Abstract
The 87-kDa antigen derived from the fungal pathogen Paracoccidioides brasiliensis can be detected in the sera of infected patients, and its levels have been shown to correlate well with response to treatment and with clinical cure. Despite its potential importance, the antigen has been poorly characterized. The 87-kDa antigen was purified to homogeneity via preparative gel electrophoresis; N-terminal amino acid sequencing revealed substantial homology with heat shock proteins (hsps) from a variety of organisms. A monoclonal antibody (MAb) raised against a Histoplasma capsulatum 80-kDa hsp showed cross-reactivity to the purified 87-kDa antigen via Western blotting, and the 87-kDa-specific MAb P1B demonstrated that the antigen was expressed at higher levels in yeast than in mycelia by the same technique. Enzyme-linked immunosorbent assay and immunofluorescence reactivity using P1B confirmed increased expression of the 87-kDa antigen during the temperature-induced transformation of mycelia to yeast. Yeast-to-mycelium transformation was accompanied by a fall in expression, although the 87-kDa antigen was clearly constitutively expressed in both phases. Immunochemical staining of tissues from patients with MAb P1B who were infected with P. brasiliensis confirmed in vivo expression of the 87-kDa antigen by yeasts, and identification of this antigen via this method appears to be a useful adjunct to other methods used to diagnose paracoccidioidomycosis.
Paracoccidioides brasiliensis is a thermally dimorphic fungus responsible for the human disease paracoccidioidomycosis (PCM), one of the most important systemic fungal mycoses in Central and South America (2, 32). It is estimated that approximately 10 million people are infected, although the majority do not show clinical symptoms (2, 32). Both immunocompromised and immunocompetent individuals may develop infection, and there is a gender bias, with significantly more men than women developing disease (1). The disease varies in severity and primarily involves the lungs, with subsequent dissemination to other organs and systems; secondary lesions may occur in the mucous membranes, skin, lymph nodes, and adrenal glands (2, 32). Two forms of the disease are recognized: the chronic form (adult type) and the acute or subacute form (juvenile type) (11).
Laboratory diagnosis of PCM is largely based on the visualization of the fungus in biopsy and clinical specimens and its isolation by culture (2). In addition, antibodies to P. brasiliensis can be detected in patient sera by serological techniques, such as complement fixation, immunodiffusion, and immunoenzymatic assays (4, 5, 30, 36). However, these tests have certain important limitations: cross-reactivity is a problem, and there are profound difficulties encountered in the proper standardization of the various tests and reagents used (16). The value of these tests in monitoring patients is also problematic, which is due in part to the diversity and complexity of the humoral response in PCM patients (22).
Different diagnostic approaches to the detection of P. brasiliensis antigen in body fluids have also been attempted, with rather conflicting results (12, 26, 27, 35). The majority of the reports indicate variation in sensitivity and specificity, which is probably due to the small number of patients evaluated. Recently, Gómez and colleagues have described the use of a novel inhibition enzyme-linked immunosorbent assay (inh-ELISA) using a specific monoclonal antibody (MAb) which recognizes an 87-kDa antigen in the sera of patients with PCM (14, 15). This inh-ELISA was useful in the early diagnosis of the mycosis, especially in immunocompromised patients, with an overall sensitivity of 80.4% and a specificity of 81.4% (14). The quantitation of the 87-kDa antigen was also useful in monitoring the follow-up of patients with acute multifocal and unifocal forms of PCM (15). However, despite clearly identifying the 87-kDa antigen as a key target for serodiagnosis, these studies did not extend to the characterization of this important antigen.
In this paper, we describe the purification and characterization of the 87-kDa P. brasiliensis antigen and its identification as a heat shock protein (hsp). Expression of the antigen during temperature shift is described, and MAb P1B, which was used to develop the original inh-ELISA (14), has also been used in the detection of P. brasiliensis yeast cells in biopsy materials of patients with PCM. This has confirmed the in vivo expression of this antigen and has demonstrated another application of MAb P1B in the diagnosis of PCM.
MATERIALS AND METHODS
Fungal strains and cytoplasmic antigen preparation
P. brasiliensis CIB339 and Histoplasma capsulatum var. capsulatum isolate Hc 1980 were obtained from the culture collection of the Corporación para Investigaciones Biológicas (CIB), Medell&ıacute;n, Colombia. P. brasiliensis and H. capsulatum cytoplasmic yeast antigens (CYA) were produced as follows: isolates were initially subcultured on slants of synthetic McVeigh-Morton (SMV) medium (31) and incubated at 36°C for 3 days; the cultures were then transferred to a 500-ml flask containing 200 ml of liquid SMV medium, which was then placed in a gyratory shaker incubator at 125 rpm and 36°C. Seven days later, the cells were killed with thimerosal (0.02% [wt/vol]) (Sigma, Poole, United Kingdom) and harvested by centrifugation at 3,000 × g for 15 min. The pellet was washed twice in phosphate-buffered saline (PBS; 0.01 M; pH 7.2) and treated with a mixture of protease inhibitors containing 1 mM phenylmethylsufonyl fluoride (Sigma), 0.1 mM pepstatin A (Sigma), 0.1 mM N-α-p-tosyl-l-phenylalanine chloromethyl ketone (Sigma), 0.2 mM N-α-p-tosyl-l-lysine chloromethyl ketone (Sigma), and 1 mM EDTA (Sigma). The pellet was frozen with liquid nitrogen and disrupted by mechanical maceration, and the homogenate was then centrifuged at 10,000 × g for 30 min at 4°C. The supernatant was recovered, and the total protein concentration was measured by the Bradford technique (Bio-Rad, Hercules, Calif.) (38).
