Abstract
The growth inhibitory effects of D-glucosamine hydrochloride (GlcNH2·HCl), D-glucosamine (GlcNH2) and N-acetyl glucosamine (NAG) on human hepatoma SMMC-7721 cells in vitro were investigated. The results showed that GlcNH2·HCl and GlcNH2 resulted in a concentration-dependent reduction in hepatoma cell growth as measured by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. This effect was accompanied by a marked increase in the proportion of S cells as analyzed by flow cytometry. In addition, human hepatoma SMMC-7721 cells treated with GlcNH2·HCl resulted in the induction of apoptosis as assayed qualitatively by agarose gel electrophoresis. NAG could not inhibit the proliferation of SMMC-7721 cells. GlcNH2·HCl exhibited antitumor activity against Sarcoma 180 in Kunming mice at dosage of 125~500 mg/kg, dose of 250 mg/kg being the best. GlcNH2·HCl at dose of 250 mg/kg could enhance significantly the thymus index, and spleen index and could promote T lymphocyte proliferation induced by ConA. The antitumor effect of GlcNH2·HCl is probably host-mediated and cytocidal.
Keywords: D-glucosamine hydrochloride, D-glucosamine, N-acetyl glucosamine, Antitumor, Human hepatoma cell, Sarcoma 180
INTRODUCTION
Chitin, a kind of poly-β(1,4)-N-acetyl-D-glucosamine, is a natural biopolymer present in the exoskeleton of crustaceans and in cell walls of fungi, insects and yeast. A series of oligomers and monosaccharides, such as GlcNH2 and NAG, can be obtained by either chemical or enzymatic hydrolysis of chitin and chitosan (Akiyama et al., 1995). Besides hydroxyl, there are other functional groups in GlcNH2 and NAG, for example, amido and acetylamino. Many derivatives can thus be obtained from them, most of which are known to have various biological activities including antitumor activities (Chen et al., 2005), increased protective effects against infection by some pathogens (Martin et al., 2003; Wang and Chen, 2005) and antimicrobial activities (Chen, 2001).
Wang et al.(2003a; 2003b) reported that NAG, GlcNH2·HCl and GlcNH2 could induce proliferation of leukemia K562 cells and make them differentiate toward macrophage. GlcNH2 at concentration of certain range could kill tumor cells without influencing normal cells (Friedman and Skehan, 1980). It is therefore postulated that combination of GlcNH2 with membrane-active drugs may have the potential to kill tumor cells, especially for neuro-oncology. Dong et al.(2004) reported that glucosamine sulphate could inhibit proliferation of leukemia HL60 cells and induce the differentiation of HL60 cells toward the granulocytic or monocytic lineage. In addition, glucosamine sulphate has been proved to be useful in the therapy of osteoarthritis (Reginster et al., 2001).
However, there is no report on the direct cytotoxicity of D-glucosamine and its derivatives to human hepatoma cell. Moreover, there are only a few reports on the antitumor activity of D-glucosamine and its derivatives in murine models. Much controversy on its mechanism still exists. This study was aimed at investigating the inhibitory effects of GlcNH2·HCl, GlcNH2 and NAG on human hepatoma SMMC-7721 cells and the antitumor activity of GlcNH2·HCl in Kunming mice implanted with solid tumor Sarcoma 180.
MATERIALS AND METHODS
Chemicals and reagents
D-glucosamine hydrochloride (GlcNH2·HCl), D-glucosamine (GlcNH2) and N-acetyl glucosamine (NAG) were prepared and purified (purity≥99%) by our lab according to previous methods (Ingle et al., 1973). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Concanavalin A (ConA) were purchased from Sigma Chemical Co. (USA). All other chemicals were analytical reagents.
Cells and culture
Human hepatoma cell line SMMC-7721 was procured from Shanghai Institute of Cell Biology, China. Kunming male mice (8-week-old) were obtained from the Shandong Academy of Medical Sciences. The cells were cultured in RPMI-1640 medium supplemented with 15% BCS, 100 U/ml penicillin and 100 U/ml streptomycin at 37 °C in a 5% CO2 atmosphere and then seeded in 96-well plates (Costar) at initial density of (5~7)×105 cells/ml and incubated for 24 h, then treated with the test glucosamine samples at final concentration of 10~1000 μg/ml respectively. Untreated cells were used as controls. Microplates were incubated at 37 °C in a 5% CO2 atmosphere for 24 to 120 h. At the indicated treatment times, observation and microphotography of cells were done by IX 70 inverted microscope (Olympus).
MTT cell viability assay
The cytotoxicity of glucosamine samples against SMMC-7721 cells in vitro was examined with MTT assay according to previously described procedures (Mosmann, 1983; Nakashima et al., 1989; Wilson et al., 1990). Briefly, 40 μl MTT (5 mg/ml) solution was added to each well of 96-well plates containing (5~7)×104 cells treated with different concentrations of GlcNH2·HCl, GlcNH2 and NAG for 24 to 120 h. The reaction was stopped after 4 h incubation by extracting the solution and adding 140 μl of 0.04 mol/L HCl in isopropanol. The absorbance of each well was measured by an ELISA reader (Multiscan MK3, Thermo Labsystems) using a test wavelength of 490 nm. Each concentration treatment was done in triplicate wells. Then the results were expressed as the inhibition ratio. δ=(A−B)/A×100%, where A and B were the absorbance of the control and sample groups after 120 h incubation respectively.
