Abstract
Exogenous administration of the GABAergic neurosteroid allopregnanolone (ALLO) can increase ethanol intake in rats and mice. In order to determine the contribution of endogenous neurosteroids (i.e., ALLO and related pregnane steroids) in the regulation of established ethanol consumption patterns in male C57BL/6J (B6) mice, the 5α-reductase (5αR) enzyme inhibitor, finasteride (FIN), was chronically administered and then subsequently withdrawn. Mice were provided daily 2-hr limited access to a 10% v/v ethanol solution (10E) and water in lickometer chambers during their dark phase. Following the establishment of stable 10E intake patterns, mice were injected (i.p.) with either vehicle (VEH; 20% w/v 2-hydroxypropyl-β-cyclodextrin; n = 8) or FIN (50 mg/kg; n = 16) for 7 days. Effects of withdrawal from FIN treatment were subsequently assessed for an additional 7 days. Ethanol intakes were significantly decreased with acute FIN treatment (days 1-3) and during early withdrawal (days 1-3). Acute FIN treatment was also associated with an extended latency to first bout, reduced first bout size, and greatly attenuated sipper contact count during the initial 20-min interval of 10E access. These findings collectively indicated that acute FIN treatment markedly attenuated the initiation of 10E consumption during the limited access sessions. The influence of FIN on 10E intake patterns was largely dissipated with chronic treatment, suggesting that compensatory changes in neurosteroid modulation of inhibitory tone may have occurred. Thus, acute FIN treatment modulated ethanol intake patterns in a manner opposite to that previously demonstrated for a physiologically-relevant, exogenous ALLO dose, consistent with the ability of a 5αR inhibitor to block ALLO biosynthesis. Manipulation of endogenous neurosteroid activity via biosynthetic enzyme inhibition or antagonism of steroid binding to the GABAA receptor may prove a beneficial pharmacotherapeutic strategy in the intervention of alcohol abuse and alcoholism.
Keywords: Alcohol, Self-administration, Drinking patterns, Lickometer, Bout microarchitecture, Neurosteroid metabolism
1. Introduction
5α-reduced pregnane neurosteroids are believed to mediate many of the nongenomic cellular and behavioral effects of their parent steroid, progesterone. Perhaps the best characterized of these pregnane neurosteroids is 3α-hydroxy-5α-pregnan-20-one (allopregnanolone; ALLO), which is synthesized by the two-step conversion of progesterone to 5α-dihydroprogesterone (5α-DHP) and of 5α-DHP to ALLO via the enzymes 5α-reductase (5αR) and 3α-hydroxysteroid dehydrogenase (3αHSD), respectively (Mellon et al., 2001). ALLO and structurally related progestin metabolites are potent positive modulators of γ-aminobutyric acid type A (GABAA) receptors (Belelli & Lambert, 2005; Lambert et al., 2003) and exhibit anxiolytic, anticonvulsant, anaesthetic, and sedative-hypnotic properties that typify the pharmacological profile of agonists for this receptor complex (for review, see Gasior et al., 1999). Recent findings are consistent with earlier predictions that endogenous ALLO levels (≤ 100 nM) would mimic the activity of this neurosteroid demonstrated at GABAA receptors in vitro (Gee et al., 1988; Morrow et al., 1987). Specifically, use of enzyme inhibition (to reduce neurosteroid biosynthesis) and novel antagonists (to prevent 5α-pregnanes from binding to their putative active site) have discerned a contributory role for endogenous pregnane neurosteroids towards inhibitory GABAergic neurotransmission within the central nervous system (Belelli & Lambert, 2005; Mennerick et al., 2004).
Ethanol exhibits a GABAmimetic profile (see Grobin et al., 1998) that overlaps extensively with the pharmacological activity and behavioral manifestations of pregnane neurosteroids. Although a putative binding pocket for alcohols and inhalants at GABAA receptors has been described (Mihic et al., 1997), the relative insensitivity of most GABAA receptor populations to a direct modulation by ethanol have hinted towards more indirect mechanisms that alter GABA-invoked inhibitory tone that include ethanol-stimulated presynaptic release of GABA (Roberto et al., 2003; 2004) and regional elevation of pregnane neurosteroids levels (Criswell & Breese, 2005). Consistent with this tenet, both systemically-administered (Barbaccia et al., 1999; Finn et al., 2004c; VanDoren et al., 2000) and orally self-administered (Finn et al., 2004c) ethanol augments brain ALLO concentrations in male rodents. These findings are in agreement with recent observations in humans documenting significantly elevated plasma ALLO levels following ethanol self-administration by male and female adolescents (Torres & Ortega, 2003; 2004).
Data supporting an interaction of ALLO and ethanol at GABAA receptors has provided the impetus for studies examining the potential contribution of endogenous ALLO levels on ethanol's effects (see Finn et al., 2004a; Morrow et al., 2001). In vivo studies have determined that the ability of an ethanol injection to increase endogenous ALLO levels was primarily of adrenal and gonadal origin, as the increase in ALLO levels was not apparent in adrenalectomized and gonadectomized rats (Khisti et al., 2003; O'Dell et al., 2004). However, a recent electrophysiological study in hippocampal tissue determined that the action of ethanol on GABAergic inhibition was biphasic, and consisted of a rapid direct effect on GABAA receptor activity and an indirect effect that appeared to be mediated by neurosteroid biosynthesis, documenting that ethanol-induced ALLO synthesis can occur in brain slices (Sanna et al., 2004). That is, pretreatment with the 5α-R inhibitor finasteride did not affect the rapid increase in IPSC amplitude and decay time induced by ethanol, while it abolished the secondary increase of both parameters that was apparent between 20 and 40 min during bath application of ethanol. This biphasic effect of ethanol on GABAergic inhibition may explain why in vivo studies report that finasteride can antagonize some, but not all, behavioral effects of ethanol (e.g., Hirani et al., 2002; Hirani et al., 2005; Khisti et al., 2004; VanDoren et al., 2000).