P. brasiliensis mycelium was grown at 22°C on slants of SMV medium and subcultured every 7 days. The cultures were transferred to a 500-ml flask containing 200 ml of liquid SMV medium, which was then placed in a gyratory shaker incubator at 125 rpm and 22°C. Cytoplasmic mycelial antigen (CMA) was produced as described above via mechanical maceration in liquid nitrogen. The protein concentration was measured by the Bradford technique (38).
SDS-PAGE, ELISA, and immunoenzyme development of Western blots.
For analysis of the fractions obtained during protein purification and characterization, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (using 10% [vol/vol] gels), ELISA, and Western immunoblotting (using polyvinylidene difluoride membranes [Immobilon P; Millipore Corporation, Watford, United Kingdom]) were used as previously described (10, 17, 19). MAbs P1B (14) and 69F (the latter being an anti-H. capsulatum hsp80 MAb [18]) were used in immunodevelopment.
Purification of the 87-kDa antigen.
Purification was performed by preparative gel electrophoresis, using the Prep-Cell system (model 491; Bio-Rad). The resolving gel of 7.5% (vol/vol) acrylamide (with 0.1% [wt/vol] SDS) was prepared and loaded with 30 mg of P. brasiliensis CYA (made up in loading buffer with 2-mercaptoethanol and boiled in water for 2 to 3 min). Two hundred sequential 2-ml fractions were collected, and then 200 μl from each fraction was precipitated with 3 volumes of chilled acetone and left at −20°C for 2 h; samples were then centrifuged at 12,000 × g for 15 min at 4°C. The resultant pellet was air dried, resuspended in loading buffer, and then run on an SDS-10% (vol/vol) PAGE gel. Proteins were visualized by Coomassie blue staining (0.1% [wt/vol]) and/or silver staining (Bio-Rad). Western blotting and ELISA with MAb P1B identified those fractions containing the 87-kDa protein, which were then selected and concentrated using centrifugal concentrators with a membrane which retained molecules greater than 1 kDa (Amicon, Stonehouse, United Kingdom). Protein concentration was measured by the Bradford technique.
N-terminal amino acid sequence.
Purified samples of 87-kDa protein (5 μg/μl), pretreated with loading buffer containing 2-mercaptoethanol and boiled for 3 min, were loaded onto an SDS-10% (vol/vol) PAGE gel (with 2 mM thioglycolic acid in the upper buffer chamber) and electrophoresed at 160 V for 1 h. The gel was then blotted onto a polyvinylidene difluoride membrane as previously described (17). The blot was stained for 1 min with 0.1% (wt/vol) Coomassie blue R and destained. The purified band was then subjected to N-terminal amino acid sequencing using Edman degradation and an Applied Biosystems (Warrington, United Kingdom) Procise sequencer (protein and nucleic acid chemistry facility, Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom).
Phase transition.
P. brasiliensis yeast cultures were grown at 36°C on SMV medium slants and subcultured every 3 days. Mycelial cultures were grown at 26°C on SMV medium slants and subcultured every 15 days. Cells from each phase were then subcultured and incubated for 48 h in the same liquid media (at the corresponding temperatures) in a series of flasks containing 200 ml with shaking at 120 rpm, and a 50-ml subsample of culture was removed (zero hour time point). The phase transition was then initiated by changing the culture temperature from 26 to 36°C for mycelium-to-yeast transition and from 36 to 26°C for the reverse process. Fifty-milliliter samples were collected at the following time points: 1.5, 3, 6, 12, 24, 48, 72, and 96 h and 7, 14, and 21 days post-temperature change. The culture samples were centrifuged, cells were collected, and the pellet was then divided into two aliquots, one of which was used to produce cytoplasmic antigen as described above. The samples were then analyzed by ELISA and immunoenzyme development of Western blots to detect expression of the 87-kDa antigen using MAb P1B. The second aliquot was embedded in optimal cutting medium (OCT) (Reichert-Jung, Nussloch, Germany) and stored in liquid nitrogen at −70°C until it was used in immunofluorescence staining.
Clinical samples.
A total of 23 biopsy samples embedded in OCT and stored in liquid nitrogen were used in immunohistochemical studies. All of the samples were obtained from Colombian patients attending the mycology laboratory, CIB. Six of these biopsy samples were obtained from patients with PCM disease (confirmed by direct examination, isolation by culture, and positive serological tests). Biopsy samples obtained from 13 different patients with other mycoses (confirmed by culture and serology) were also evaluated; 6 of the patients had histoplasmosis, 3 had sporotrichosis, 2 had lobomycosis, and 2 had aspergillosis (both of the last two samples were taken postmortem, from lung and brain, respectively). Negative controls consisting of four biopsy samples from healthy individuals were also studied.
