Abstract
Only a few cell lines have been infected with prions, offering limited genetic diversity and sensitivity to several strains. Here we report that cultured neurospheres expressing cellular prion protein (PrPC) can be infected with prions. Neurosphere lines isolated from the brains of mice at embryonic day 13–15 grow as aggregates and contain CNS stem cells. We produced neurosphere cultures from FVB/NCr (FVB) mice, from transgenic (Tg) FVB mice that overexpress mouse PrP-A (Tg4053), and from congenic FVB mice with a targeted null mutation in the PrP gene (Prnp0/0) and incubated them with the Rocky Mountain Laboratory prion strain. While monitoring the levels of disease-causing PrP (PrPSc) at each passage, we observed a dramatic rise in PrPSc levels with time in the Tg4053 neurosphere cells, whereas the level of PrPSc decayed to undetectable levels in cell cultures lacking PrP. PrPSc levels in cultures from FVB mice initially declined but then increased with passage. Prions produced in culture were transmissible to mice and produced disease pathology. Intracellular aggregates of PrPSc were present in cells from infected cultures. The susceptibility of neurosphere cultures to prions mirrored that of the mice from which they were derived. Neurosphere lines from Tg4053 mice provide a sensitive in vitro bioassay for mouse prions; neurosphere lines from other Tg mice overexpressing PrP might be used to assay prions from other species, including humans.
Keywords: CNS stem cells, in vitro bioassay, neurodegeneration, scrapie, transgenic mice
Prion diseases are transmissible neurodegenerative disorders of protein conformation. This group of diseases includes kuru, Creutzfeldt–Jakob disease, Gerstmann–Sträussler–Scheinker syndrome, and fatal insomnia in humans; scrapie in sheep and goats; and bovine spongiform encephalopathy (1). Prion diseases are characterized by spongiform degeneration of the brain, neuron loss, and astrocytic gliosis (2). The key event in prion replication is the posttranslational conversion of normal cellular forms of prion protein (PrPC) to pathological, alternatively folded disease-causing PrP (PrPSc) isoforms, which comprise the infectious particle (3, 4).
Much evidence for the central role of PrP in prion disease has come from studies using genetically modified mice. For example, overexpression of transgene-encoded PrP shortens the incubation time by increasing the supply of PrPC available for interaction with PrPSc (5, 6). Similarly, the absolute dependence on PrPC for prion replication was demonstrated by the resistance of PrP gene-ablated [FVB.129-Prnptm1Zrch (Prnp0/0)] mice to infection with scrapie prions (7, 8). Although both natural and engineered genetic variations among mice have provided important resources for prion research, incubation time studies are expensive and can require hundreds of days for completion. An additional limitation of whole animal studies is the difficulty of directly addressing cellular and biochemical mechanisms involved in prion replication and pathogenesis. Cell lines from diverse transgenic (Tg) lines and inbred strains used to study prions could offer more rapid and economical approaches. Unfortunately, only a few cell lines have proven to be permissive for prion infection, demonstrating that PrP expression is necessary but not sufficient for prion replication (9–11). Among the relatively few cell lines that can maintain prion replication, mouse neuroblastoma cell line N2a has been the most useful for studying the cell biology of prion replication. However, for unknown reasons, only a small percentage of N2a cells replicate prions when exposed to the scrapie agent (12), and this replication is unstable (11, 12). Similar limitations exist for other permanent cell lines susceptible to prion infection, including PC12 rat pheochromocytoma cells (13, 14), spontaneously immortalized hamster brain cells (15), the T-antigen immortalized GT1 hypothalamic neuron line (16), and T-antigen immortalized cells of the central and peripheral nervous systems (17, 18). Some nonneuronal cells, such as PrPC-transfected epithelial cells (19) and fibroblast cell lines NIH/3T3 and L29 (20, 21), are susceptible to transient prion infection. Primary cultures, particularly of terminally differentiated neurons, have failed to support prion replication but may be susceptible to toxic effects of PrPSc or PrP-derived peptides (17).
In view of the foregoing results, we explored alternative cell culture systems. Both the embryonic and adult mammalian CNS possess stem cells that can proliferate, self-renew, and differentiate into the three primary cell types of the CNS (22–25). Neurosphere cultures can be readily isolated from fetal brain and are enriched in CNS stem-cell activity. Here we report isolation of neurosphere cultures from non-Tg FVB/NCr (FVB) mice, FVB-Tg(MoPrP-A)4053 (Tg4053) mice overexpressing mouse PrPC, and FVB.Prnp0/0 mice congenic for a targeted null mutation in the PrP gene. Both FVB and Tg4053 neurospheres express PrPC and can be infected with mouse-passaged scrapie prions. The Tg4053 neurospheres are highly sensitive to prion infection, raising the possibility that neurosphere cultures may serve as an alternative to prion bioassays in mice.
Results
PrPC Is Expressed in Neurosphere Cultures.
Susceptibility to prion disease requires the expression of host PrPC (8), levels of which were analyzed by immunoblotting. Cell blot analysis of FVB, Prnp0/0, and Tg4053 neurospheres clearly demonstrates the higher level of PrPC expression in Tg4053 neurospheres compared with FVB neurospheres (Fig. 1A). No PrP immunostaining was observed in Prnp0/0 neurospheres, making them an ideal negative control for persistence of PrPSc in subsequent infection studies. Dot blot analysis indicates that Tg4053 neurospheres express ≈4- to 8-fold more PrPC than FVB neurospheres (Fig. 1B). Western blot analysis confirmed the higher PrPC levels in Tg4053 than in FVB neurospheres and showed that most PrPC in FVB and Tg4053 neurospheres is diglycosylated, as is the case in the mice from which they were derived (Fig. 1C).
