It is also worthwhile to test multiple urine samples collected at shorter intervals, e.g., several samples within one day, from the same patient in order to understand the nature of the intermittent release of Leptospira antigen into the urine and to speed up the laboratory diagnosis by the antigen detection assay.
Clinical features of Leptospira infection vary greatly from mild, flu-like symptoms to fatal hemorrhages and renal failure. The finding of Leptospira antigen by MAb-based dot-ELISA in the urine sample of 1 of the 120 apparently healthy inhabitants of the area of disease endemicity might be explained as a subclinical infection in that individual, or it is also possible that this person might have been recovering from acute leptospirosis and was in the stage of Leptospira carriage or shedding. It has been reported that humans may harbor Leptospira in the kidneys and urine for a certain period after recovery from the illness, although long-term carriage seems uncommon (3). It has been documented also that in the tropical region where other acute febrile illnesses are common, leptospirosis may be clinically misdiagnosed as other infections and vice versa. Unfortunately, the urine sample of the individual in question was not adequate for WB analysis to reveal specific reactive bands. Urine samples from 3 of the 26 patients who were diagnosed with other illnesses gave positive antigen test results. These patients were clinically diagnosed with melioidosis, due to the similarity of clinical features and the high and concurrent endemicity of the two diseases in the northeast of Thailand and because they were seronegative for Leptospira antibodies by IgM Dipstick assay. WB analysis of the SDS-PAGE-separated concentrated urine samples of these three patients against MAb LD5 and MAb LF9 revealed proper antigen-antibody reactive bands at approximately 35 kDa and about 38 kDa, respectively (data not shown), indicating that these three urine samples contained specific antigens of pathogenic Leptospira; thus, the patients had leptospirosis but were misdiagnosed as having melioidosis.
Besides offering higher sensitivity, the urine antigen test by dot-ELISA has many advantages over the current methodologies for leptospirosis diagnosis. These include no requirement for maintaining living Leptospira cultures; no need for invasive procedures to be performed on the patients upon specimen collection; and the facts that several samples can be tested at the same time without significant increase in the turnaround time, that the results are read visually without a microscope or spectrophotometer, and that the test results can be preserved for a retrospective study or other purposes. The dot-ELISA is simple, inexpensive, and rapid (not longer than 95 min). It can be performed in areas where laboratory facilities are limited.
Acknowledgments
We acknowledge with thanks financial support from the Thailand Research Fund (TRF), Bangkok, Thailand; Science Development and Management Company Ltd. (SDM), Bangkok, Thailand; and the National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency (NSTDA), Ministry of Sciences, Technology, and Environment, Thailand.
Thanks are due to Montip Getayakamin, AFRIMS, Bangkok, Thailand, for the supply of BALB/c mice; Duangporn Poonsuksombat, AFRIMS, for her kind help in Leptospira culturing; and Mark Roselieb of SDM for reading the manuscript. Many heterologous antigens were kind gifts from Varee Vongchotikul and Pornsawan Amarapal Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Information on leptospirosis in Thailand that was provided by Departments of Epidemiology, Communicable Diseases Control and Medical Sciences, Ministry of Public Health, Thailand, is greatly appreciated.