Immunohistochemistry.
Super frost microscope slides (Labcraft, Houston, Tex.) were coated with 20 μl of 0.1% (vol/vol) poly-l-lysine (Sigma). P. brasiliensis cells and human tissues previously embedded in OCT were sectioned in a cryostat (Figocut 2700; Reichert-Jung, Nussloch, Germany), and sections were collected on coated slides. The fungal sections were then fixed by a 10-min immersion in cold acetone. All sections were stored at −70°C until they were used.
Indirect immunofluorescence.
The slides were transferred to Tris-acetate buffer (TAB) (0.05 M Tris, 0.1 M NaCl, 0.05% [vol/vol] Tween 20, 0.05% [vol/wt] Brij-35 [polyoxyethylene {24} lauryl ether], 0.25% [vol/vol] glacial acetic acid, 0.01% [wt/vol] thimerosal), and pretreated with unconjugated goat anti-mouse immunoglobulin G (IgG) Fc (Jackson, West Globe, Pa.), at a 1:10 dilution in TAB, for 2 h at room temperature. This solution was replaced after the slide was washed with primary antibody (MAb P1B; diluted 1:50 in TAB), and the slides were then incubated for 1 h at 37°C. The slides were washed with three changes of fresh TAB and incubated for 30 min at 37°C with rat liver acetone-absorbed fluorescein isothiocyanate-conjugated anti-mouse IgG Fc (Jackson) diluted 1:20 in TAB. Prior to its use, free fluorescein isothiocyanate particles were eliminated from the conjugate by a 30-min incubation with rat liver acetone powder (Sigma) at room temperature, followed by centrifugation (1,000 × g for 5 min). After further washes with fresh TAB, the slides were mounted in a 50% (vol/vol) glycerol-PBS (pH 7.5) buffer. A negative control in which the primary antibody was replaced with PBS was also used.
Immunoperoxidase staining.
Sections were treated with PBS containing 0.3% (vol/vol) hydrogen peroxide for 30 min at room temperature. The slides were then placed in a humid chamber, transferred to TAB for 10 min, and blocked by a 10-min incubation with blocking solution (normal swine serum diluted 1:5 in TAB-0.4% [vol/vol] Triton X-100), at room temperature. The slides were washed in TAB and incubated for 1 h at 37°C with MAb P1B (dilution 1:50) in blocking solution. After three TAB washes, the slides were incubated for 1 h with the secondary antibody (peroxidase-conjugated goat anti-mouse IgG [Dakopatts, Glostrup, Germany]) diluted 1:10 in blocking solution. The slides were incubated for 30 min at room temperature with mouse peroxidase-antiperoxidase (PAP) complex (Dakopatts) diluted 1:20 in TAB. After further washes in TAB, positive staining was determined by 15-min incubation with 3,3" diaminobenzidine (Sigma) substrate (1 mg/ml) in 0.1 M PBS-0.3% (vol/vol) hydrogen peroxide. After final TAB washes, the slides were counterstained with Mayer's hematoxylin (Sigma) for 90 s, dehydrated, cleared in xylene, and mounted with Permount mounting medium (Fisher Scientific Co., Burlington, N.J.). The negative control was as described above.
RESULTS
Purification of the 87-kDa antigen and N-terminal amino acid sequencing.
A total of 40 of the 200 fractions (numbers 50 to 90) collected from the Prep-Cell system were found to contain the 87-kDa protein when assessed by SDS-PAGE and Western immunoblotting. Pure protein was found in fractions 72 to 85 (data not shown); the total protein content of these fractions was 0.5 mg, which was obtained from an initial quantity of 30 mg of P. brasiliensis CYA (giving an approximate yield of 1.6%). The purification of this protein has been repeated in triplicate without variation, demonstrating the reproducibility of the method.
Microsequencing revealed extensive homology of the N-terminal amino acid sequence of the purified 87-kDa antigen from P. brasiliensis with various members of the hsp70 family from various sources (Table 1). The highest homology found was with an hsp70 protein previously described from P. brasiliensis (76%) (7). N-terminal sequencing of the protein was repeated three times without variation in the sequence obtained.
TABLE 1.
N-terminal amino acid sequence comparison of 87-kDa protein with most homologous proteins from BLAST search results
| Protein source | Sequencea | % Identityb |
|---|---|---|
| P. brasiliensis 87-kDa antigen | APAIGIDLKTTYQVIGIDL | |
| 11019 | ||
| P. brasiliensis hsp70 | APAIGIDLGTTYSCVGI | 76 |
| 218 | ||
| Cladosporium herbarum hsp 70 | APAIGIDLGTTYSCVGI | 76 |
| 218 | ||
| Ajellomyces capsulata hsp70 | APAVGIDLGTTYSCVGI | 65 |
| 218 |
Identical residues are underlined.
Percent sequence identities in amino acid overlap.
Immunoenzyme recognition of Western blots of 87-kDa antigen.