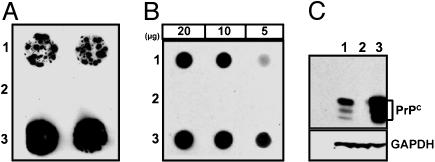
Fig. 1.
PrPC expression in neurosphere cultures from FVB (1), Prnp0/0 (2), and Tg4053 (3) mice. (A) Cell blots of neurospheres grown on tissue culture-treated coverslips, transferred to nitrocellulose, and immunostained with anti-PrP. (B) Estimation of PrP concentration by dot blotting. Cell lysates with 20, 10, or 5 μg of protein from neurospheres were transferred by vacuum blotting onto nitrocellulose for immunodetection of PrP with indirect chemiluminescence. (C) PrPC is normally glycosylated. Lysates of neurospheres containing 40 μg of protein were electrophoresed, Western blotted, and stained with anti-PrP. The blot was stripped and reprobed with anti-GAPDH to normalize for loading or transfer differences. Fab D18 was used to detect PrP in all cases.
Neurosphere Cultures Likely Contain CNS Stem Cells.
Immunofluorescence showed coexpression of PrPC and nestin, a commonly used marker for CNS stem cells (26), in our neurosphere cultures (see Fig. 6, which is published as supporting information on the PNAS web site). Most nestin-stained cells are also positive for PrPC in FVB neurosphere cultures at passage 3. Similar results were obtained with Tg4053 neurosphere lines but with more intense staining for PrP. As expected, neurospheres isolated from Prnp0/0 mice do not stain with anti-PrPC antibodies but express nestin, similar to FVB cultures. Most cells in the neurosphere cultures also expressed vimentin, another commonly used marker for CNS stem cells. Some of the cells adherent to the coverslips expressed glial fibrillary acidic protein, a glial cell marker, or microtubule-associated protein-2, a marker for the neuronal pathway. These observations suggest that our neurosphere cultures contain CNS stem cells, as expected from the results of others (22, 23, 27). However, stem-cell activity was not formally tested.
Neurospheres Expressing PrPC Can Replicate Prions.
Having established the expression of PrPC in neurosphere cultures, we tested their susceptibility to prion infection. FVB, Tg4053, and Prnp0/0 cultures at passage 3 were incubated with a 50-fold dilution of Rocky Mountain Laboratory (RML) prions for 4 days. At the end of 4 days, the neurospheres were washed and split 1:4. Every 3–4 days, one-half of the medium was replaced with fresh medium. To detect the persistence and de novo production of proteinase K (PK)-resistant PrPSc in infected cultures, cells growing on plastic coverslips were blotted and tested for the presence of PK-resistant PrPSc. All three neurosphere lines showed PrPSc at 12 days postinfection (dpi) (Fig. 2A Left), whereas control neurospheres that were not exposed to RML prions had no PrPSc. By 24 dpi, little PrPSc remained in Prnp0/0 neurospheres, whereas PrPSc was readily detected in FVB and Tg4053 cultures (Fig. 2A Center). By 36 dpi and two passages, no PK-resistant PrPSc was seen in Prnp0/0 neurospheres, but substantial amounts of PK-resistant PrPSc were found in Tg4053 neurospheres; fewer PrPSc-positive neurospheres were found in FVB cultures, but PrPSc was clearly present.
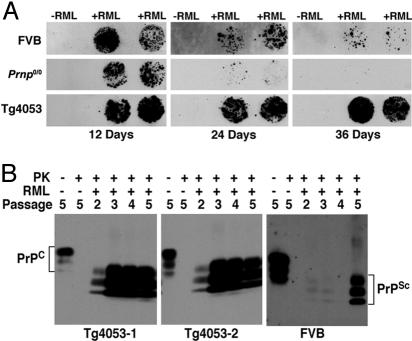
Fig. 2.
Persistence and generation of PrPSc in neurospheres incubated with RML prions. FVB, Prnp0/0, and Tg4053 neurospheres at passage 3 were incubated for 4 days with a 50-fold dilution of 10% RML-infected mouse brain homogenate (+RML), washed, and passaged as described in Results. Controls incubated with isolate diluent (−RML) were treated similarly. (A) PrPSc persists temporarily in Prnp0/0 neurospheres but replicates in cells expressing PrPC. To detect PrPSc, duplicate cell blots were treated with PK and GdnSCN before staining with anti-PrP Fab D18. Cells were harvested at 12, 24, and 36 dpi, counted from the time the brain homogenate was removed. The availability of Prnp0/0 neurospheres allows persistence of PrPSc in the inoculum to be distinguished from active replication. (B) Production of PrPSc increases with passage. Cell lysates containing 500 μg of protein from FVB and Tg4053 cultures were digested with 5 μg/ml PK to detect PrPSc. Undigested cell lysates (40 μg of protein) were run for detection of PrPC. The rows labeled PK, RML, and Passage indicate whether the sample was PK treated (+) or not (−), whether the culture was incubated with RML prions (+) or diluent (−), and the passage number after incubation, respectively. Passage 2 is 36 dpi, passage 3 is 50 dpi, and passage 4 is 64 dpi; passage 5 is 81 dpi for Tg4053 and 137 dpi for FVB cultures. The blot of lysates from FVB neurospheres, which express ≈8-fold less PrPC than Tg4053 cultures, was exposed longer than the Tg4053 blots. Note the low levels of PrPSc in FVB neurosphere cultures until the fifth passage.