A compelling interplay between ethanol and 5α-pregnane neurosteroids (particularly, ALLO and pregnanolone) has been repeatedly demonstrated in drug discrimination paradigms, with ALLO and pregnanolone typically exhibiting full substitution for the ethanol training dose (Bowen et al., 1999; Engel et al., 2001; Grant et al., 1996). Limited evidence also suggests that acute ALLO pretreatment reinstates ethanol-appropriate lever responding following extinction in rats (Nie & Janak, 2003). However, a recent study demonstrated that ALLO failed to modify ethanol-induced conditioned place preference, thereby suggesting that this neurosteroid does not alter ethanol's positive motivational effects within this paradigm (Gabriel et al., 2004). This finding contrasts earlier studies conducted within an operant procedure that reported ALLO-elicited increases in ethanol-appropriate lever presses with and without concurrent availability of an alternate (sucrose) reinforcer (Janak & Gill, 2003). An initial report from our laboratory demonstrated that ALLO dose-dependently enhanced self-administration of unsweetened ethanol solutions in male B6 mice during the initial hour of a 2-hr preference drinking procedure (Sinnott et al., 2002). A subsequent multiple-dose characterization of acute ALLO pretreatment on ethanol intake patterns in male B6 mice (Ford et al., 2005) found that low doses of ALLO significantly increased ethanol intake during the 2-hr drinking session. Additionally, ALLO dose-dependently promoted consumption initiation by augmenting the total licks exhibited during the initial 5 minutes of ethanol access (Ford et al., 2005). Collectively these studies support the notion that ALLO likely contributes to ethanol discrimination, reinstatement, and self-administration under both operant and 2-bottle choice conditions.
Taking into consideration the aforementioned observations and their indication of an interaction between ALLO and multiple ethanol-related behaviors, we hypothesized that endogenous 5α-pregnane neurosteroids modulate ethanol self-administration under physiologically-relevant conditions. Thus, the goal of the current study was to determine whether pharmacological suppression of endogenous ALLO levels with FIN would invoke alterations in ethanol intake patterns that were commensurate with an overall reduction in ethanol consumption. Based upon previous work examining the influence of exogenously applied ALLO (Ford et al., 2005; Sinnott et al., 2002), it was predicted that FIN treatment would reduce total ethanol intake and allay the onset (initiation) of self-administration within a drinking session.
2. Materials and Methods
2.1. Animals
Twenty-four male C57BL/6J (B6) mice of approximately 6 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME). Each mouse was individually housed and acclimated to a reverse light/dark schedule (12hr/12hr; lights off at 0900 hrs) for a minimum of 7 days. All mice were provided ad libitum access to rodent chow and tap water in lickometer chambers (see below). Mice were weighed and handled daily throughout acclimation and experimental phases of the study. The local Institutional Animal Care and Use Committee approved all procedures in accordance with the guidelines described in the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council of the National Academies, 2003).
2.2. Apparatus
Custom lickometer chambers were designed as previously described (Ford et al., 2005). Briefly, a four-walled Plexiglas chamber with a hinged top and an elevated, stainless steel wire floor contained two small access ports that permitted access to drinking tubes. The raised floor and perforated top allowed for sufficient ventilation. Stainless steel sippers (Ancare, Bellmore, NY) were adjoined to modified polystyrene serological pipettes (VWR, Tualatin, OR), which allowed for volume measurements to the nearest 0.05 ml. The steel wire floor of the chamber and each metal sipper generated an open electrical circuit wired to a lickometer device (MED Associates, Inc., St. Albans, VT), which permitted the collection of cumulative sipper contact (lick) records for the ethanol solution and water. Lickometers were interfaced to an IBM compatible computer running MED-PC IV software (MED Associates, Inc.).
2.3. Two-Bottle Preference Procedure
The 2-hr limited access time (1100-1300 hrs; starting 2 hr after lights out) was selected based on a pilot study of continuous access that indicated a 2- to 3-fold increase in sipper contacts for a 10% ethanol (10E) solution during the third hour of the dark phase when compared to modest light phase activity. Furthermore, a newly characterized drinking model has documented that greater ethanol intakes are achieved in B6 mice when access begins either 2 or 3 hrs after lights out (when compared to 1 hr; Rhodes et al., 2005). Each day, mice were removed from their lickometer chambers (homecages), weighed, and placed immediately back into their chambers. Mice were then provided 2-hr access to a drug sipper tube containing 10E and a second sipper tube filled with tap water. Upon the establishment of stable 10E intakes, as determined by <10% variability in ethanol dose over 3 consecutive sessions, the mean ± SEM of the 10E dose consumed was 3.03 ± 0.16 g/kg. The 10E sippers were counterbalanced between the left and right sides across lickometer chambers to control for side preferences.
2.4. Injection Habituation and Finasteride Treatment
Each mouse was habituated to daily vehicle injections (VEH; 20% w/v 2-hydroxypropyl-β-cyclodextrin; 0.01 ml/g; i.p.; Cerestar USA, Inc., Hammond, IN) administered at the conclusion of each 2-hr drinking session until stable intake patterns were re-established according to the above mentioned criterion. Mice were then designated to one of two treatment groups that were balanced for ethanol dose (g/kg) consumed. One group received daily systemic injections of finasteride (FIN; 50 mg/kg; n = 16) whereas the other group was administered VEH injections (n = 8) over a period of 7 days. Following chronic treatment, both groups received an additional 7 days of VEH injections to assess withdrawal from FIN. The FIN dose was selected based on pilot studies conducted in our laboratory which demonstrated that a 50 mg/kg dose decreased plasma and brain ALLO levels by 66% and 80%, respectively, at a 24-hr post-injection time point (Finn et al., 2004a). Furthermore, this FIN dose has been found to be effective in altering ethanol-related behaviors (Gorin et al., 2005; Hirani et al., 2005). Because the peak effect of 50 mg/kg FIN on endogenous ALLO levels was at 24-hrs post-injection, treatments were administered 22-hr prior to each drinking session (i.e., conclusion of the prior session).