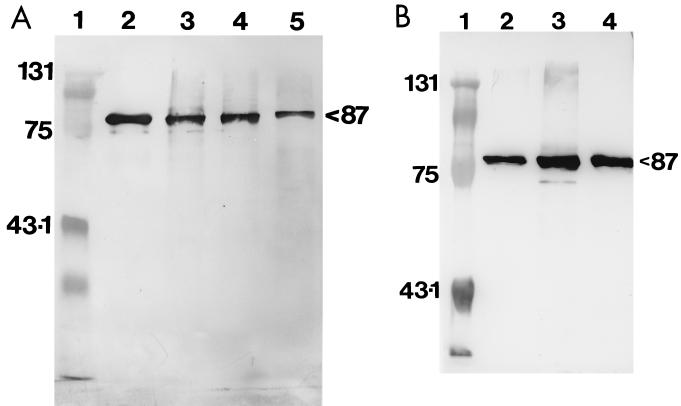
MAb 69F, which had previously been raised against an hsp80 protein from H. capsulatum (18), demonstrated cross-reactivity with the 87-kDa antigen in P. brasiliensis CMA and CYA and with the pure protein (Fig. 1A). However, reactivity was more intense against CYA and pure protein (at equivalent protein concentrations). MAb 69F recognized H. capsulatum CYA as a positive control. MAb P1B demonstrated reactivity to the 87-kDa antigen in P. brasiliensis CMA and CYA and to the pure protein (Fig. 1B). The recognition of the antigen by the MAb was much greater in CYA than in CMA (at equivalent protein concentrations).
FIG. 1.
(A) Immunoenzyme reactivity of MAb 69F on Western blots with cytoplasmic antigens and the 87-kDa purified protein of P. brasiliensis. Lane 1, Bio-Rad molecular mass marker (in kilodaltons); lane 2, H. capsulatum CIB1980 CYA (positive control); lane 3, P. brasiliensis CIB339 CMA; lane 4, P. brasiliensis CIB339 CYA; lane 5, purified 87-kDa protein. All lanes were loaded with 5 μg total protein. (B) Immunoenzyme reactivity of MAb P1B on Western blots with CMA, CYA, and pure 87-kDa protein of P. brasiliensis. Lane 1, Bio-Rad molecular mass marker (in kilodaltons); lane 2, P. brasiliensis CIB339 CMA; lane 3, P. brasiliensis CIB339 CYA; lane 4, 87-kDa purified protein. All lanes were loaded with 5 μg total protein.
Comparison of expression of the 87-kDa protein during phase transformation.
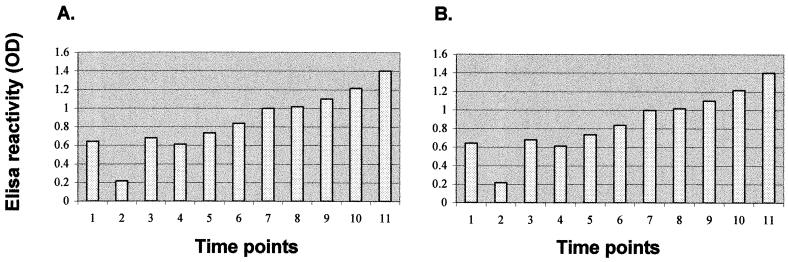
Indirect ELISA with MAb P1B was used to analyze the 87-kDa protein expression in CMA and CYA during phase transformation of P. brasiliensis. Mycelium-to-yeast conversion induced by temperature shift from 26 to 36°C was characterized by a gradual increase in the expression of the 87-kDa protein (as measured by ELISA reactivity) at equivalent total protein concentrations (Fig. 2A) However, there was an initial transient fall in expression at 1.5 h.
FIG. 2.
ELISA detection of 87-kDa protein with MAb P1B during phase transformation. (A) P. brasiliensis CIB339 mycelial cells subjected to temperature shift from 26 to 36°C; (B) P. brasiliensis CIB339 yeast cells subjected to temperature shift from 36 to 26°C. Time points: 1, zero h; 2, 1.5 h; 3, 3 h; 4, 6 h; 5, 12 h; 6, 24 h; 7, 48 h; 8, 72 h; 9, 96 h; 10, 7 days; 11, 14 days. OD, optical density.
When yeast cells were subjected to a temperature shift from 36 to 26°C, a marked decrease in synthesis of the 87-kDa protein was detected at equivalent total protein concentrations (Fig. 2B); the lowest level of expression was observed 96 h after temperature shift. A subsequent increase in the level of 87-kDa protein expression was observed after 7 days, when the majority of yeast cells had already differentiated into mycelial elements.