Independently isolated cultures from the three mouse lines gave similar results. Western immunoblots show that PrPSc increased from passage 2 to passage 3 after exposure and was maintained at high levels thereafter in two Tg4053 neurosphere isolates (Fig. 2B Left and Center).
Replication of PrPSc in Neurospheres Correlates with PrPC Expression.
Infected FVB neurospheres also produced PrPSc but to a lesser extent than Tg4053 cultures (Fig. 2B Right). At passages 2–4 after exposure, FVB neurospheres replicated PrPSc more slowly than Tg4053 cultures. FVB cultures were allowed to grow to a density similar to that of Tg4053 neurospheres, and, at passage 5, a dramatic increase in PrPSc level was observed. Infected Tg4053 neurospheres continue to produce high levels of PrPSc for at least 12 passages after exposure (data not shown); this passage history represents an ≈108-fold dilution of the original RML-infected brain homogenate. Infected Tg4053 neurospheres can be cryopreserved and produce PrPSc with thawing (data not shown). Tg4053 cultures are highly susceptible to infection and replicate PrPSc from passage 3 to passage 14. There were no obvious differences in cell morphology or growth rates between infected and uninfected cultures.
Cells in Infected Cultures Contain Intracellular Aggregates of PrPSc.
Previous results from several laboratories indicate that PrPSc accumulates intracellularly (15, 18, 28). Immunofluorescent detection of PrP on fixed, permeabilized single-cell preparations (Fig. 3A) and on cells grown on poly-l-lysine-coated coverslips (Fig. 3B) from infected and uninfected Tg4053 neurospheres was performed with or without denaturation by guanidine thiocyanate (GdnSCN). Cells from infected cultures treated with GdnSCN before anti-PrP staining appeared different from uninfected cultures and from nondenatured, infected cells. Fluorescence was more intense and granular in GdnSCN-treated infected cells (Fig. 3 A Lower Right and B Lower) than in the other samples. The single-cell preparations were used to quantify differences in fluorescence intensity in individual cells among the groups. In uninfected cells, no significant difference was seen in PrP immunostaining intensity between denatured and nondenatured samples (Fig. 3C). In contrast, the intensity of PrP immunostaining was significantly (ANOVA; F = 37.896; P < 0.0001) greater in denatured, infected cells compared with nondenatured, infected samples and with uninfected cultures (Fig. 3C). This increased staining and punctate distribution from infected, denatured cells indicate accumulation of PrPSc because the epitopes detected by the D13 and D18 Fabs are buried in undenatured PrPSc (29). The bright punctate staining provides a marker for infected cells. In contrast to ScN2a cells, where only a minority of the cells produce PrPSc, >95% of the cells in infected Tg4053 neurosphere cultures are positive for PrPSc (see Fig. 7, which is published as supporting information on the PNAS web site). The high proportion of infected cells can also be seen in cell blots, where nearly all Ponceau-staining entities transferred to nitrocellulose are positive for PK-resistant PrPSc (Fig. 4).
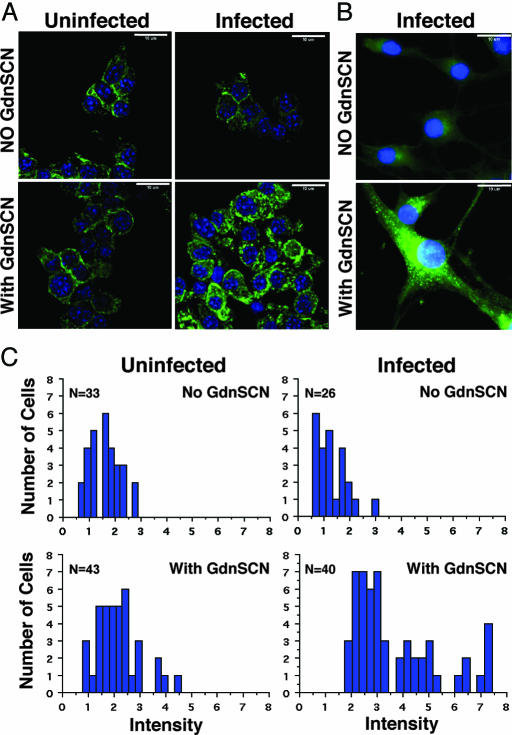
Fig. 3.
Intracellular aggregates of PrPSc in infected Tg4053 neurospheres. (A) Uninfected (Left) and infected (Right) Tg4053 neurospheres at passage 5 (81 dpi) were harvested and triturated to obtain single cells/small cell clumps. Cells were fixed, permeabilized, and denatured (Lower) or not (Upper) with GdnSCN. The cells were applied to albuminized slides for indirect immunofluorescent staining using anti-PrP Fab D18. Nuclei were stained with DAPI (blue). The D18 epitope is poorly accessible in nondenatured PrPSc. Exposure of all panels is equivalent. (Scale bars, 10 μm.) (B) To assess better the intracellular localization of PrPSc, infected Tg4053 neurospheres were dissociated and grown on poly-l-lysine-treated slides for 4 days, denatured or not with GdnSCN. PrP was stained with D18 (green); nuclei were stained with DAPI (blue). (Scale bars, 10 μm.) (C) Quantitative analysis of the fluorescence intensity of individual cells, some of which are shown in A, as described in Materials and Methods. Histograms show the number of cells plotted against the intensity in arbitrary units; n indicates the number of cells analyzed. PrP immunofluorescence was significantly greater in infected, GdnSCN-treated Tg4053 neurosphere cells than in infected cells without denaturation or in uninfected cultures, indicating the accumulation of PrPSc aggregates in infected cells (ANOVA; F = 37.896; P < 0.0001).