2.5. Blood Ethanol Concentration (BEC)
BECs were assayed at the conclusion of the 2-hr drinking sessions corresponding to the 7th finasteride treatment and the 7th day of withdrawal from treatment. On each occasion, a 20 μl blood sample was obtained from the intra-orbital sinus of each mouse. The blood samples were processed as previously described (Gallaher et al., 1996). In brief, each blood sample was added to a microcentrifuge tube containing 50 μl of cold 5% ZnSO4 and then was placed on ice. Subsequent to the collection of all samples, 50 μl of 0.3N Ba(OH)2 and 300 μl of deionized water were added to each sample tube. The samples were then agitated and centrifuged for 5 minutes at 12,000 rpm. Supernatants were assayed for BEC by gas chromatography. Seven pairs of external standards with known ethanol concentrations (ranging from 0.25-4.00 mg/ml) were analyzed in parallel to construct a standard curve from which unknown concentrations of samples were interpolated.
2.6. Drugs
The ethanol solution (10% v/v; Pharmco Products, Brookfield, CT) was prepared by dilution of a 200 proof stock in tap water. Finasteride [1,(5α)-androstan-4-aza-3-one-17β-(N-tert-butyl-carboxamide)] was purchased from Steraloids Inc (Newport, RI) and was solubilized in 20% w/v 2-hydroxypropyl-β-cyclodextrin at a stock concentration of 5 mg/ml, which permitted an injection volume of 0.01 ml/g body weight.
2.7. Statistical Analysis
The ethanol dose (g/kg) consumed was calculated based on the 10E volume (ml) depleted throughout the drinking session and body weight recorded immediately prior to the session start. Ethanol preference ratios were derived from the 10E volume consumed divided by the total fluid volume (10E plus water). Independent cumulative sipper contact (lick) records for the 10E and water drinking tubes were generated by MED-PC IV software (MED Associates, Inc.) and compiled by a custom data analysis program that calculated several consumption pattern endpoints: total sipper contacts (licks), bout frequency, bout size (licks), bout length (min), inter-bout interval (IBI; min), bout lick rates (licks/min), and latency to first bout (min). The male B6 mice in this study consistently exhibited ethanol preference ratios approximating 0.95, and concomitant total session water licks averaged 100 or less, thereby precluding any meaningful analysis of bout dynamics and consumption patterns for water. Based upon our previous work in rat (Ford et al., 2002) and mouse (Ford et al., 2005) self-administration models, an ethanol bout was experimentally defined as a minimum of 20 licks with no more than a 60 second pause between successive licks. The reported bout lick rates were derived from the average rate of all bouts expressed, and did not reflect IBIs.
All statistical analyses were performed with the SigmaStat version 2.03 software package (SPSS Inc., Chicago, IL), all figures and cumulative records were generated with the Sigma Plot 2001 software package (SPSS Inc.), and temporal distribution analysis of sipper contacts (licks) was facilitated by the SoftCR for Windows program (MED Associates, Inc.). The SigmaPlot linear regression function (based on least squares fit of data points) was utilized to derive the correlation between BECs versus ethanol dose (g/kg). Based on a preliminary analyses of daily ethanol dose (g/kg) consumed by FIN-and VEH-treated mice across the experimental time course (see Results section for more details), FIN effects on within-group drinking pattern measures were delineated into treatment phases, and then subsequently evaluated by one-way repeated measures ANOVA [factor = treatment phase: baseline, acute FIN treatment (days 1 & 3), chronic FIN treatment (days 4-7), early withdrawal (days 1 & 3), and protracted withdrawal (days 4-7)]. A two-way repeated measures ANOVA was similarly implemented to identify possible within-group factorial interactions [treatment phase x session interval (20-min bins)] for the temporal distribution of sipper contacts (licks). If a statistically significant interaction was detected, a subsequent analysis of Simple Main effects for treatment phase within each 20-min interval was conducted. When appropriate, pair-wise differences were determined by the Fisher’s least significant difference multiple comparisons procedure. For all statistical analyses, significance was set at p ≤ 0.05.
The VEH-treated group also was examined for the effects of treatment phase on drinking measures. As values in the VEH-treated group did not change significantly across time (see Results section), collapsed VEH treatment values representative of all experimental sessions for each drinking pattern variable are depicted in Table 1 and Figure 1. Within each 20-min interval, t-tests compared the temporal distribution of 10E sipper contacts between the collapsed VEH control and the FIN-treated baseline groups. A similar t-test approach was implemented to compare overall drinking pattern variables between the collapsed VEH control and baseline groups.
Table 1.