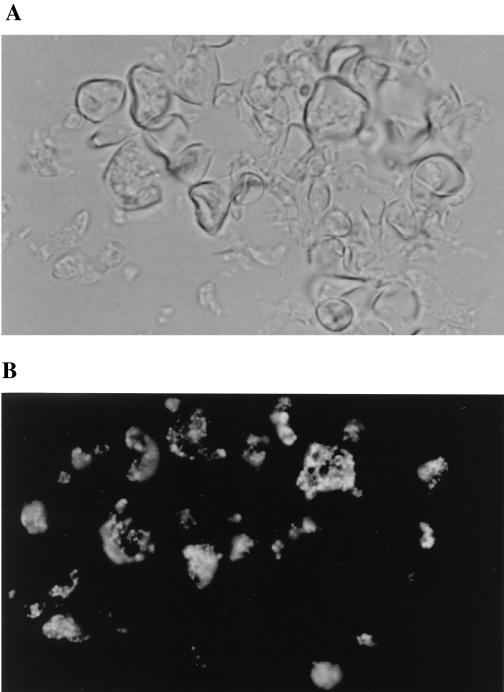
MAb P1B was also used to study the expression of the 87-kDa protein in P. brasiliensis cells before and during phase transformation by immunofluorescence staining. Yeast cultures grown at 36°C demonstrated intense staining of the cytoplasm, with a granular appearance (Fig. 3). Apparently “empty” cells (i.e., devoid of cytoplasm) did not stain. When yeast cultures were subjected to a temperature shift at 26°C, the developing mycelia were much less reactive to MAb P1B than any residual yeast cells within the transforming culture (data not shown). Similarly, mycelial cultures incubated at 36°C for 21 days showed that the cytoplasmic reactivity of mycelia was much weaker than that of yeast cells (data not shown). Negative control slides were unreactive (data not shown)
FIG. 3.
Immunofluorescence reactivity of P. brasiliensis cells using MAb P1B at dilution of 1:10. P. brasiliensis CIB339 yeast cells grown at 36°C are shown by phase-contrast (A) and immunofluorescence (B) microscopy (magnification, ×40).
Immunohistochemical identification of the 87-kDa antigen in biopsy material.
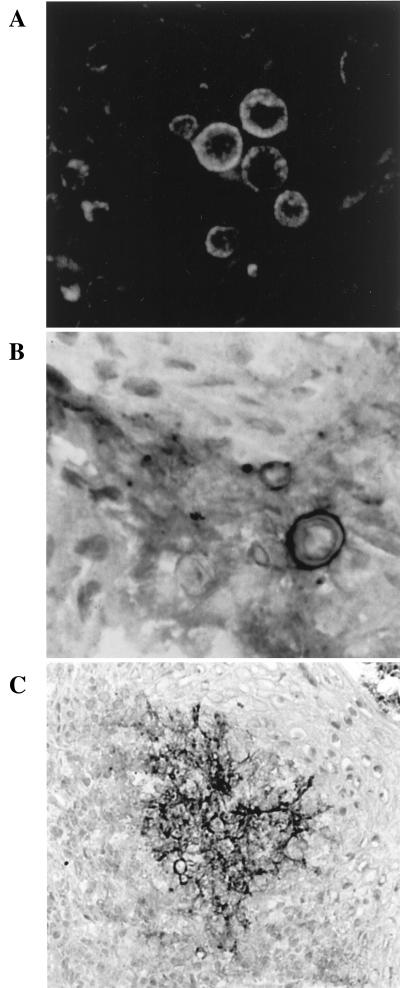
The immunohistochemistry results using MAb P1B to stain biopsy specimens from patients with PCM by different immunochemical techniques are summarized in Table 2. MAb P1B-labeled yeast cells in biopsy specimens from infected PCM patients were brightly stained by immunofluorescence, although a variation in the staining intensity among different yeast cells was observed (Fig. 4A). The fluorescence labeling was distributed towards the periphery of the cell, with a generalized granular appearance. Generally, cells stained with immunoperoxidase demonstrated a pattern similar to that observed via immunofluorescence (Fig. 4B). In some of the biopsy specimens, extracellular labeling was seen, apparently within granulomas (Fig. 4C). In terms of the specificity of reaction, MAb P1B showed cross-reactivity only to the biopsy material of patients with aspergillosis (data not shown); all other biopsy materials, from patients with heterologous mycoses and from controls, were negative.
TABLE 2.
Immunohistochemical detection of P. brasiliensis with MAb P1B in infected human tissue and in normal controls
| Patient group and no. | Tissue | IFa | PAP |
|---|---|---|---|
| PCM | |||
| 1 | Skin | Positive | Positive |
| 2 | Oral mucosa | Positive | Positive |
| 3 | Oral mucosa | Positive | Positive |
| 4 | Oral mucosa | Positive | Positive |
| 5 | Skin | Positive | Positive |
| 6 | Skin | Positive | Positive |
| Histoplasmosis (n = 6) | Oral mucosa, skin | Negative | Negative |
| Sporotrichosis (n = 3) | Skin | Negative | Negative |
| Lobomycosis (n = 2) | Skin | Negative | Negative |
| Aspergillosis (n = 2) | Lung, brain | Positive | Positive |
| Normal controls (n = 4) | Skin | Negative | Negative |
IF, immunofluorescence.
FIG. 4.
Immunohistochemical recognition of the 87-kDa antigen in biopsy specimens from PCM patients with MAb P1B. (A) Immunofluorescence staining of P. brasiliensis cells (magnification, ×40); (B) PAP staining of P. brasiliensis yeast cells (magnification, ×40); (C) PAP staining of P. brasiliensis cells showing extracellular labeling within the biopsy tissue (magnification, ×10).