DNA extraction and agarose gel electrophoresis
DNA was isolated as described previously (Sambrook and Russell, 2001). Briefly, SMMC-7721 cells treated with GlcNH2·HCl, GlcNH2, NAG and untreated cells were collected and washed with ice-cold PBS. Then, the cells were lysed in a solution containing 50 mmol/L Tris-HCl (pH 8.0), 10 mmol/L EDTA, 10 mmol/L NaCl, 0.5% SDS and 0.5 mg/ml proteinase K and were incubated at 60 °C for 1 h. After the addition of RNase A (final concentration 0.25 mg/ml), the cells were incubated at 37 °C for 1 h. The lysate was extracted with equal volume of phenol/chloroform (1:1, v/v) and then precipitated with ethanol. The extracted DNA samples were dissolved in TE buffer and the amount of DNA equivalent to content of (3~5)×105 cells was electrophoresed at 50 V in 1% agarose gel. The presence of DNA in the gel was visualized by ethidium bromide and was photographed under UV illumination (YLN2K, YALIEN Co.).
Cell cycle analysis
After washing twice with PBS, cells (1×106) treated and untreated with GlcNH2·HCl were fixed in 70% ice-cold ethanol immediately for 4 h. Fixed cells were washed in phosphate-buffered saline with 0.1% Triton-X and then stained with 20 μg/ml propidium iodide (Sigma, USA) and 200 U/ml RNase A (Lin et al., 1996). The DNA content was measured by FACS Vantage Flow Cytometer (Becton Dickinson, USA) and analyzed by ModFit LT software.
In vivo antitumor activity assay
Sarcoma 180 tumor ascites (2×107~6×107 cells/ml) were subcutaneously inoculated into 8-week-old Kunming male mice armpit at 0.2 ml/mouse. The mice were divided randomly into control and GlcNH2·HCl groups. GlcNH2·HCl dissolved in saline was orally administered to the tumor-bearing mice once daily for 10 d at 24 h after tumor inoculation. The same volume of saline was orally administered to the control mice. The tumor was allowed to grow on the mice for 10 d before it was removed from the animal and weighed. The spleen and thymus of the mice were also removed and weighed to obtain the index of the spleen and thymus. The antitumor activity of GlcNH2·HCl was expressed as an inhibition ratio calculated as [(A−B)/A]×100%, where A and B were the average tumor weights of the control and treated groups, respectively. Spleen index (mg/g)=spleen weight/body weight, thymus index (mg/g)=thymus weight/body weight.
Mitogenic response assay
For mitogenic response assay, splenic lymphocytes (8×106 per well) from tumor-bearing Kunming mice administered with indicated doses of GlcNH2·HCl and the control mice were co-cultured in the presence of ConA (7.0 μg/ml) in 96-well culture plates for 72 h. Cytotoxicity to lymphocyte proliferation was examined by MTT assay. The cultures were incubated with 0.5 μg/ml MTT solution for the last 4 h incubation. Then, the supernatants of the cultures were mixed with 100 μl of dimethyl sulfoxide (DMSO). The absorbance of each well was measured by an ELISA reader (Multiscan MK3, Thermo Labsystems) using a test wavelength of 570 nm. The proliferation of lymphocyte=A−B, where A was the OD570 of well with ConA, and B was the OD570 of well without ConA.
Statistical analysis
Data are presented as the arithmetic mean±standard deviation (SD) of triplicate samples. Statistical analysis was performed using SPSS 10.0. ANOVA was used to analyze statistical comparisons between groups. Differences with P-values less than 0.05 were considered to be statistically significant.
RESULTS
Inhibitory effects of glucosamine samples on the proliferation of SMMC-7721 cells in vitro
It was found that control cells achieved a plateau phase after an incubation of 96 h. The cell viability at 120 h approximated that at 96 h. From 24 h to 72 h, the cell viability of SMMC-7721 treated with different concentrations of GlcNH2·HCl and GlcNH2 kept rising as the control, but the cell viability declined obviously after 72 h at concentrations of 500 μg/ml and 1000 μg/ml and became minimum at 120 h. The growth of cells treated with different concentrations of NAG was similar to that of the control.
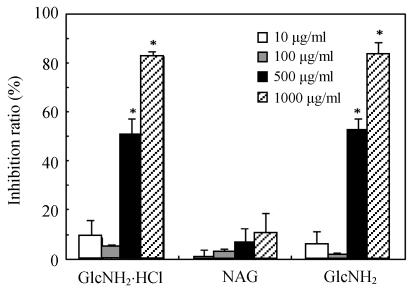
Fig.1 shows the inhibition of cell growth after treatment with GlcNH2·HCl, GlcNH2 and NAG at the concentrations of 10~1000 μg/ml for 120 h. Treatment with GlcNH2·HCl and GlcNH2 for 120 h resulted in a concentration-dependent inhibition in SMMC-7721 cell growth. The inhibition ratios against SMMC-7721 cells of GlcNH2·HCl and GlcNH2 at concentration of 500 μg/ml were 50% and 52%, and reached 82% and 83% at concentration of 1000 μg/ml, respectively. However, NAG hardly showed growth inhibition even at the concentration of 1000 μg/ml.