Effects of finasteride treatment and withdrawal on ethanol intake and bout microarchitecture. VEH Control values represent the collapsed mean ± SEM across all treatment phases for n = 8 VEH-treated mice. All other value represent the mean ± SEM of each treatment phase for n = 16 FIN-treated mice.
| Treatment Phase |
||||||
|---|---|---|---|---|---|---|
| VEH Control | Baseline | Acute Treatment | Chronic Treatment | Early Withdrawal | Protracted Withdrawal | |
| General Measures | ||||||
| 10E Dose (g/kg) | 3.09 ± 0.09 | 3.00 ± 0.17 | 2.41 ± 0.19*** | 2.84 ± 0.13 | 2.40 ± 0.18*** | 3.00 ± 0.17 |
| 10E Licks | 1090 ± 63 | 1151 ± 62 | 950 ± 69*** | 1073 ± 43 | 906 ± 66*** | 1092 ± 63 |
| Water Licks | 97 ± 38 | 116 ± 30 | 175 ± 41* | 105 ± 26 | 102 ± 30 | 104 ± 43 |
| Total Fluid Intake (ml) | 1.09 ± 0.09 | 1.10 ± 0.05 | 0.96 ± 0.05** | 1.06 ± 0.04 | 0.93 ± 0.06*** | 1.12 ± 0.05 |
| Preference Ratio | .96 ± .02 | .96 ± .01 | .87 ± .05*** | .95 ± .02 | .93 ± .02 | .94 ± .03 |
| Body Weights (g) | 26.2 ± 0.3 | 27.9 ± 0.6 | 28.0 ± 0.6 | 27.8 ± 0.6 | 27.8 ± 0.6 | 27.9 ± 0.5 |
| Total Session 10E Bout Dynamics | ||||||
| Bout Frequency | 11.5 ± 1.0 | 10.6 ± 0.7 | 10.9 ± 0.8 | 11.3 ± 0.5 | 9.2 ± 1.0* | 9.7 ± 0.6 |
| Bout Size (licks) | 100 ± 5 | 115 ± 7 | 84 ± 5*** | 98 ± 5* | 108 ± 9 | 116 ± 7 |
| Inter-bout Interval (min) | 11.7 ± 1.4 | 12.2 ± 1.2 | 13.0 ± 2.8 | 10.5 ± 0.6 | 16.8 ± 2.2* | 13.9 ± 1.3 |
| Bout Length (min) | 0.90 ± 0.10 | 1.04 ± 0.09 | 0.68 ± 0.08*** | 0.70 ± 0.07*** | 0.74 ± 0.08*** | 0.94 ± 0.10 |
| Lick Rate (licks/min) | 316 ± 21 | 296 ± 20 | 343 ± 22* | 354 ± 21** | 344 ± 22* | 320 ± 19 |
| First 10E Bout Dynamics | ||||||
| Latency to 1st Bout (min) | 1.16 ± 0.36 | 1.37 ± 0.27 | 4.36 ± 1.52* | 1.89 ± 0.66 | 2.30 ± 1.01 | 1.24 ± 0.20 |
| Size of 1st Bout (licks) | 212 ± 28 | 177 ± 25 | 118 ± 19* | 170 ± 22 | 180 ± 26 | 224 ± 31 |
| Length of 1st Bout (min) | 2.22 ± 0.20 | 1.92 ± 0.27 | 1.54 ± 0.38 | 1.90 ± 0.32 | 1.76 ± 0.28 | 2.43 ± 0.38 |
| Rate of 1st Bout (licks/min) | 152 ± 13 | 183 ± 32 | 200 ± 43 | 155 ± 23 | 164 ± 28 | 152 ± 31 |
p ≤ .05,
p < .01
p < .001 versus within-group Baseline values for FIN-treated mice.
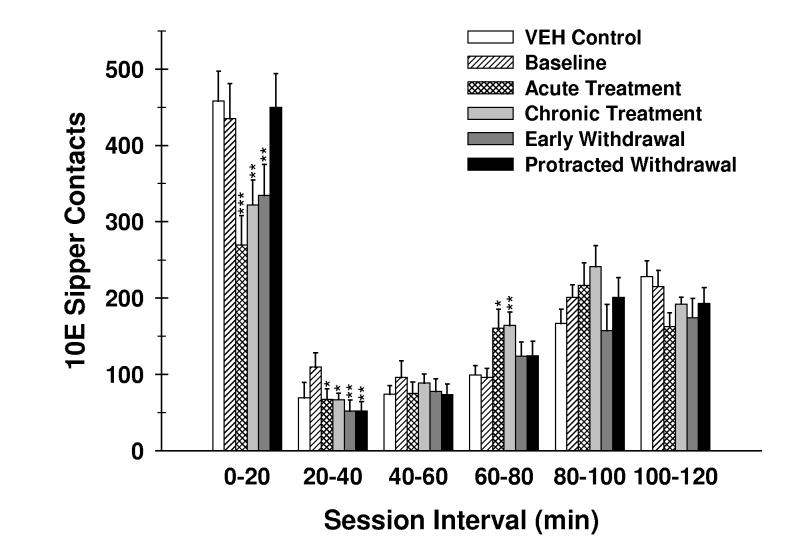
Fig. 1.
Effects of finasteride treatment and withdrawal on the temporal distribution of 10E sipper contacts (licks). The number of 10E licks that occurred during each 20-minute session interval throughout the 2-hr drinking session is shown. Collapsed values for the VEH-treated mice (VEH control) are shown for non-statistical comparison. Vertical bars represent the mean ± SEM of licks for 8 VEH-treated and 16 FIN-treated mice. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001 versus within-subject baseline of FIN-treated mice for the respective session interval.
On limited occasions an obvious discordance between total licks and the ethanol dose (g/kg) consumed by individual mice was apparent, indicating that either a computer detection error occurred or a mouse pawed/grabbed a drinking sipper, ultimately resulting in an unrepresentative record of cumulative sipper contacts. A licks per milliliter (licks/ml) quality assurance criterion was imposed to pinpoint and eliminate these data points from further analysis. The acceptable range of licks/ml values determined for the current study was 1142 ± 544 (mean ± 2 standard deviations). The drinking patterns of one mouse from treatment days 3 and 6 and one mouse from withdrawal day 5 (different mouse on each occasion) were disqualified based on this criterion.