REFERENCES
- 1.Adler, B., A. M. Murphy, S. A. Locarnini, and S. Faine. 1980. Detection of specific anti-leptospiral immunoglobulins M and G in human serum by solid-phase enzyme-linked immunosorbent assay. J. Clin. Microbiol. 11:452-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arimitsu, Y., E. Kmet, Y. Ananyina, G. Baranton, I. R. Ferguson, L. Smythe, and W. J. Terpstra. 1994. Evaluation of one-point microcapsule agglutination test for diagnosis of leptospirosis. Bull. W. H. O. 72:395-399. [PMC free article] [PubMed] [Google Scholar]
- 3.Bal, A. E., C. Gravekamp, R. A. Hartskeerl, J. De Meza-Brewster, H. Korver, and W. J. Terpstra. 1994. Detection of leptospires in urine by PCR for early diagnosis of leptospirosis. J. Clin. Microbiol. 32:1894-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchuin, N., H. Appassakij, S. Sarasombath, S. Manatsathit, B. Rungpitarangsi, P. Komolpit, and T. Sukosol. 1987. Detection of Salmonella typhi protein antigen in serum and urine: a value for diagnosis of typhoid fever in an endemic area. Asian Pac. J. Allerg. Immunol. 5:155-159. [PubMed] [Google Scholar]
- 5.Chaicumpa, W., P. Srimanote, Y. Sakolvaree, T. Kalambaheti, M. Chongsa-nguan, P. Tapchaisri, B. Eampokalap, P. Moolasart, G. B. Nair, and P. Echeverria. 1998. Rapid diagnosis of cholera caused by Vibrio cholerae O139. J. Clin. Microbiol. 36:3595-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaicumpa, W., W. Thin-Inta, S. Khusmith, P. Tapchaisri, P. Echeverria, T. Kalambaheti, and M. Chongsa-Nguan. 1988. Detection with monoclonal antibody of Salmonella typhi antigen 9 in specimens from patients. J. Clin. Microbiol. 26:1824-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaicumpa, W., Y. Ruangkunaporn, D. Burr, M. Chongsa-Nguan, and P. Echeverria. 1992. Diagnosis of typhoid fever by detection of Salmonella typhi antigen in urine. J. Clin. Microbiol. 30:2513-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, C. D., A. D. Alexander, and L. C. Murphy. 1957. Evaluation of the hemolytic test in the serodiagnosis of human leptospirosis. J. Infect. Dis. 101:201-218. [DOI] [PubMed] [Google Scholar]
- 9.Daher, E., D. M. Zanetta, M. B. Cavalcante, and R. C. Abdulkader. 1999. Risk factors for death and changing patterns in leptospirosis acute renal failure. Am. J. Trop. Med. Hyg. 61:630-634. [DOI] [PubMed] [Google Scholar]
- 10.Douglin, C. P., C. Jordan, R. Rock, A. Hurley, and P. N. Levett. 1997. Risk factors for severe leptospirosis in the parish of St. Andrew, Barbados. Emerg. Infect. Dis. 3:78-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont, H., D. Dupont-Perdrizet, J. L. Perie, S. Zehner-Hansen, B. Jarrige, and J. B. Daijardin. 1997. Leptospirosis: prognostic factors associated with mortality. Clin. Infect. Dis. 25:720-724. [DOI] [PubMed] [Google Scholar]
- 12.Faine, S. 1982. Guidelines for the control of leptospirosis. World Health Organization, Geneva, Switzerland.
- 13.Farr, R. W. 1995. Leptospirosis. Clin. Infect. Dis. 21:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Galton, M. M., D. K. Powers, A. M. Hall, and R. G. Cornell. 1958. A rapid microscopic-slide screening test for the serodiagnosis of leptospirosis. Am. J. Vet. Res. 19:505-512. [PubMed] [Google Scholar]
- 15.Gussenhoven, G. C., M. A. W. G. van der Hoorn, M. G. A. Goris, W. J. Terpstra, R. A. Hartskeerl, B. W. Mol, C. W. van Ingen, and H. L. Smits. 1997. LEPTO Dipstick, a dipstick assay for detection of Leptospira specific immunoglobulin M antibodies in human sera. J. Clin. Microbiol. 35:92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, R. C., and V. G. Harris. 1967. Differentiation of pathogenic and saprophytic leptospires. J. Bacteriol. 94:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kee, S. H., I. S. Kim, M. S. Choi, and W. H. Chang. 1994. Detection of leptospiral DNA by PCR. J. Clin. Microbiol. 32:1035-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Levett, P. N., and C. U. Whittington. 1998. Evaluation of the indirect hemagglutination assay for diagnosis of acute leptospirosis. J. Clin. Microbiol. 36:11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masri, S. A., P. T. Nguyen, S. P. Gale, C. J. Howard, and S. C. Jung. 1997. A polymerase chain reaction assay for the detection of Leptospira spp. in bovine semen. Can. J. Vet. Res. 61:15-20. [PMC free article] [PubMed] [Google Scholar]