Fig. 1.
Inhibition of SMMC-7721 cells proliferation by various concentrations of GlcNH2·HCl, GlcNH2 and NAG for 120 h (* P<0.05)
Changes in cell morphology
As shown in Figs.2a~2c, the control cell was morphologically hexagon-like, and its cytoplasm was clear. Adjacent cells were kept compact and there was no contact inhibition. Figs.2d~2i demonstrate the morphological changes in SMMC-7721 cells treated with GlcNH2·HCl and GlcNH2 at concentration of 1000 μg/ml. Cells turned fibriform after 72 h treatment. Many granules occurred in cytoplasm, and cell boundary became blurred after 120 h incubation. Besides fragments, agglomerate chromatin was also observed.
Fig. 2.
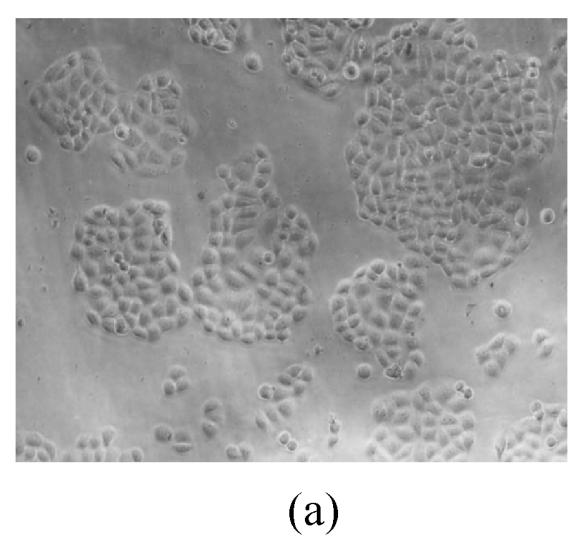
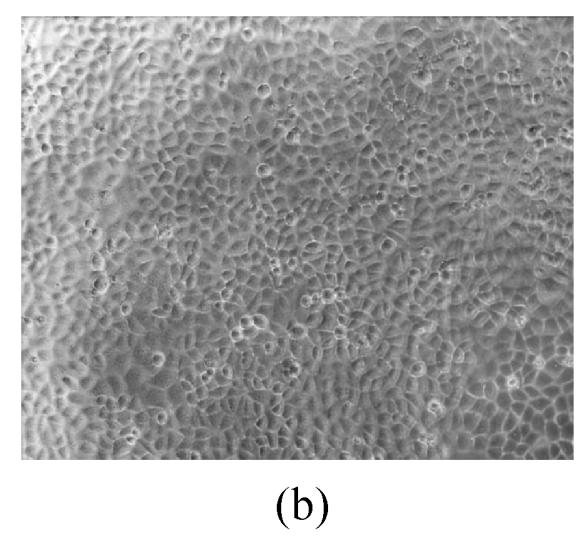
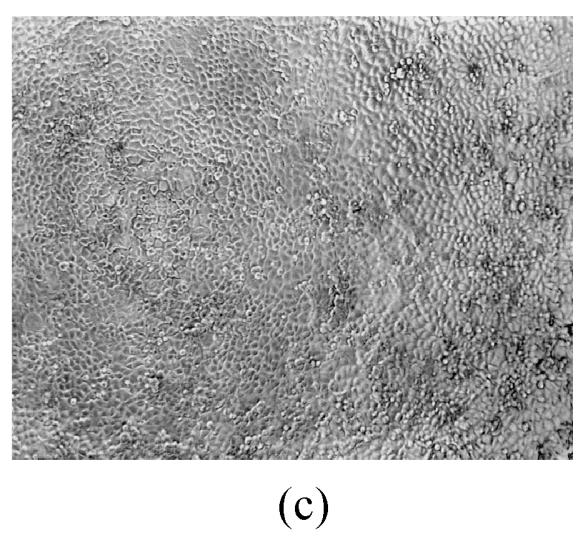
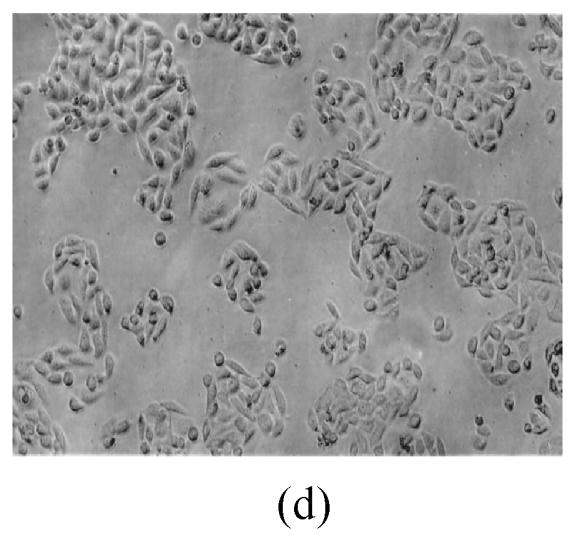
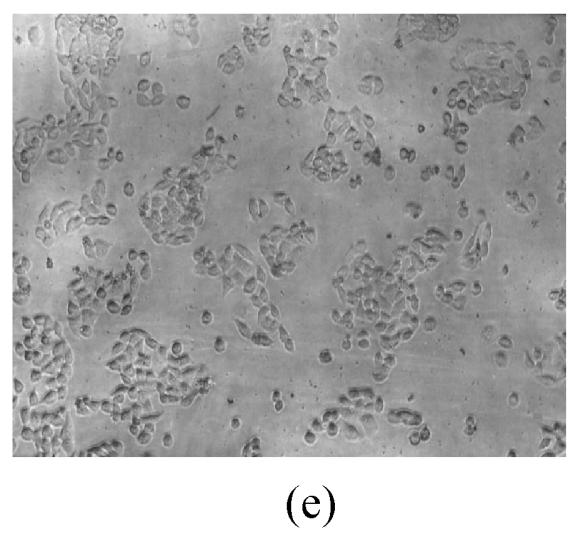
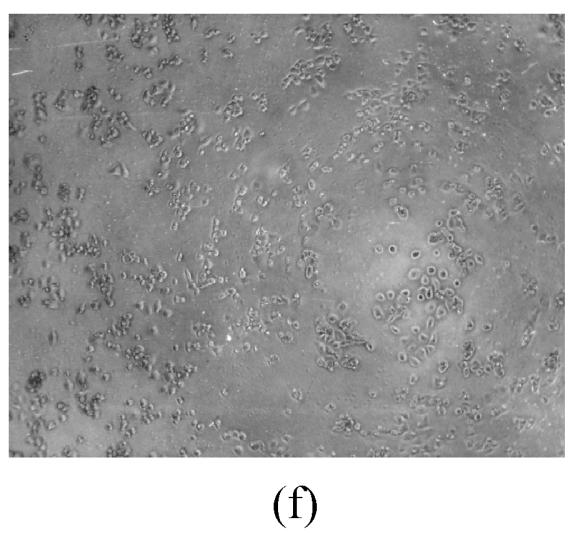
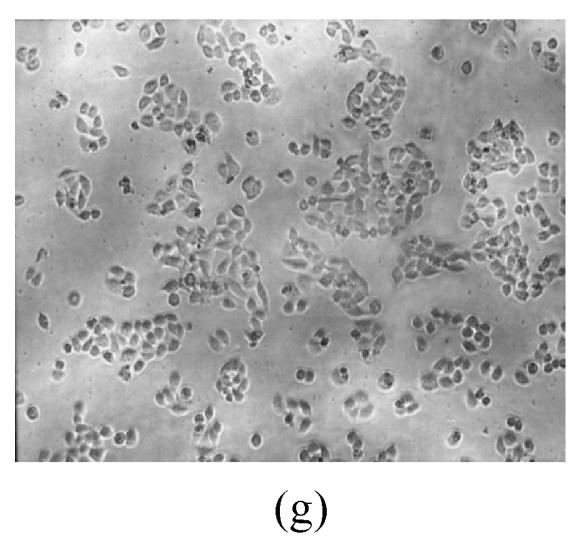
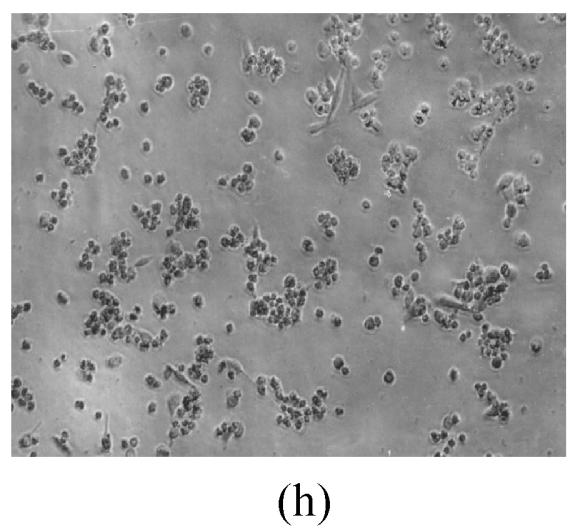
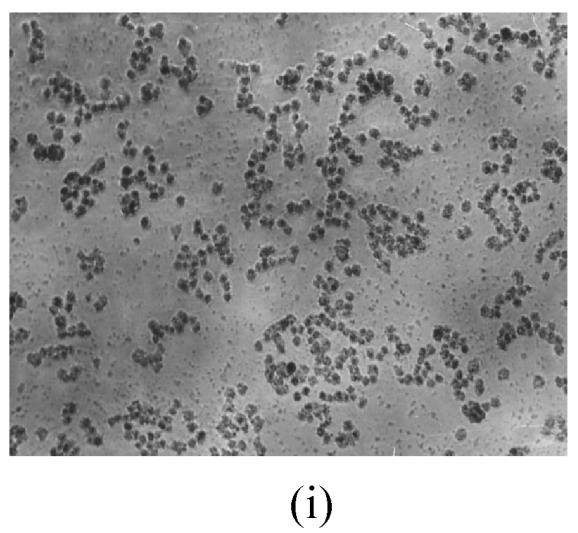
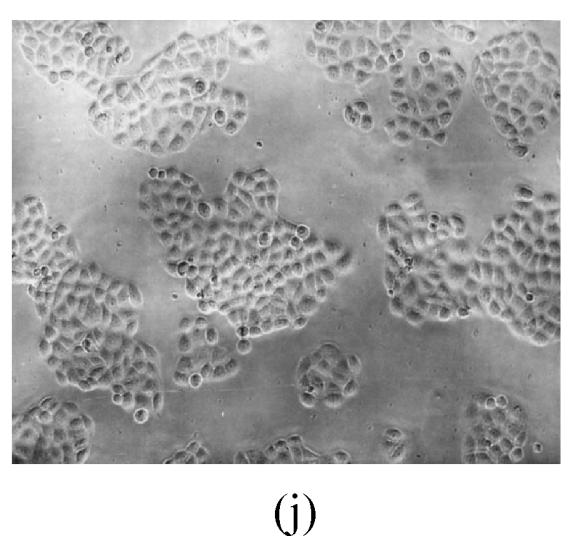
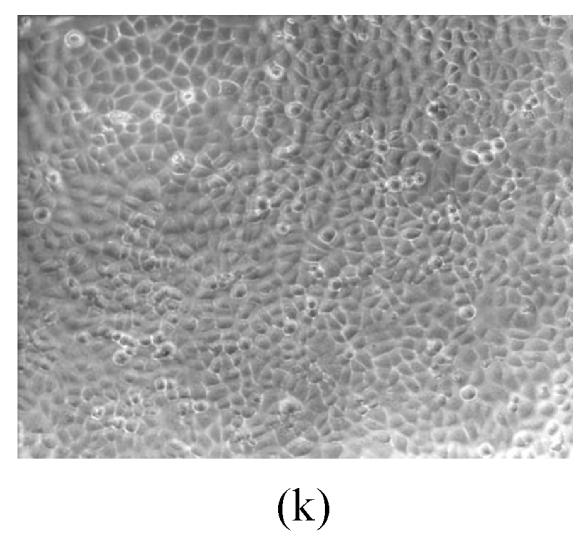
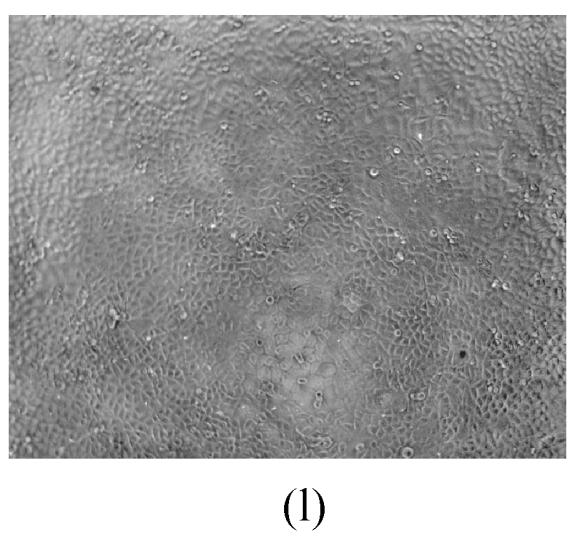
The pictures of SMMC-7721 cells treated or untreated with the test glucosamine samples. (a), (b), (c) Cells untreated for incubation of 24, 72, 120 h, respectively; (d), (e), (f) Cells treated with 1000 μg/ml GlcNH2·HCl for 24, 72, 120 h, respectively; (g), (h), (i) Cells treated with 1000 μg/ml GlcNH2 for 24, 72, 120 h, respectively; (j), (k), (l) Cells treated with 1000 μg/ml NAG for 24, 72, 120 h, respectively
In contrast, the cell growth of NAG group was similar to that of the control as shown in Figs.2j~2l.
DNA fragmentation pattern
DNA integrity of SMMC-7721 cells treated with 500 μg/ml GlcNH2·HCl was analyzed to assess apoptosis. Fig.3 shows that these cells did not display the inter-mucleosomal DNA fragmentation pattern characteristic of apoptosis after incubation for 72~168 h. Lanes C-E contained DNA samples of GlcNH2·HCl-treated cells and showed a slight smear.
Fig. 3.