Data from the second day of finasteride treatment and from the second day of withdrawal were not included in statistical analyses. A late session start (≈1.5 hrs) due to facility emergency on the second treatment day resulted in a spike in ethanol intake within both the VEH- and FIN-treated groups, consistent with a recent report that a later start time generates differing intake levels (Rhodes et al., 2005). All drinking pattern data on withdrawal day #2 was lost due to a computer programming complication.
3. Results
3.1. Ethanol Dose vs. Sipper Contacts Correlation and Delineation of Treatment Phases
A significant positive correlation between ethanol licks and ethanol dose was found (r = .894,p < .001,n= 356) throughout the experimental time course. The goodness of fit (r2) of the regression line was 0.799, indicating that 80% of the variation in 10E dose (g/kg) could be accounted for by the variation in 10E licks.
A one-way repeated measure ANOVA for the daily 10E dose (g/kg) consumed by the FIN-treated group (excluding treatment day 2 and withdrawal day 2 as noted above) was initially run as a general survey of treatment and withdrawal effects over the experimental time course. A statistically significant influence of session on 10E dose was detected [F(12,180) = 4.36; p < .001]. Significant reductions in 10E dose were noted on FIN treatment days 1 (p < .01) and 3 (p < .001) as well as on withdrawal days 1 (p < .001) and 3 (p < .01) when compared to a within-group 3-day baseline measure (data not shown). Based on these statistical observations, all FIN-treated group variables were presented as the following treatment phases: baseline (days 1-3), acute treatment (days 1 & 3), chronic treatment (days 4-7), early withdrawal (days 1 & 3), and protracted withdrawal (days 4-7).
A similar analysis in the VEH-treated group revealed no statistically significant differences in the 10E dose consumed between drinking sessions [F(12,84) = 1.49; p = .143]. Ethanol intakes exhibited by the VEH-treated groups were 2.96 ± 0.21, 2.97 ± 0.21, 3.05 ± 0.24, 2.89 ± 0.26, and 3.39 ± 0.30 g/kg/2-hrs during the baseline, acute treatment, chronic treatment, early withdrawal, and protracted withdrawal experimental phases, respectively. Since no within group differences were observed, a collapsed VEH treatment value representative of all experimental sessions for each drinking pattern variable was determined and is provided for non-statistical comparison to the various treatment phase values of FIN-treated mice. However, t-tests also confirmed that drinking pattern variables (Table 1) and 10E sipper contacts within each 20-min interval (Figure 1) were not significantly different between the collapsed VEH control and the baseline values of the FIN-treated mice.
3.2. Effects of Finasteride on Ethanol Intake and Preference Ratio
Statistically significant influences of treatment phase for both 10E dose [F(4,60) = 14.89; p < .001] and 10E licks [F(4,60) = 9.43; p < .001] were detected. Acute FIN treatment significantly attenuated ethanol dose (p < .001) and 10E licks (p < .001) by 20% and 17%, respectively, when compared to baseline values (Table 1). Reductions in these ethanol intake measures largely subsided with chronic treatment. The early FIN withdrawal phase was associated with significantly attenuated 10E dose (p < .001) and 10E licks (p < .001) versus baseline levels. Notably, 10E dose and licks were completely restored to baseline quantities throughout the protracted withdrawal phase.
Although a significant main effect of treatment phase on water licks [F(4,60) = 2.98; p < .05] was observed, this effect was restricted to a significant elevation of 51% during the acute FIN treatment phase (p < .05) when compared to baseline water licks (Table 1). Total fluid intakes (TFIs) were also significantly influenced by treatment phase [F(4,60) = 8.14; p < .001], with significant decreases of 13% and 15% occurring during acute treatment (p < .01) and early withdrawal (p < .001), respectively, when compared to baseline TFIs. The amounts of TFI throughout all treatment phases mirrored the fluctuations in ethanol dose and licks (Table 1), thereby suggesting that the impact of FIN treatment and withdrawal on TFI were largely driven by differences in 10E consumption. A statistically significant impact of treatment phase on ethanol preference ratio [F(4,60) = 4.29; p < .01] was evident, with acute treatment significantly lowering ethanol preference by 9% versus the baseline ratio. This finding, in conjunction with the significant enhancement in water licks during this treatment phase, would suggest that the FIN-induced reduction in 10E dose consumed was neither attributable to a generalized suppression of all fluids nor due to an overall malaise (as the absence of body weight changes for FIN-treated mice throughout the experimental time course would indicate; Table 1).
3.3. Effects of Finasteride on Mean Bout Measures
Statistically significant main effects of treatment phase on both ethanol bout frequency [F(4,60) = 3.56; p < .05] and bout size [F(4,60) = 8.20; p < .001] were detected. Bout frequency was significantly diminished by 13% only during early FIN withdrawal (p < .05) when compared to baseline bout incidences (Table 1). In contrast, mean bout sizes were significantly suppressed only during acute (p < .001) and chronic (p < .05) FIN treatment versus baseline measures (Table 1). A significant influence of treatment phase on inter-bout interval (IBI) was also found [F(4,60) = 3.23; p < .05], with a significant lengthening of IBI by 38% during early FIN withdrawal (p < .05), when compared to baseline durations. These findings indicate that the similar reductions in ethanol intake observed during acute FIN treatment and early FIN withdrawal were mediated by different bout micro-architectural components (i.e., bout size decreases during acute treatment versus bout frequency reductions and IBI extensions during early withdrawal).