DISCUSSION
The purification of the 87-kDa protein, as accomplished by preparative gel electrophoresis, was relatively straightforward and reproducible. The purity of the antigen was confirmed by the observation of a single band on silver-stained gels and by the collection of consistent N-terminal amino acid sequence data. The latter clearly demonstrated that the 87-kDa antigen has a high homology with previously reported members of the hsp70 family. The greatest homology was with a previously identified P. brasiliensis hsp (7). However, at this time we cannot be sure of the relationship between the 87-kDa antigen and this hsp, although the apparent differences in relative molecular weight and sequence heterogeneity suggest that although they are related they are not identical.
The recognition of the 87-kDa protein by MAb 69F, which was previously produced against a known hsp from H. capsulatum (18), confirms the N-terminal sequence data. Interestingly the epitope against which MAb 69F was raised has been defined as including an o-glycosylated linkage (18), strongly implying that this structure is also present on the 87-kDa antigen. These carbohydrate structures are commonly associated with extracellular molecules, and certainly o-glycosylated hsps are not unknown (33). This clearly raises the possibility that the 87-kDa antigen is an exported hsp. Indeed, our immunohistochemical studies, which demonstrated the apparent release of the antigen in situ within granulomas, and the previous inh-ELISA studies, which showed that the 87-kDa antigen was detectable in sera, confirm that it can be found extracellularly. However, the possibility remains that this antigen could be released as a consequence of cell death, as has been suggested for other fungal hsps by Mathews et al. (23), and thus, this question requires further study.
The level of expression of the 87-kDa antigen can be modified by temperature changes, as was confirmed by ELISA, Western immunoblotting, and immunofluorescence staining; this behavior is also consistent with our identification of the antigen as an hsp. Thus, when mycelial cells were incubated at 36°C, expression of the 87-kDa antigen was increased; this pattern of expression parallels the attendant mycelium-to-yeast transformation. In the same way, yeast cultures incubated at 26°C showed a significant decrease in the expression of this protein. However, in common with many hsps described thus far (8, 21), the 87-kDa protein is clearly constitutively expressed, because it can be detected at lower levels, as shown by Western blotting and ELISA, in mycelia which have not been subject to temperature shift.
hsp expression has been widely studied in a variety of fungi; these proteins are essential for cell survival following external stress and for cell growth (25, 28, 29, 37). The N-terminal data would suggest that the 87-kDa antigen is a member of the hsp70 family, proteins which are thought to play a role in folding or unfolding of denatured or misfolded proteins (20). Interestingly, in Saccharomyces cerevisiae, different subsets of hsp70 molecules may exist, each one performing different roles, with some being induced in response to an increase in temperature and others being expressed almost exclusively at lower temperatures (9). The P. brasiliensis 87-kDa antigen would appear to be closer to the former group, with the important provision that it is expressed at low levels even in mycelia not previously subjected to heat shock, thus suggesting that it plays a role even under “normal” conditions (9, 21). Interestingly the level of expression of the 87-kDa protein after temperature shift (from 26 to 36°C) also showed an initial transient fall at 1.5 h; this phenomenon has been described for the expression of other hsp70 proteins (25). hsps are, of course, closely associated with the phenomenon of dimorphism common to fungi such as P. brasiliensis, H. capsulatum, and Blastomyces dermatitidis, and the factors involved in their reversible transformation have been extensively studied (13, 21, 25, 34); it is well known that changes in temperature induce increases in the expression of specific genes, which in turn activate and regulate the morphological changes characteristic of dimorphism.
hsps have previously been shown to be important markers of disease in several fungal infections (24, 39, 40, 41), including Candida albicans infection, and some of them have been used in the diagnosis of infectious diseases (3, 6, 23, 25), so it is perhaps not surprising that the P. brasiliensis 87-kDa antigen should be a member of this group of proteins. Although hsps are highly conserved among species, it appears that MAb P1B, which has been used successfully in diagnosis and follow-up of patients (14, 15), is directed against an epitope on the P. brasiliensis antigen which has a certain degree of heterogeneity. However, at least part of this epitope appears to be shared with a similar molecule derived from Aspergillus fumigatus; this would account for the cross-reaction seen in the biopsy material containing Aspergillus hyphae and for the previously identified cross-reactivity with this species documented in inh-ELISA (14). It is worth noting, however, that in terms of immunohistochemical diagnosis, the detection of the 87-kDa antigen by P1B in biopsy material is potentially a useful adjunct to the in situ identification of P. brasiliensis. The immunohistochemical diagnosis of PCM is an underdeveloped area of research, and it is worth noting that the only apparent cross-reactive species is A. fumigatus. This species is unlikely to be misidentified as P. brasiliensis, at least on purely morphological grounds. Thus, we believe that P1B could be more broadly applied to in situ identification. As for the 87-kDa antigen itself, we now plan follow-up studies to clone the gene encoding the protein, in order to define the precise epitope recognized by P1B and to define epitopes that are completely unique to this molecule. This will allow further development of more specific diagnostic tests incorporating the detection of the 87-kDa P. brasiliensis hsp.