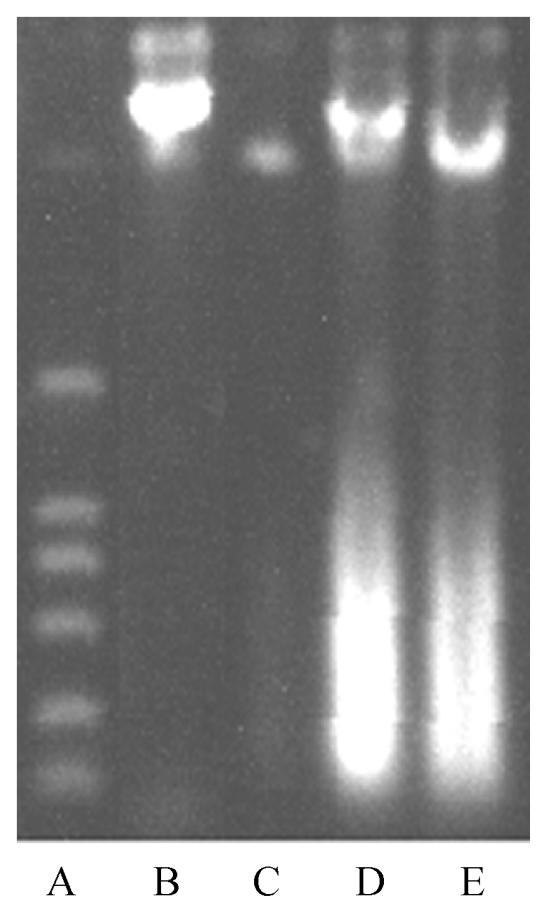
Agarose gel electrophoresis of DNA extracted from control cells and cells treated with 500 μg/ml GlcNH2·HCl for 72, 120 and 168 h
A: DNA marker; B: Control; C: 72 h; D: 120 h; E: 168 h
Effect of GlcNH2·HCl on cell cycle changes of SMMC-7721 cells
Cells treated with 500 μg/ml GlcNH2·HCl for 72 h were arrested in S phase and had decreased number of cells in G2 phase (Table 1). Increase in GlcNH2·HCl concentration and treatment time may increase the percentage of cells with apoptotic DNA content measurement (data not shown).
Table 1.
Cell cycle analysis of SMMC-7721 cells after treatment with 500 μg/ml GlcNH2·HCl for 72 h
| Group | G0/G1 (%) | S (%) | G2/M (%) |
| Control | 74.43±1.68 | 18.11±0.86 | 7.46±1.37 |
| GlcNH2·HCl | 70.61±0.93 | 28.44±1.41 | 0.95±0.45 |
Antitumor activity of GlcNH2·HCl in vivo
Table 2 shows the antitumor activity of GlcNH2·HCl in vivo. GlcNH2·HCl at intermediate dose (250 mg/kg) had the highest inhibition ratio on Sarcoma 180 tumor growth and that the inhibition ratio declined at higher dose (500 mg/kg). GlcNH2·HCl at dose of 250 mg/kg could also promote obviously the T lymphocyte proliferation induced by ConA, as well as the thymus index and spleen index. It suggests that GlcNH2·HCl might enhance body immune function at a certain dose.
Table 2.
Effects of GlcNH2·HCl on tumor growth and immune function in Sarcoma 180 tumor-bearing mice
| Group | Dose ((mg/kg)·d) | Tumor weight (g) | Inhibition ratio (%) | Thymus index (mg/g) | Spleen index (mg/g) | Lymphocyte transformation (OD570) |
| Normal control | – | – | – | 3.13±0.81 | 5.67±1.58 | 0.16±0.04 |
| Tumor control | – | 0.97±0.41 | – | 2.87±0.39 | 9.51±2.13 | 0.13±0.04 |
| GlcNH2·HCl | 125×10 | 0.70±0.46* | 27.84 | 3.11±0.54* | 9.65±2.31 | 0.16±0.11 |
| 250×10 | 0.64±0.15* | 34.02 | 3.21±1.01* | 9.89±1.01* | 0.17±0.03* | |
| 500×10 | 0.69±0.38* | 29.33 | 3.14±1.00* | 10.44±1.70* | 0.20±0.05* |
P<0.05 vs tumor control
DISCUSSION
Studies showed that the antitumor activities of chitin and chitosan oligomers on the growth inhibition of tumor cells occurred via an immuno-enhancing effect (Tsukada et al., 1990). The study reported herein revealed the direct inhibitory effects of GlcNH2·HCl and GlcNH2 on the proliferation of human hepatoma cell line SMMC-7721 in vitro might involve cell cycle arrest, though the exact mechanism of action is still unknown.
Glucosamine could be converted to uridine diphosphate-N-acetyl glucosamines. This sugar is used for O-linked glycosylation of several proteins, including chromatin proteins, transcription factors, nuclear pore proteins, and certain types of cytoskeletal proteins, leading to alteration in their biological activity (Kearse and Hart, 1991). Furthermore, glucosamine has been demonstrated to play an important role in the detoxification of liver and kidney, and have biological activities such as liver-protecting and antimicrobial activities in vivo (Muzzarelli, 1993). Such properties of glucosamine led us to hypothesize that hexosamine would be involved in the glycosylation of nucleoplasmic and cytoplasmic proteins, which is a regulatory modification (Reason et al., 1992). In addition, our results are similar to those reported in other studies, which indicates that glucose and glucosamine could induce autocrine TGF-β activity (Kolm-Litty et al., 1998).
The lack of inter-nucleosomal DNA degradation as shown in Fig.3 could suggest the occurrence of necrosis. However, the lower percentage of apoptotic cells could remain undetectable as DNA fragments in the gel electrophoretic analysis (Kosmider et al., 2004). Borner et al.(1997) reported that an increase of membrane permeability would result in the loss of small DNA fragments. Ormerod et al.(1994) concluded that apoptotic cells would eventually undergo ‘secondary’ necrosis and the consequent non-specific degradation of their DNA would account for the slight smearing of the DNA upon gel electrophoresis. These suggest that apoptotic cell death did not require degradation of DNA into nucleosomal-sized fragments (Schulze-Osthoff et al., 1994; Bortner et al., 1995; Sakahira et al., 1999).