Fig. 4.
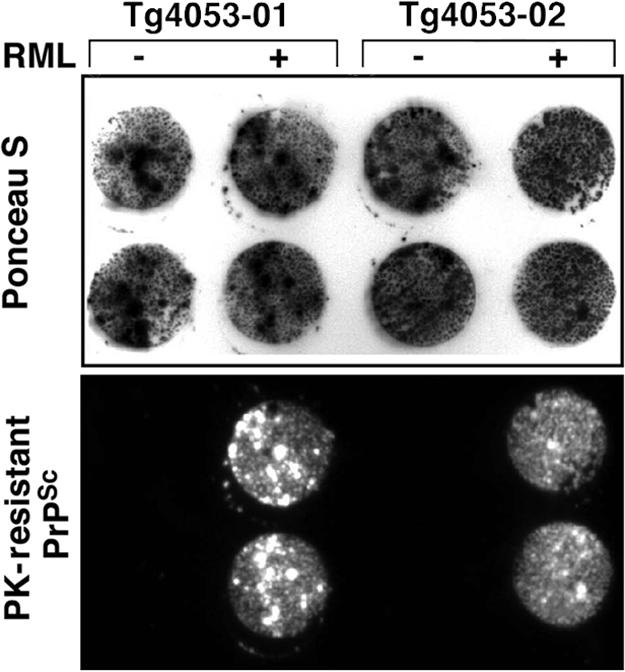
Most Tg4053 neurospheres in infected cultures produce PrPSc. Two lines of Tg4053 neurospheres were infected, or not, at passage 9 with RML prions. Six passages later, infected (+) and noninfected (−) cells were grown on tissue culture-treated coverslips, and cell blots were prepared. Before immunostaining, the membrane was stained with Ponceau S (0.1% Ponceau S in 1% acetic acid), followed by several washes in picopure water, and photographed (Upper). Ponceau stain was removed by several washes in lysis buffer followed by incubation with PK, denaturation with GdnSCN, and immunostaining with anti-PrP Fab D18 antibody. Chemiluminescence shows PK-resistant PrPSc localization (Lower). Comparison of Ponceau S and PrPSc-positive spots indicates that nearly all entities that transferred to the membrane from infected cultures were positive for PrPSc.
PrPSc Replicating in Tg4053 Neurosphere Cultures Is Infectious.
To determine whether PrPSc replicating in Tg4053 neurospheres is infectious, we inoculated cell lysates containing 4 μg of protein into Tg4053 and FVB mice. All inoculated Tg4053 and FVB mice developed disease with mean incubation times of 75.4 ± 3.8 and 171 ± 0 days, respectively (Table 1); 40 μg of the brain homogenate containing RML prions produced disease in 50 ± 2 and 127 ± 2 days in Tg4053 and FVB mice, respectively (4, 5). Mice inoculated with Tg4053 neurosphere lysates developed neuropathology typical of mouse RML prions (see Fig. 8A, which is published as supporting information on the PNAS web site). In contrast, all mice injected with lysates (40 μg of protein) of Prnp0/0 neurosphere cultures exposed to RML prions remained healthy and showed no pathological changes in their brains. Western blot analysis demonstrated the presence of PK-resistant PrPSc with a glycoform profile similar to that of the original RML inoculum (see Fig. 8B). As expected, formation of PK-resistant PrP isoforms in Tg4053 neurospheres was accompanied by the production of infectious prions.
Table 1.
Cell lysates from Tg4053 neurosphere cultures, but not from Prnp0/0 cultures, infected with RML prions transmit disease to mice
| Mice inoculated | Inoculum (total protein) | Incubation time (no. sick/no. inoculated) |
|---|---|---|
| Tg4053 | Infected Tg4053 cell lysate (4 μg) | 75 ± 3.8 days (5/5) |
| Tg4053 | Infected Prnp0/0 cell lysate (40 μg) | — (0/5)* |
| Tg4053 | RML brain homogenate (40 μg) | 50 ± 2 days (16/16)† |
| FVB/NCr | Infected Tg4053 cell lysate (4 μg) | 171 ± 0 days (5/5) |
| FVB/NCr | Infected Prnp0/0 cell lysate (40 μg) | — (0/5)* |
| FVB/NCr | RML brain homogenate (40 μg) | 127 ± 2 days (18/18)‡ |
Neurosphere Cultures as a Prion Bioassay.
RML prions in mouse brain homogenate (18 μg of protein per μl) diluted 50-, 500-, 5,000-, or 50,000-fold were incubated in triplicate with two independent isolates of Tg4053 neurospheres. Dot blot analysis was conducted on neurosphere lysates starting at passage 2 (36 dpi) and continuing through passage 5 (76 dpi) (Fig. 5). At passage 2, PK-resistant PrPSc was detected in 20 μg of lysate from both cell lines infected with lower dilutions (1:50 and 1:500) of RML prions. Little, if any, PK-resistant PrPSc was detected in 80 μg of protein from neurospheres incubated with RML prions diluted 50,000-fold at passage 2, although comparable amounts of PrPC were present in all samples. On subsequent passages, the level of PK-resistant PrPSc produced by cultures exposed to high dilutions of prions increased (Fig. 5, passages 3–5). Importantly, most cultures exposed to RML diluted 50,000-fold, which showed very little PrPSc at passages 2 and 3, had detectable PrPSc in 20 μg of protein at passage 4, which increased with additional passages. These findings demonstrate that prions in Tg4053 neurosphere cultures replicate and spread from cell to cell.