Acknowledgments
We thank the Overseas Research Student Awards Scheme (ORSAS), London, United Kingdom; the Wellcome Trust, London, United Kingdom; and Colciencias, Bogotá, Colombia, for their financial support.
REFERENCES
- 1.Aristizabal, B. H., K. V. Clemons, D. A. Stevens, and A. Restrepo. 1998. Morphological transition of Paracoccidioides brasiliensis conidia to yeast cells : in vivo inhibition in females. Infect. Immun. 66:5587-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brummer, E., E. Castañeda, and A. Restrepo. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6:89-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnie, J. P. 1991. Developments in the serological diagnosis of opportunistic fungal infections. J. Antimicrob. Chemother. 28:23-33. [DOI] [PubMed] [Google Scholar]
- 4.Camargo, Z. P., J. L. Guesdon, E. Drouhet, and L. Improvisi. 1984. Enzyme linked immunosorbent assay (ELISA) in paraccocidioidomycosis. Comparison with counterimmunoelectrophoresis and erythroimmunoassay. Mycopathologia 88:31-37. [DOI] [PubMed] [Google Scholar]
- 5.Cano, L. E., and A. Restrepo. 1987. Predictive value of serologic tests in the serodiagnosis and follow up of patients with paracoccidioidomycosis. Rev. Inst. Med. Trop. Sao Paulo 29:276-283. [DOI] [PubMed] [Google Scholar]
- 6.Crampin, A. C., and R. C. Matthews. 1993. Application of the polymerase chain reaction to the diagnosis of candidosis by amplification of an HSP 90 gene fragment. J. Med. Microbiol. 39:233-238. [DOI] [PubMed] [Google Scholar]
- 7.Da Silva, S. P., M. I. Borges-Walmsley, I. Silva Pereira, C. M. de Almeida Soares, A. R. Walmsley, and M. S. Soares Felipe. 1999. Differential expression of an Hsp70 gene during transition from the mycelial to the infective yeast form of the human pathogenic fungus Paracoccidioides brasiliensis. Mol. Microbiol. 31:1039-1050. [DOI] [PubMed] [Google Scholar]
- 8.Estruch, F. 2000. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 20:469-486. [DOI] [PubMed] [Google Scholar]
- 9.Feige, U., and B. S. Polla. 1994. Heat shock proteins: the Hsp70 family. Experientia 50:979-986. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa, J., A. J. Hamilton, M. A. Bartholomew, L. E. Fenelon, and R. J. Hay. 1990. Preparation of species-specific murine monoclonal antibodies against the yeast phase of Paracoccidioides brasiliensis. J. Clin. Microbiol. 28:1766-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco, M., M. R. Montenegro, R. P. Mendes, S. A. Marquez, N. L. Dillon, and N. G. S. Mota. 1987. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev. Soc. Bras. Med. Trop. 20:129-132. [DOI] [PubMed] [Google Scholar]
- 12.Freitas-da-Silva, G., and M. C. Roque-Barrera. 1992. Antigenemia in paracoccidioidomycosis. J. Clin. Microbiol. 30:381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldani, L. Z., M. Picard, and A. M. Sugar. 1994. Synthesis of heat-shock proteins in mycelia and yeast forms of Paracoccidioides brasiliensis. J. Med. Microbiol. 40:124-128. [DOI] [PubMed] [Google Scholar]
- 14.Gómez, B. L., J. I. Figueroa, A. J. Hamilton, B. Ortiz, M. A. Robledo, R. J. Hay, and A. Restrepo. 1997. Use of monoclonal antibodies in diagnosis of paracoccidioidomycosis: new strategies for detection of circulating antigens. J. Clin. Microbiol. 35:3278-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez, B. L., J. I. Figueroa, A. J. Hamilton, S. D|$$|Aa|fiez, M. Rojas, A. M. Tobón, R. J. Hay, and A. Restrepo. 1998. Antigenemia in patients with paracoccidioidomycosis: detection of the 87-kilodalton determinant during and after antifungal therapy. J. Clin. Microbiol. 36:3309-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton, A. J. 1998. Serodiagnosis of histoplasmosis, paracoccidioidomycosis and penicilliosis marneffei; current status and future trends. Med. Mycol. 36:351-364. [PubMed] [Google Scholar]
- 17.Hamilton, A. J., M. A. Bartholomew, L. E. Fenelon, J. I. Figueroa, and R. J. Hay. 1990. A murine monoclonal antibody exhibiting high species specificity for Histoplasma capsulatum var. capsulatum. J. Gen. Microbiol. 136:331-335. [DOI] [PubMed] [Google Scholar]
- 18.Jeavons, L., L. Hunt, and A. Hamilton. 1994. Immunochemical studies of heat shock protein 80 of Histoplasma capsulatum. J. Med. Mycol. 32:47-57. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Mager, W. H., and P. Moradas Ferreira. 1993. Stress response of yeast. Biochem. J. 290:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maresca, B., and G. S. Kobayashi. 1995. Hsps in dimorphic fungi, p. 119-130. In R. Matthews and J. P. Burnie (ed.), Heat shock proteins in fungal infections. R. G. Landes Company, Georgetown, Tex.