Significant main effects of treatment phase on bout length [F(4,60) = 7.73; p < .001] and lick rate [F(4,60) = 3.27; p < .05] were identified. Acute and chronic treatment and early withdrawal of FIN significantly truncated bout length by 29-35% (p < .001) and were associated with lick rates that were significantly accelerated by 16-20% (p < .05) versus baseline values. In contrast to changes in 10E (dose and licks) and water (licks) intakes (Table 1), effects of FIN on bout length and lick rate persisted throughout the chronic treatment phase.
3.4. Effects of Finasteride on First Bout Dynamics
The assessment of mean 10E bout parameters (above) describes overall session drinking patterns that largely reflect the manner in which ethanol self-administration was maintained throughout the 2-hr access period. In order to assess the influence of FIN treatment and withdrawal on the onset of 10E consumption, characteristics specific to the first ethanol bout were also evaluated. Treatment phase significantly altered the latency to first bout [F(4,60) = 2.50; p = .05], which was primarily due to the 2.2-fold delay in the latency to first bout with acute FIN treatment when compared to baseline conditions (Table 1). Treatment phase significantly influenced the size of first bout [F(4,60) = 4.84; p < .01]. Acute FIN treatment significantly diminished the first bout size (p < .05) by 33% versus baseline levels (Table 1). Together, the observations regarding the size and latency to first bout indicated that acute FIN treatment, but not chronic exposure or withdrawal from FIN, curtailed the initiation of ethanol self-administration during the limited access sessions. Length and rate of the first ethanol bout were unaffected by treatment phase.
3.5. Effects of Finasteride on the Temporal Distribution of Sipper Contacts (Licks)
An evaluation of the temporal distribution of 10E sipper contacts throughout the 2-hr limited access sessions was conducted. Two-way repeated measure ANOVA revealed a statistically significant treatment phase x session interval interaction [F(20,300) = 3.84; p < .001] for 10E sipper contacts (Fig. 1). Subsequent analyses within each 20-min session interval yielded statistically significant effects of treatment phase within the 0-20 min [F(4,60) = 10.04; p < .001], 20-40 min [F(4,60) = 3.44; p < .05], and 60-80 min [F(4,60) = 2.55; p < .05] intervals. Within the initial session interval, significant declines in 10E sipper contacts occurred during acute (p < .001) and chronic (p < .01) FIN treatment and early withdrawal (p < .01) when compared to the baseline levels of FIN-treated mice (0-20 min interval; Fig. 1). Notably, the marked 38% drop in 10E sipper contacts that occurred during acute FIN treatment within the first session interval (Fig.1) coincided with the significant attenuation of first bout size and the large increase in latency to first bout (see Table 1). Suppression of 10E sipper contacts persisted during minutes 20-40 of the limited access session, with significant decreases in the acute (p < .05) and chronic (p < .05) FIN treatment as well as early (p < .01) and protracted (p < .01) FIN withdrawal phases. Surprisingly, 10E sipper contacts were significantly elevated as a result of acute (p < .05) and chronic (p < .01) FIN treatment when compared to baseline values during the 60-80 min session interval (Fig. 1). Overall, the influence of FIN treatment and subsequent withdrawal on 10E sipper contacts during minutes 0-40 was suggestive of a pronounced dampening of ethanol intake initiation whereas acute and chronic FIN treatment were associated with a comparably modest elevation in the maintenance of 10E sipper contacts later in the drinking session.
3.6. BECs During Chronic Treatment and Protracted Withdrawal
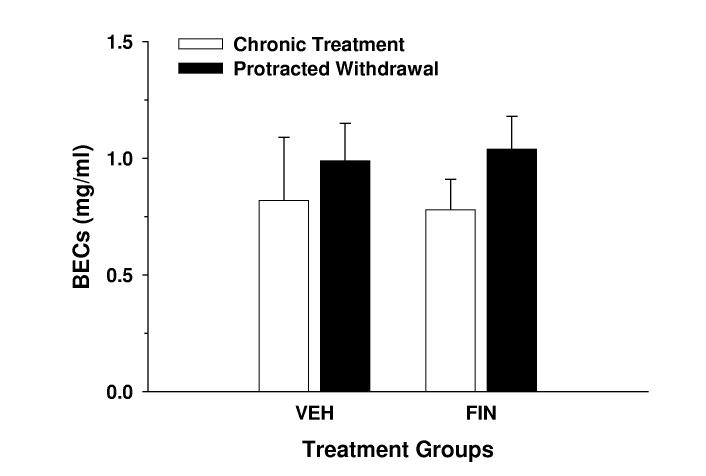
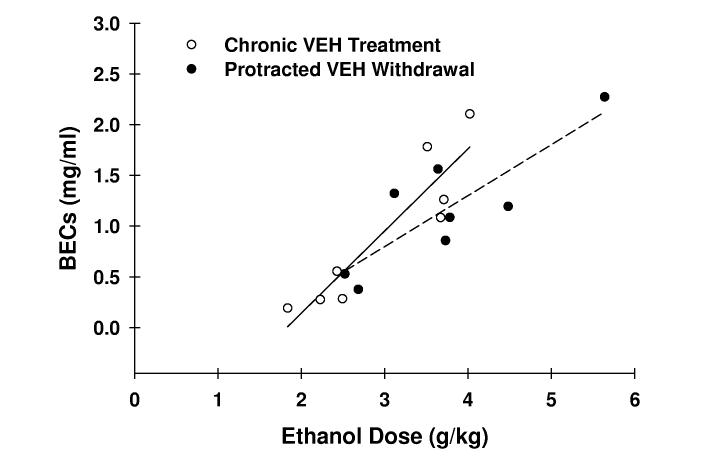
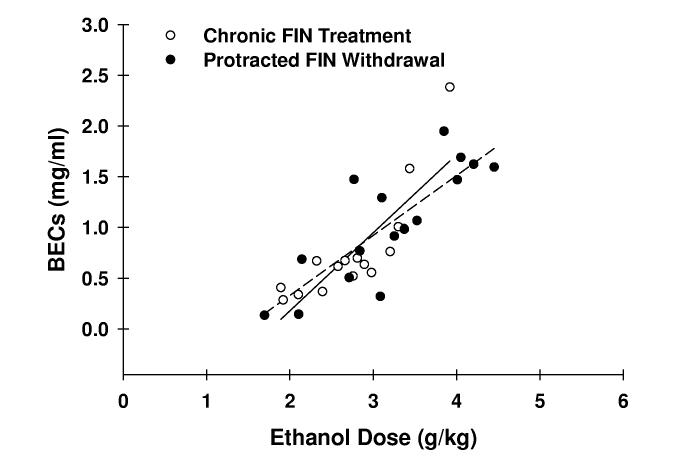
Although a 2-way repeated measure ANOVA failed to identify a significant treatment group x treatment phase interaction for BECs, a significant influence of treatment phase [F(1,22) = 4.31; p = .05] was found. The end-session BECs on day 7 of protracted withdrawal were 20-25% greater than those exhibited on day 7 of chronic treatment (Fig. 2A). These BEC observations were consistent with the 13-18% difference in 10E dose consumed between these respective days (data not shown). Linear regression analyses revealed significant positive correlations between ethanol dose and BEC in both VEH- and FIN-treated mice, respectively, during both chronic treatment (r = .913, p < .01, n = 8; r = .834, p < .001, n = 16) and protracted withdrawal (r = .844, p < .01, n = 8; r = .824, p < .001, n = 16) phases (Fig. 2C). Furthermore, comparison of regression line slopes by t-test revealed no statistical difference in the relationship between ethanol dose and BEC between chronic treatment and protracted withdrawal phases within the VEH or FIN treatment groups.