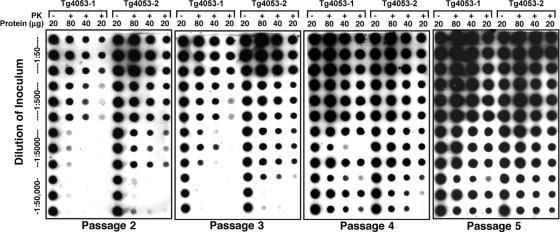
Fig. 5.
PrPSc levels increase with passage in Tg4053 cultures, indicating that neurospheres can serve as a prion bioassay. Two independent Tg4053 isolates were incubated in triplicate with four 10-fold dilutions of 10% RML-infected brain homogenate starting at 1:50. At each passage shown, cell lysates were prepared and either left undigested (−) or digested (+) with PK. Undigested lysate containing 20 μg of protein and PK-digested lysates originally containing 20, 40, or 80 μg of protein were blotted and immunostained for PrP after denaturation with GdnSCN. At high dilutions of RML, the PrPSc signal increased with passage.
Discussion
Neurosphere cultures offer the advantages of both primary cultures and established cell lines. Several cell lines, N2a or GT1 for example, can be infected and maintain prion replication over many passages. Much of our knowledge of the cell biology of prion replication comes from experiments using such cell lines (9, 11, 12, 15), but the number of independently isolated lines is limited and thus impedes analysis of the mechanisms underlying genetic differences in susceptibility to infection. Prion replication in the neurosphere lines described in this report reflects the prion susceptibility of the mice from which they were derived. Using well-established procedures to derive neurosphere/CNS stem-cell cultures, we have produced neurosphere lines from 10 mouse strains and Tg lines as well as the three lines reported here. Neurospheres provide the choice of genotypes offered by primary cultures and stable replication of prions over many passages, as indicated by our results. Both FVB and Tg4053 neurospheres were able to be infected soon after they were established (passage 3), when growth was slower, and remained infectible over at least nine additional passages.
Prion replication takes longer to become established in FVB than in Tg4053 neurospheres. In mice, the length of the incubation time is inversely proportional to the level of PrPC expression (5, 6). It is not known whether the decreased incubation time (and increased rate of prion replication) in Tg mice overexpressing PrP is due to increased efficiency of infection resulting in more cells initially infected, to faster replication in each infected cell, or both. After incubation with RML-infected brain homogenates, neurospheres from FVB, Tg4053, and Prnp0/0 mice show different time courses for PrPSc immunostaining (Fig. 2). In cell blots, PrPSc-positive neurospheres/cell clumps persist in the Prnp0/0 line for 24 days but decline to undetectable levels with passage. Similar results also were reported with use of primary cultures of Prnp0/0 cerebellar neurons in which infectivity persisted at substantial levels for ≈28 days postinoculation; however, these cultures could be maintained for little more than a month (17). In contrast, cell blots of Tg4053 neurospheres show much more PK-resistant immunostaining at 36 dpi than can be explained by residual inoculum, and high levels of PrPSc continue to be produced with repeated passage. Cell blots of FVB cultures also show production of PrPSc but at much lower levels than Tg4053 neurospheres (Fig. 2). Our results suggest that infection is more rapidly established in Tg4053 neurospheres than in FVB cultures.
Klohn et al. (30) established a cell blot assay using a subclone of N2a cells; at high dilutions of RML prions, few cells were initially infected, but on subsequent passage, the prions spread to additional cells. A similar spread appears to occur in our FVB and Tg4053 neurosphere cultures. Our results suggest that high levels of PrPC in neurospheres increase the rate of PrPSc formation, in accord with earlier studies in Tg mice (6). Whether Tg4053 neurospheres can be infected with lower doses of prions than FVB neurospheres remains to be established.
In contrast to our results in which infected neurosphere cultures remained healthy and stably infected, the primary cultures infected with sheep scrapie prions consistently showed apoptosis (17). These primary neuronal cultures were maintained only for 28 days and could not be subpassaged, making it difficult to distinguish de novo-generated infectivity from the original inoculum. Neurospheres may be the first primary culture system to propagate prions stably similar to the few immortalized cell lines that are capable of replicating mouse prions.
Our results also demonstrate that prions can replicate in cells with CNS stem-cell properties; whether replication occurs in endogenous stem cells of infected animals is not known. Although we have not tested stem-cell activity in our cultures, similar neurosphere cultures have been shown to be capable of differentiation into the three major cell types of the CNS: neurons, astrocytes, and oligodendrocytes (22, 25, 27). Most cells in our neurosphere cultures express vimentin, which has been found in human fetal brain cell cultures (31) and is a commonly used marker for CNS stem cells, and nestin, a major cytoskeletal protein in neuronal progenitor cells (26). Most cells in neuroepithelium are nestin-positive before neurogenesis (32). Thus, our neurosphere cultures are likely to possess cells with CNS stem-cell properties. The presence of adherent cells that are highly positive for the glial marker glial fibrillary acidic protein or for the neuronal marker microtubule-associated protein-2 supports this view.