- 22.Martins, R., S. Marquez, M. Alves, D. Fecchio, and M. Franco. 1997. Serological follow-up of patients with paracoccidioidomycosis treated with itraconazole using dot-blot, ELISA and Western-blot. Rev. Inst. Med. Trop. Sao Paulo. 39:261-269. [DOI] [PubMed] [Google Scholar]
- 23.Matthews, R. 1995. Hsps in candidiasis, p. 1-92. In R. Matthews and J. P. Burnie (ed.), Heat shock proteins in fungal infections. R. G. Landes Company, Georgetown, Tex.
- 24.Matthews, R. C. 1992. Candida albicans HSP 90: link between protective and auto immunity. J. Med. Microbiol. 36:367-370. [DOI] [PubMed] [Google Scholar]
- 25.Matthews, R. C., B. Maresca, J. P. Burnie, A. Cardona, L. Carratu, S. Conti, G. S. Deepe, A. M. Florez, S. Franceschelli, E. Garcia, L. S. Gargano, G. S. Kobayashi, J. G. McEwen, B. L. Ortiz, A. M. Oviedo, L. Polonelli, J. Ponton, A. Restrepo, and A. Storlazzi. 1998. Stress proteins in fungal diseases. Med. Mycol. 36:45-51. [PubMed] [Google Scholar]
- 26.Mendes-Giannini, M. J., J. P. Bueno, M. A. Shikanai-Yasuda, A. W. Ferreira, and A. Masuda. 1989. Detection of the 43,000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J. Clin. Microbiol. 27:2842-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendes-Giannini, M. J. S., G. B. Del Negro, and A. M. Siqueira. 1994. Serodiagnosis, p. 345-363. In M. Franco, C. Da Silva Lacaz, A. Restrepo, and G. del Negro (ed.), Paracoccidioidomycosis. CRC Press, Inc., Boca Raton, Fla.
- 28.Miao, B., J. Davis, and E. A. Craig. 1999. The HSP70 family—an overview, p. 3-13 In M. J. Gething (ed.), Guidebook to molecular chaperones and protein-folding catalysts. Oxford University Press, Oxford, England.
- 29.Mosser, D. D., A. W. Caron, L. Bourget, A. Meriin, M. Y. Sherman, R. I. Monimoto, and B. Massie. 2000. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 20:7146-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz, B. L., S. D|$$|Aa|fiez, M. E. Urán, J. M. Rivas, M. Romero, V. Caicedo, A. Restrepo, and J. G. McEwen. 1997. Use of the 27-kilodalton recombinant protein from Paracoccidioides brasiliensis in serodiagnosis of paracoccidioidomycosis. Clin. Diagn. Lab. Immunol. 5:826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Restrepo, A., and B. E. Jimenez. 1980. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J. Clin. Microbiol. 12:279-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Restrepo, A. 2000. Paracoccidioides brasiliensis, p. 2768-2772. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 33.Russo, P., N. Kalkkinen, H. Sareneva, J. Paakkola, and M. Makarow. 1992. A heat shock gene from Saccharomyces cerivisae encoding a secretory glycoprotein. Proc. Natl. Acad. Sci. USA 89:3671-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salem-Izacc, S. M., R. S. A. Jesuino, W. A. Brito, M. Pereira, M. S. S. Felipe, and C. M. A. Soares. 1997. Protein synthesis patterns of Paracoccidioides brasiliensis isolates in stage-specific forms and during cellular differentiation. J. Med. Vet. Mycol. 35:205-211. [PubMed] [Google Scholar]
- 35.Salina, M. A., M. A. Shikanai-Yasuda, R. Poncio Mendes, B. Barraviera, and M. J. S. Mendes-Giannini. 1998. Detection of circulating Paracoccidioides brasiliensis antigen in urine of paracoccidioidomycosis patients before and during treatment. J. Clin. Microbiol. 36:1723-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taborda, C. P., and Z. P. Camargo. 1994. Diagnosis of paracoccidioidomycosis by dot blot immunobinding assay for antibody detection using the purified and specific antigen gp43. J. Clin. Microbiol. 32:554-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toone, W. M., and N. Jones. 1998. Stress-activated signalling pathways in yeast. Genes Cells 3:485-498. [DOI] [PubMed] [Google Scholar]
- 38.Walker, J. M. 1994. Basic protein and peptide protocols, vol. 32, p. 9-34. Humana Press Inc., Totowa, N.J. [Google Scholar]
- 39.Weigl, E., P. Kopeèek, M. Raška, and Š. Hradilová. 1999. Heat shock proteins in immune reactions. Folia Microbiol. 44:561-566. [DOI] [PubMed] [Google Scholar]
- 40.Zügel, U., and S. H. E. Kaufmann. 1999. Immune response against heat shock proteins in infectious diseases. Immunobiology 201:22-35. [DOI] [PubMed] [Google Scholar]
- 41.Zügel, U., and S. H. E. Kaufmann. 1999. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 12:19-39. [DOI] [PMC free article] [PubMed] [Google Scholar]