Our investigation also revealed that NAG hardly showed any inhibitory effect on SMMC-7721 cells. It implies the NH2 group or positive NH2 group would be necessary for the inhibitory effect of glucosamine in vitro. A possible ionic interactions between the positively-charged NH2 group in GlcNH2·HCl and negatively-charged sialic acid residue on tumor cell surface would possibly result in a change in the ionic environment of the cell membrane, which is important in maintaining cell integrity and cell growth (Olsen et al., 1989; Santini et al., 1997; Schipper et al., 1996). Thus, it is the lack of the amino group in NAG that may be responsible for its non-inhibitory effect on SMMC-7721 cells. Moreover, GlcNH2·HCl or GlcNH2 may be incorporated into the synthetic pathway of glycoproteins, and subsequently interferes with the normal cell glucose metabolism and protein synthetic rates (Chandy and Sharma, 1990).
The real role of GlcNH2·HCl in the body of tumor-bearing mice has remained unknown. The antitumor activity of GlcNH2·HCl may be due to its cytocidal and immunomodulating properties. It was reported that the median lethal intravenous dose of GlcNH2·HCl to a mouse is 1100 mg/kg (Ouyang et al., 2000), which is much higher than the high dose of 500 mg/kg in our in vivo experiments. Borderline efficacy could be attributed to the highest inhibition ratio of medium dose, and doses between 250~500 mg/kg should be set to find the exact dose having the best antitumor activities in vivo. The pharmacokinetics of GlcNH2·HCl in vivo should also be studied in further investigations.
It is known that apoptotic breakdown of cellular structures is largely mediated by caspases. Caspase-3 is involved in the cleavage of Nuclear Mitotic Apparatus protein and lamins either directly or by activating other proteases (Hengartner, 2000; Kivinen et al., 2005). It can be detected at different stages of apoptosis. Hence, caspase-3 will be detected to clarify the plausible cellular mechanisms of the antiproliferative activity of GlcNH2·HCl in our further study. The effect of GlcNH2·HCl on immune function in tumor-bearing mice will also be more investigated.
Footnotes
Project (No. 2001AA625050) supported by the Hi-Tech Research and Development Program (863) of China
References
- 1.Akiyama K, Kawazu K, Kobayashi A. A novel method for chemo-enzymatic synthesis of elicitor-active chitosan oligomers and partially N-deacetylated chitin oligomers using N-acylated chitotrioses as substrates in a lysozyme-catalyzed transglycosylation reaction system. Carbohydrate Research. 1995;279:151–160. doi: 10.1016/0008-6215(95)00288-X. [DOI] [PubMed] [Google Scholar]
- 2.Borner MM, Jonocourt F, Hotz MA. Similarity of apoptosis induction by 2-chlorodeoxyadenosine and cisplatin in human mononuclear blood cells. British Journal of Cancer. 1997;76(11):1448–1454. doi: 10.1038/bjc.1997.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bortner CD, Cidlowski NBE, Oldenburg JA. The role of DNA fragmentation in apoptosis. Trends in Cell Biology. 1995;5(1):21–26. doi: 10.1016/S0962-8924(00)88932-1. [DOI] [PubMed] [Google Scholar]
- 4.Chandy T, Sharma CP. Chitosan―as a biomaterial. Journal of Biomaterials, Artificial Cells & Artificial Organs. 1990;18(1):1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 5.Chen X. Study of antimicrobial effects of D-glucosamine hydrochloride. Fine Chemicals. 2001;18(2):78–79. (in Chinese) [Google Scholar]
- 6.Chen Q, Yang F, Du YG. Synthesis of a C-3-symmetric (1→6)-N-acetyl-beta-D-glucosamine octadecasaccharide using click chemistry. Carbohydrate Research. 2005;340(16):2476–2482. doi: 10.1016/j.carres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Dong BX, Wang Z, Liang R, Chen XQ, Huang GS, Qiao Y, Wang AQ. Inhibition of proliferation and induction of differentiation of leukemia cell line HL60 by glucosamine sulphate. Journal of the Fourth Military Medical University. 2004;25(5):424–427. (in Chinese) [Google Scholar]
- 8.Friedman SJ, Skehan P. Membrane-active drugs potentiate the killing of tumor cell by D-glucosamine. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(2):1172–1176. doi: 10.1073/pnas.77.2.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengartner MG. The biochemistry of apoptosis. Nature. 2000;407(6805):770–775. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 10.Ingle TR, Vaidya SH, Pai MU. Production of D-glucosamine hydrochlo ride (GAH) from fish canning waste. Research and Industry. 1973;18(2):54–56. [Google Scholar]