Fig. 2.
BECs during chronic finasteride treatment and protracted withdrawal. In panel A, the mean ± SEM of BECs are depicted for VEH-treated (n = 8) and FIN-treated (n = 16) mice and represent levels determined at the conclusion of the 2-hr drinking session on finasteride treatment day 7 and on withdrawal day 7. The relationship between BEC and ethanol dose in VEH- (panel B; n = 8) and FIN-treated mice (panel C; n = 16) was also assessed. The solid and dashed lines denote linear regressions for the chronic treatment and protracted withdrawal phases, respectively.
4. Discussion
The present study was the first to demonstrate the effects of FIN treatment and its subsequent withdrawal on ethanol self-administration patterns. One major finding was that acute FIN treatment and early FIN withdrawal resulted in significantly reduced 10E intakes throughout the 2-hr limited access sessions. Notably, the observed decreases in 10E intake without concomitant declines in water intake and changes in body weight suggested that the reduction in 10E intakes was not due to a generalized suppression of drinking or feeding behavior. A second major finding was that the diminished 10E intake during acute FIN treatment was primarily attributable to a decline in mean bout size whereas intake decreases that coincided with early FIN withdrawal were accompanied by a reduced bout frequency and an enlarged IBI. The divergence in bout microarchitectural components mediating the attenuated 10E intakes during acute FIN treatment versus early FIN withdrawal would indicate that different regulatory mechanisms of ethanol consumption behavior were at play during each of these treatment phases. Third, it was determined that bout lengths and lick rates, unlike other 10E bout parameters, were persistently modulated throughout acute and chronic FIN treatment, thereby indicating that drinking pattern dynamics were not entirely tolerant to continuous FIN exposure as the largely restored 10E intakes during chronic treatment would have suggested. Lastly, the parallel observations of an extended latency to first bout, suppressed first bout size, and reduced 10E sipper contact count throughout the first 20-min interval of access indicated that acute FIN treatment markedly dampened the onset of ethanol self-administration.
It was prognosticated that FIN treatment would influence ethanol consumption patterns in a manner that was consistent with the depletion of endogenous ALLO concentrations (or exert the opposite effects of exogenous ALLO administration). Although the role of endogenous ALLO levels has not directly been tested, acute administration of multiple doses of exogenous neurosteroid was demonstrated to modulate ethanol self-administration patterns in male B6 mice (Ford et al., 2005). In contrast to FIN treatment, a 3.2 mg/kg ALLO dose, which was previously reported to manifest physiologically-relevant plasma levels of this steroid (Finn et al., 1997), significantly augmented total session 10E intake. Furthermore, exogenous ALLO dose-dependently elevated sipper contacts within the initial 5 minutes of access and increased the size of the first bout by as much as 130% (Ford et al., 2005). Consistent with these findings, a 3 mg/kg ALLO dose increased operant responding and subsequent ethanol presentations in male rats, an effect that was partly attributable to an increase in the initial run of lever responding that occurred early in the session (Janak et al., 1998). Collectively, these previous observations with ALLO treatment indicated an enhanced onset of ethanol self-administration. This influence of exogenous ALLO on drinking onset is in stark contrast to the current observations that acute FIN treatment suppressed ethanol consumption onset. Thus, the prediction that FIN would modulate drinking patterns in a manner opposite to exogenously administered ALLO was largely realized during the acute treatment phase. Future studies will be required to further examine the selectivity of finasteride's modulation of ethanol intake by determining the effects of this enzyme inhibitor on consumption of sweet (e.g., sucrose or saccharin) or bitter (e.g., quinine) solutions.