Immunofluorescent staining after GdnSCN denaturation allowed us to determine that almost all cells in infected Tg4053 cultures produce PrPSc. The subcellular distribution of PrPSc has been difficult to determine because of the poor immunoreactivity of native PrPSc and the deleterious effect of GdnSCN on cell morphology (28). Previous work has shown that PrPSc accumulates as aggregates in the cytoplasm of ScN2a cells (15). Similar results were also obtained in prion-infected peripheral neuroglial cells from sheep (18). A GFP–PrP fusion protein, which binds to PrPSc but does not itself convert to PrPSc, shows a similar distribution without the need for denaturants (33). Denaturation by GdnSCN followed by immunostaining showed a significant increase in fluorescence intensity and revealed punctate deposits in infected Tg4053 neurosphere cultures. Over 95% of the cells in infected Tg4053 cultures showed this pattern of staining, which was not seen in uninfected cultures. This finding is in contrast to persistently infected N2a cell cultures, in which a low fraction of the cells contains PrPSc. Even highly prion-susceptible subclones of N2a are unstable, with decreasing numbers of cells infected on repeated passaging (12, 30, 34).
In summary, PrPSc-positive Tg4053 neurospheres were readily detected 56 days after inoculation with RML-infected brain homogenate at a dilution of 1:50,000. This result suggests that neurospheres can be used as a sensitive bioassay for mouse prions. Neurospheres produced from Tg mice may offer a bioassay not only for mouse prions but also for all other prions, including those from cattle and humans. How rapid a neurosphere bioassay can be made is unclear. In Fig. 5, no additional sensitivity was obtained over that found at the fourth passage by a fifth passage. Neurospheres may not only provide a novel system for the bioassay of prion infectivity but may also offer new approaches to studying the replication of prions as well as the spread of prions from one cell to another.
Materials and Methods
Mice.
FVB, Prnp0/0, and Tg4053 mice were used. Prnp0/0 mice, which lack PrP, and Tg4053 mice, which express ≈8-fold higher levels of PrPC than non-Tg mice, have been described previously (5, 8). Mice were bred and housed at the McLaughlin Research Institute.
Prion Isolates.
The RML isolate of the Chandler prion strain was a 10% (wt/vol) brain homogenate from clinically ill CD-1 mice as described previously (35, 36). Lysates from neurosphere cultures to test for prion infectivity were produced by washing the cells in sterile PBS and subjecting them to three cycles of freeze–thaw to kill the cells. They were then passed successively through smaller-gauge needles (18–20-22–25-27 gauge) and stored at −80°C. Protein concentration in the lysates was determined by the bicinchoninic acid assay as recommended by the manufacturer (Pierce).
Antibodies.
Recombinant anti-PrP Fabs D18 and D13 have been described previously (37). These humanized anti-PrP Fabs were used at 0.5 μg/ml for immunoblots and 5 μg/ml for immunocytochemistry; binding was detected by using goat anti-human F(ab)2 polyclonal antibody conjugated with either peroxidase or fluorescein (Pierce). Mouse monoclonal antibodies to nestin, vimentin, and the housekeeping gene GAPDH were diluted as recommended by the manufacturer (Chemicon); peroxidase- or Alexa Fluor 546-conjugated goat anti-mouse IgG (H and L chains; Molecular Probes) was used as the secondary antibody.
Neurosphere Isolation.
Isolation of neurosphere lines used methods similar to those described previously (24, 27). Embryos from mice were harvested at embryonic day 13 (E13) to E15, where E0 is the day the postcoital vaginal plug forms. The brains were removed and transferred to a 35-mm plate containing serum-free DMEM supplemented with 2 mM glutamine, 100 units of penicillin, and 100 μg/ml streptomycin (Invitrogen). Cells were mechanically dissociated by trituration with a 200-μl Gilson pipette, and the resulting cell suspension was filtered through a 45-μm cell strainer (Falcon). The cells were centrifuged at 100 × g, and the pellet was resuspended in neurobasal medium with N2 supplement (GIBCO/Invitrogen), 2 mM l -glutamine, penicillin, streptomycin, 20 ng/ml epidermal growth factor and 10 ng/ml basic fibroblast growth factor (both human recombinant from GIBCO/Invitrogen), and 10 ng/ml mouse recombinant leukemia inhibitor factor (Chemicon). Cells harvested from individual fetal brains were suspended in 15 ml of medium in non-tissue culture-treated T75 flasks (Nunc) and cultured for 2 days in a humidified incubator at 37°C with 5% CO2 in air. Nonadherent cell clusters were collected and recultured in uncoated T75 flasks. After 4–7 days of culture, distinct spheres of cells (neurospheres) were observed. Neurosphere cultures were fed every 3–4 days by replacing 7–10 ml of old medium with fresh medium and were passaged by mechanical dissociation and washing when the medium started to change color from red to orange/yellow followed by reculturing at a 1:4 dilution (every 10–40 days). At every passage, some cells or cell clumps initially attach to the substrate but grow as mounds of cells that later detach to become neurospheres (see Fig. 9, which is published as supporting information on the PNAS web site). During culture, extensive attachment was prevented by gently knocking the flasks every other day. The property of dissociated neurospheres to attach was exploited for immunostaining by plating cells at low density on tissue culture-grade coverslips, chamber slides, or poly-l-lysine-coated coverslips.
Immunocytochemistry.