- 21.Merien, F., P. P. Amouriaux, P. Perolat, G. Baranton, and I. Saint Girons. 1992. Polymerase chain reaction for detection of Leptospira spp. in clinical samples. J. Clin. Microbiol. 30:2219-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milner, A. R., K. B. Jackson, K. Woodruff, and I. J. Smart. 1985. Enzyme-linked immunosorbent assay for determining specific immunoglobulin M in infections caused by Leptospira interrogans serovar hardjo. J. Clin. Microbiol. 22:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappas, M. G., W. R. Ballou, M. R. Gray, E. T. Takafuji, R. N. Miller, and W. T. Hockmeyer. 1985. Rapid serodiagnosis of leptospirosis using the IgM-specific dot-ELISA: comparison with the microscopic agglutination test. Am. J. Trop. Med. Hyg. 34:346-354. [DOI] [PubMed] [Google Scholar]
- 24.Quang, N. N., P. Tapchaisri, M. Chongsa-Nguan, V. V. Cao, T. T. Doan, Y. Sakolvaree, P. Srimanote, and W. Chaicumpa. 1997. Diagnosis of enteric fever caused by Salmonella spp. in Vietnam by a monoclonal antibody-based dot-blot ELISA. Asian Pac. J. Allerg. Immunol. 15:205-212. [PubMed] [Google Scholar]
- 25.Sehgal, S. C., M. V. Murhekar, and A. P. Sugunan. 1995. Outbreak of leptospirosis with pulmonary involvement in north Andaman. Indian J. Med. Res. 102:9-12. [PubMed] [Google Scholar]
- 26.Silva, M. V., P. M. Nakamura, E. D. Carmargo, L. Batista, A. J. Vaz, E. C. Romero, and A. P. Brandao. 1997. Immunodiagnosis of human leptospirosis by dot-ELISA for the detection of IgM, IgG and IgA antibodies. Am. J. Trop. Med. Hyg. 56:650-655. [DOI] [PubMed] [Google Scholar]
- 27.Smits, H. L., C. K. Eapen, S. Sugathan, M. Kuriakose, M. H. Gasem, C. Yersin, D. Sasaki, B. Pujianto, M. Vestering, T. H. Abdoel, and G. C. Gussenhoven. 2001. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin. Diagn. Lab. Immunol. 8:166-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulzer, C. R., J. W. Glosser, F. Rogers, W. L. J. Jones, and M. Fris. 1975. Evaluation of an indirect hemagglutination test for the diagnosis of human leptospirosis. J. Clin. Microbiol. 2:218-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terpstra, W. J., G. J. Schoone, and J. ter Schegget. 1986. Detection of leptospiral DNA by nucleic acid hybridisation with 32P- and biotin-labelled probes. J. Med. Microbiol. 22:23-28. [DOI] [PubMed] [Google Scholar]
- 30.Thiermann, A. B. 1984. Leptospirosis: current development and trends. J. Am. Vet. Med. Assoc. 184:722-725. [PubMed] [Google Scholar]
- 31.Thiermann, A. B. 1984. Isolation of leptospires in diagnosis of leptospirosis. Mod. Vet. Pract. 65:758-759. [PubMed] [Google Scholar]
- 32.Torten, M., E. Shenberg, and J. van der Horden. 1966. The use of immunofluorescence in the diagnosis of human leptospirosis. J. Infect. Dis. 166:537-543. [DOI] [PubMed] [Google Scholar]
- 33.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner, L. H. 1968. Leptospirosis II. Trans. R. Soc. Trop. Med. Hyg. 62:880-899. [DOI] [PubMed] [Google Scholar]
- 35.Van Eys, G. J. J. M., C. Gravekamp, M. J. Gerritsen, W. Quint, M. T. E. Cornelissen, J. ter Schegget, and W. J. Terpstra. 1989. Detection of leptospires in urine by polymerase chain reaction. J. Clin. Microbiol. 27:2258-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watt, G., L. M. Alquiza, L. P. Padre, M. L. Tuazon, and L. W. Laughlin. 1988. The rapid diagnosis of leptospirosis: a prospective comparison of the dot enzyme-linked immunosorbent assay and the genus-specific microscopic agglutination test at different stages of illness. J. Infect. Dis. 157:840-842. [DOI] [PubMed] [Google Scholar]
- 37.Winslow, W. E., D. J. Merry, M. C. Pirc, and P. Devine. 1997. Evaluation of enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody in diagnosis of human leptospiral infection. J. Clin. Microbiol. 35:1938-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yersin, C., P. Bovet, F. Merien, J. Clement, M. Laille, M. Van Ranst, and P. Perolat. 2000. Pulmonary haemorrhage as a predominant cause of death in leptospirosis in Seychelles. Trans. R. Soc. Trop. Med. Hyg. 94:71-76. [DOI] [PubMed] [Google Scholar]
- 39.Zaki, S. R., and W. J. Shieh. 1996. Leptospirosis associated with outbreak of acute febrile illness and pulmonary haemorrhage, Nicaragua, 1995. Lancet 347: 535-536. [DOI] [PubMed] [Google Scholar]