- 11.Kearse KP, Hart GW. Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(5):1701–1705. doi: 10.1073/pnas.88.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivinen K, Kallajoki M, Taimen P. Caspase-3 is required in the apoptotic disintegration of the nuclear matrix. Experimental Cell Research. 2005;311(1):62–73. doi: 10.1016/j.yexcr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor β1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. Journal of Clinical Investigation. 1998;101(1):160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosmider B, Osiecka R, Ciesielska E, Szmigiero L, Zyner E, Ochocki J. Induction of apoptosis and necrosis in lymphocytes by the cis-Pt(II) complex of 3-aminoflavone in comparison with cis-DDP. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2004;558(1-2):169–179. doi: 10.1016/j.mrgentox.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Lin PS, Ho KC, Yang SJ. Tirapazamine (SR 4233) interrupts cell cycle progression and induces apoptosis. Cancer Letters. 1996;105(2):249–255. doi: 10.1016/0304-3835(96)04292-9. [DOI] [PubMed] [Google Scholar]
- 16.Martin GG, Castro C, Moy N, Rubin N. N-acetyl-D-glucosamine in crustacean hemocytes; possible functions and usefulness in hemocyte classification. Invertebrate Biology. 2003;122(3):265–270. [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–56. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Muzzarelli RAA. In vivo Biochemical Significance of Chitin-Based Medical Items. In: Dumitriu S, editor. Polymeric Biomaterials. New York: Marcel Dekker; 1993. pp. 179–197. [Google Scholar]
- 19.Nakashima T, Uemura T, Maehara Y, Sugimachi K. Succinate dehydrogenase inhibition test for evaluating head and neck tumors. Oncology. 1989;46(3):162–168. doi: 10.1159/000226707. [DOI] [PubMed] [Google Scholar]
- 20.Olsen R, Schwartzmiller D, Weppner W, et al. Biomedical Applications of Chitin and Its Derivatives. In: Skjak-Braek G, Anthonsen T, Sandford P, editors. Chitin and Chitosan. London: Elsevier; 1989. pp. 813–828. [Google Scholar]
- 21.Ormerod MG, O’Neill CF, Robertson D, Harrap KR. Cisplatin induces apoptosis in a human ovarian carcinoma cell line without concomitant internucleosomal degradation of DNA. Experimental Cell Research. 1994;211(2):231–237. doi: 10.1006/excr.1994.1082. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang PK, Zhang GD, Qi JY, et al. Handbook of Chemical Products. 3rd Ed. Beijing: Chemical Industry Press; 2000. p. 368. (in Chinese) [Google Scholar]
- 23.Reason AJ, Morris HR, Panico M, Marais R, Treisman RH, Haltiwanger RS, Hart GW, Kelly WG, Dell A. Localization of O-GlcNAc modification on the serum response transcription factor (SRF) Journal of Biological Chemistry. 1992;267(24):16911–16921. [PubMed] [Google Scholar]
- 24.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE, Gossett C. Long-term effects of glucosamine sulphate on osteoarthritis progression: a radomised, placebo-controlled clinical trial. The Lancet. 2001;357(9252):251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 25.Sakahira H, Enari M, Ohsawa Y, Uchiyama Y, Nagata S. Apoptotic nuclear morphological change without DNA fragmentation. Current Biology. 1999;9(10):543–546. doi: 10.1016/S0960-9822(99)80240-1. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Russell D. Molecular Cloning: A Laboratry Manual. New York: Cold Spring Harbor Lab(CSHL) Press; 2001. pp. 463–468. [Google Scholar]
- 27.Santini MT, Cametti C, Indovina PL, Morelli G, Donelli G. Polylysine induces changes in membrane electrical properties of K562 cells. Journal of Biomedical Materials Research. 1997;35(2):165–174. doi: 10.1002/(SICI)1097-4636(199705)35:2<165::AID-JBM4>3.3.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Schipper NG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharmaceutical Research. 1996;13(11):1686–1692. doi: 10.1023/A:1016444808000. [DOI] [PubMed] [Google Scholar]
- 29.Schulze-Osthoff K, Walczak H, Dröge W, Krammer PH. Cell nucleus and DNA fragmentation are not required for apoptosis. Journal of Cell Biology. 1994;127(1):15–20. doi: 10.1083/jcb.127.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukada K, Matsumoto T, Aizawa K, Tokoro A, Naruse R, Suzuki S, Suzuki M. Antimetastatic and growth-inhibitory effects of N-acetylchitohexaose in mice bearing Lewis lung carcinoma. Japanese Journal of Cancer Research. 1990;81(3):259–265. doi: 10.1111/j.1349-7006.1990.tb02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang SH, Chen JC. The protective effect of chitin and chitosan against Vibrio alginolyticus in white shrimp Litopenaeus vannamei. Fish & Shellfish Immunology. 2005;19(3):191–204. doi: 10.1016/j.fsi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Qiao Y, Huang GS, Wang AQ, Zhang YQ, Feng JL, Yang GR, Guo Y, Liang R. Glucosamine and glucosamine hydrochloride induced leukemia cell line K562 differentiation into macrophage. Chinese Pharmacological Bulletin. 2003;19(3):290–293. (in Chinese) [Google Scholar]
- 33.Wang Z, Qiao Y, Huang GS, Wang AQ, Zhang YQ, Feng JL, Yang GR, Guo Y, Liang R. Induction of macrophagic differentiation of leukemia cell line K562 by N-acetyl-D-glucosamine. Journal of the Fourth Military Medical University. 2003;24(1):46–48. (in Chinese) [Google Scholar]
- 34.Wilson JK, Sargent JM, Elgie AW, Hill JG, Taylor CG. A feasibility study of the MTT assay for chemosensitivity testing in ovarian malignancy. British Journal of Cancer. 1990;62(2):189–194. doi: 10.1038/bjc.1990.258. [DOI] [PMC free article] [PubMed] [Google Scholar]