To determine whether neurosphere cells express PrPC and other markers, neurospheres were dissociated by trituration and grown for 4–5 days on tissue culture-treated glass chamber slides. Cells were fixed with 4% paraformaldehyde for 30 min followed by three 10-min washes with PBS. The cells then were permeabilized by incubation in 0.3% Triton X-100 in PBS for 5 min at room temperature followed by three 5-min washes with PBS and blocking with 10% normal goat serum. Primary antibodies were added and incubated overnight at 4°C. The cells were washed three times and incubated with the appropriate secondary antibodies for 1 h at room temperature, washed three times, rinsed with water, and coverslipped with antifade mounting medium (Molecular Probes). Fluorescence was detected and digital images were taken with a Nikon TE2000 photomicroscope. The epitopes detected by D18 and D13 Fabs are buried in PrPSc, so denaturation is required for PrPSc detection (38, 39). For in situ detection of PrPSc in neurosphere cultures, the neurospheres were triturated, filtered, fixed, permeabilized, and denatured with 3 M GdnSCN. Cell suspensions were adhered to an albumin-coated microscope slide; PrP was detected as described earlier. Nuclei were counterstained with DAPI. For quantification of PrP immunofluorescence, stacks of images along the z axis were acquired with a Quantix-57, 12-bit cooled CCD camera (Photometrics, Tucson, AZ) and metamorph software (Molecular Devices). For quantitative comparisons, samples were analyzed during the same session by using identical acquisition settings. Image stacks were deconvoluted with autodeblur (AutoQuant Imaging, Troy, NY), and regions were drawn around individual nonoverlapping cells. The regions were transferred to the original image stack, and PrP fluorescence intensity was measured in the best focus plane by using metamorph. For qualitative illustrations, the in-focus planes of the image stack were background subtracted and corrected for shading before generating a maximum projection.
Immunoblots.
Cell blots.
The cell blot technique has been described (12). Briefly, cells adherent to plastic coverslips were transferred to a nitrocellulose membrane saturated with lysis buffer (150 mM NaCl/10 mM Tris, pH 7.5/0.5% sodium deoxycholate/0.5% Triton X-100). The membrane was dried, either left undigested or incubated with PK, denatured with 3 M GdnSCN, and immunostained.
Western blots.
Neurosphere lysates were clarified by centrifugation at 500 × g, and the protein concentration was determined by the bicinchoninic acid assay. For PrPSc detection, 500 μg of total protein was treated with 5 μg of PK at 37°C for 1 h, followed by addition of Pefabloc (Fluka) to a final concentration of 4 mM. Insoluble proteins were collected by centrifugation at ≈18,000 × g for 30 min at room temperature, resuspended in sample buffer, boiled for 5 min, subjected to SDS/PAGE, and transferred to a nitrocellulose membrane. PrP was detected by using D13 or D18 Fab and chemiluminescence (SuperSignal West Pico Kit; Pierce). Samples for PrPC detection were not treated with PK. Blots were stripped and reprobed with anti-GAPDH to normalize protein loading and transfer.
Dot blots.
Cell lysates either left untreated or treated with PK were loaded into a 96-well, dot blot apparatus and transferred onto a nitrocellulose membrane by applying vacuum. The membrane was air dried, probed with anti-PrP, and developed as described for Western blots.
Prion-Incubation Time.
Mice were inoculated intracerebrally by using a 26-gauge needle with 20 μl of brain homogenate or culture lysate under isoflurane anesthesia. Inoculated mice were examined for neurological dysfunction once every week for the first month after inoculation and three times per week thereafter as described (36). Brains were harvested from terminally ill mice and their controls; one-half was immediately frozen for biochemical analyses, and the other half was fixed in 10% buffered formalin solution for histopathology.
Supplementary Material
Acknowledgments
We thank Dr. William Provance of the McLaughlin Research Institute Imaging Core Facility for valuable assistance with fluorescence microscopy and quantification. This work was funded by grants from the National Prion Research Program, U.S. Department of Defense (Grants DAMD17-03-1-0321 and DAMD17-03-1-0425) and from the National Institutes of Health (Grants NS41997, AG02132, AG10770, and AG021601), U.S. Public Health Service.
Abbreviations
- PrP
prion protein
- Prnp0/0
FVB.129-Prnptm1Zrch
- Tg
transgenic
- FVB
FVB/NCr
- Tg4053
FVB-Tg(MoPrP-A)4053
- RML
Rocky Mountain Laboratory
- PK
proteinase K
- dpi
days postinfection
- GdnSCN
guanidine thiocyanate
- En
embryonic day n.
References
- 1.Prusiner S. B. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeArmond S. J., Prusiner S. B. Am. J. Pathol. 1995;146:785–811. [PMC free article] [PubMed] [Google Scholar]
- 3.Prusiner S. B. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 4.Telling G. C., Parchi P., DeArmond S. J., Cortelli P., Montagna P., Gabizon R., Mastrianni J., Lugaresi E., Gambetti P., Prusiner S. B. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 5.Carlson G. A., Ebeling C., Yang S. L., Telling G., Torchia M., Groth D., Westaway D., DeArmond S. J., Prusiner S. B. Proc. Natl. Acad. Sci. USA. 1994;91:5690–5694. doi: 10.1073/pnas.91.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A., et al. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 7.Prusiner S. B., Groth D., Serban A., Koehler R., Foster D., Torchia M., Burton D., Yang S. L., DeArmond S. J. Proc. Natl. Acad. Sci. USA. 1993;90:10608–10612. doi: 10.1073/pnas.90.22.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bueler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissman C. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 9.Harris D. A. Curr. Issues Mol. Biol. 1999;1:65–75. [PubMed] [Google Scholar]
- 10.Weissmann C., Enari M., Klohn P. C., Rossi D., Flechsig E. Proc. Natl. Acad. Sci. USA. 2001;99(Suppl. 4):16378–16383. doi: 10.1073/pnas.172403799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enari M., Flechsig E., Weissmann C. Proc. Natl. Acad. Sci. USA. 2001;98:9295–9299. doi: 10.1073/pnas.151242598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosque P. J., Prusiner S. B. J. Virol. 2000;74:4377–4386. doi: 10.1128/jvi.74.9.4377-4386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubenstein R., Deng H., Race R. E., Ju W., Scalici C. L., Papini M. C., Kascsak R. J., Carp R. I. J. Gen. Virol. 1992;73:3027–3031. doi: 10.1099/0022-1317-73-11-3027. [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein R., Carp R. I., Callahan S. M. J. Gen. Virol. 1984;65(Pt 12):2191–2198. doi: 10.1099/0022-1317-65-12-2191. [DOI] [PubMed] [Google Scholar]
- 15.Taraboulos A., Serban D., Prusiner S. B. J. Cell Biol. 1990;110:2117–2132. doi: 10.1083/jcb.110.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatzl H. M., Laszlo L., Holtzman D. M., Tatzelt J., DeArmond S. J., Weiner R. I., Mobley W. C., Prusiner S. B. J. Virol. 1997;71:8821–8831. doi: 10.1128/jvi.71.11.8821-8831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cronier S., Laude H., Peyrin J. M. Proc. Natl. Acad. Sci. USA. 2004;101:12271–12276. doi: 10.1073/pnas.0402725101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archer F., Bachelin C., Andreoletti O., Besnard N., Perrot G., Langevin C., Le Dur A., Vilette D., Baron-Van Evercooren A., Vilotte J. L., et al. J. Virol. 2004;78:482–490. doi: 10.1128/JVI.78.1.482-490.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilette D., Andreoletti O., Archer F., Madelaine M. F., Vilotte J. L., Lehmann S., Laude H. Proc. Natl. Acad. Sci. USA. 2001;98:4055–4059. doi: 10.1073/pnas.061337998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vorberg I., Raines A., Priola S. A. J. Biol. Chem. 2004;279:29218–29225. doi: 10.1074/jbc.M402576200. [DOI] [PubMed] [Google Scholar]
- 21.Vorberg I., Raines A., Story B., Priola S. A. J. Infect. Dis. 2004;189:431–439. doi: 10.1086/381166. [DOI] [PubMed] [Google Scholar]
- 22.Gage F. H. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 23.McKay R. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds B. A., Weiss S. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 25.Uchida N., Buck D. W., He D., Reitsma M. J., Masek M., Phan T. V., Tsukamoto A. S., Gage F. H., Weissman I. L. Proc. Natl. Acad. Sci. USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lendahl U., Zimmerman L. B., McKay R. D. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds B. A., Tetzlaff W., Weiss S. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris D. A. Clin. Microbiol. Rev. 1999;12:429–444. doi: 10.1128/cmr.12.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peretz D., Williamson R. A., Matsunaga Y., Serban H., Pinilla C., Bastidas R. B., Rozenshteyn R., James T. L., Houghten R. A., Cohen F. E., et al. J. Mol. Biol. 1997;273:614–622. doi: 10.1006/jmbi.1997.1328. [DOI] [PubMed] [Google Scholar]
- 30.Klohn P. C., Stoltze L., Flechsig E., Enari M., Weissmann C. Proc. Natl. Acad. Sci. USA. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messam C. A., Hou J., Major E. O. Exp. Neurol. 2000;161:585–596. doi: 10.1006/exnr.1999.7319. [DOI] [PubMed] [Google Scholar]
- 32.Frederiksen K., McKay R. D. J. Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barmada S. J., Harris D. A. J. Neurosci. 2005;25:5824–5832. doi: 10.1523/JNEUROSCI.1192-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishida N., Harris D. A., Vilette D., Laude H., Frobert Y., Grassi J., Casanova D., Milhavet O., Lehmann S. J. Virol. 2000;74:320–325. doi: 10.1128/jvi.74.1.320-325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandler R. L. Lancet. 1961;i:1378–1379. doi: 10.1016/s0140-6736(61)92008-6. [DOI] [PubMed] [Google Scholar]
- 36.Carlson G. A., Kingsbury D. T., Goodman P. A., Coleman S., Marshall S. T., DeArmond S., Westaway D., Prusiner S. B. Cell. 1986;46:503–511. doi: 10.1016/0092-8674(86)90875-5. [DOI] [PubMed] [Google Scholar]
- 37.Peretz D., Williamson R. A., Kaneko K., Vergara J., Leclerc E., Schmitt-Ulms G., Mehlhorn I. R., Legname G., Wormald M. R., Rudd P. M., et al. Nature. 2001;412:739–743. doi: 10.1038/35089090. [DOI] [PubMed] [Google Scholar]
- 38.Peretz D., Williamson R. A., Legname G., Matsunaga Y., Vergara J., Burton D. R., DeArmond S. J., Prusiner S. B., Scott M. R. Neuron. 2002;34:921–932. doi: 10.1016/s0896-6273(02)00726-2. [DOI] [PubMed] [Google Scholar]
- 39.Williamson R. A., Peretz D., Pinilla C., Ball H., Bastidas R. B., Rozenshteyn R., Houghten R. A., Prusiner S. B., Burton D. R. J. Virol. 1998;72:9413–9418. doi: 10.1128/jvi.72.11.9413-9418.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.