In contrast to the pronounced FIN-elicited changes in drinking patterns resulting from acute treatment, these effects largely dissipated with a further continuation of FIN administration (see Table 1). One possible explanation for the waning influence of chronic FIN on consumption parameters could be an attenuated pharmacological efficacy of FIN to block ALLO biosynthesis (i.e., tolerance). However, previous work in rats would suggest that this was likely not the case. Concas and colleagues demonstrated that estrus female rats treated either acutely (19 hrs post-treatment) or daily for 7 days with 25 mg/kg FIN exhibited persistent reductions in plasma (41-49%) and cerebral cortical (46-53%) concentrations of ALLO (Concas et al., 1998).
Another possible explanation for the diminished impact of chronic FIN treatment could have been the occurrence of compensatory alterations in the expression levels of GABAA receptor subunits that altered sensitivity to ethanol. For example, treatment with and subsequent withdrawal from progesterone (and hence ALLO) has previously been associated with a 3-fold increase in expression of the α4 subunit protein within the hippocampus (Smith et al., 1998). Furthermore, it has been demonstrated that recombinant GABAA receptors containing a α4β2δ subunit composition are exquisitely sensitive to very low concentrations (1-3 mM) of ethanol; an effect that is extinguished with substitution of other subunits for either the α4 or δ subunits (Sundstrom-Poromaa et al., 2002). It is plausible that a FIN-invoked “withdrawal” of ALLO in the current study may have similarly impacted expression of α4 and other GABAA receptor subunits over time (i.e., δ subunit; see Follesa et al., 2004), thus resulting in an altered sensitivity to ethanol. This interpretation, although speculative in nature, would be consistent with the demonstration of elevated ethanol intake in women suffering from premenstrual syndrome and the associative progesterone withdrawal that takes place (McLeod et al., 1994).
In a recent characterization of a limited access drinking model in B6 mice, Cronise and colleagues found that 1.0 mg ethanol/ml blood and greater concentrations produced ataxia and quantifiable signs of intoxication (Cronise et al., 2005). BECs consistently ranged from ∼0.8-1.0 mg/ml throughout the current study, suggesting that mice likely experienced the intoxicating effects of ethanol during the drinking sessions. In fact, on a couple of occasions it was observed that mice became immobile after exhibiting copious 10E sipper contacts early within the limited access session. Based on a previous report from our laboratory, there was some concern that chronic FIN treatment may have influenced ethanol pharmacokinetics, and hence the BECs achieved. In a 72-hr ethanol vapor exposure paradigm used to induce ethanol dependence, male B6 mice treated with 50 mg/kg FIN for 4 days exhibited BECs that were 33% lower than similarly exposed mice receiving vehicle treatment (Finn et al., 2004b). However, this decrease in BEC was not apparent until the fourth injection of FIN (there was no significant difference in BEC after the initial three FIN injections). Recently, our laboratory found that an acute FIN pretreatment of 50 mg/kg failed to alter the BECs generated 2-hrs following an injection of a 4 g/kg dose of ethanol (Gorin & Finn, 2005). In the current study, BECs were monitored on the 7th day of chronic FIN exposure and then again at the conclusion of the protracted withdrawal phase. Regression slopes derived from the relationship between ethanol doses and resultant BECs were not different between treatment phases. These data, in conjunction with the data indicating that a single FIN injection did not alter BEC, suggest that ethanol pharmacokinetics were likely unaltered during chronic FIN exposure under the current experimental conditions.
One recent report employing a conditioned place preference procedure demonstrated a significant suppression of activity counts following a 30-min pretreatment with 50 mg/kg FIN (Gabriel et al., 2004). A second report documented a significant increase in the Majchrowicz intoxication rating scale following a 1.5 hr pretreatment with an identical dose of FIN (Khisti et al., 2004). Taken together, these earlier findings suggest that a sedative effect of FIN could have confounded interpretation of FIN's effects on ethanol self-administration patterns in the present study. However, the significant enhancement of water intake (licks) that occurred during acute FIN treatment would indicate that a generalized depression of all behaviors was avoided. Furthermore, other rodent studies have demonstrated no effect of this FIN dose on either the total number of squares entered on an open field grid (Frye & Walf, 2002) or immobility time during a Porsolt's forced swim test (Hirani et al., 2002) when the pretreatment time was extended to up to 2.0 hrs. By pretreating mice with FIN 22-hrs prior to behavioral testing in the present study, we were likely able to minimize this potential confound.
Notably, progesterone (P), testosterone (T) and deoxycorticosterone (DOC) are all substrates of the 5αR enzyme. It is possible that the observed effects of FIN on ethanol consumption patterns could represent a compound influence of inhibition of T and DOC as well as P metabolism. Furthermore, FIN treatment may have shunted these steroid precursors towards an alternate biosynthetic pathway. In support of this conjecture, an examination of female rats in estrus following FIN treatment exhibited P levels that were increased by 2-fold within 2-hrs, but then returned to basal levels by 19-hrs and throughout sub-chronic FIN treatment (Concas et al., 1998). One prominent metabolic end-product for both P and T is estradiol (E2). Although it has been demonstrated that E2 administration to ovariectomized female rats (Ford et al., 2002) and male mice (Hilakivi-Clarke, 1996) enhances ethanol consumption, another study in male rats demonstrated an E2-invoked decrease in ethanol intake (Juarez et al., 2002). Assessment of plasma steroid concentrations during chronic FIN treatment in a future study would help to either discount or affirm this shunting hypothesis.
Several recent studies employing human subjects have directly and indirectly demonstrated a modulatory role for neurosteroids and their metabolism in relation to ethanol's subjective effects, early withdrawal and recovery from dependence. First, FIN was found to diminish the subjective response to self-administered ethanol by moderate drinkers, with particular effectiveness noted in individuals homozygous for the common A-allele variant of the GABRA2 gene encoding the GABAA receptor α2 subunit (Pierucci-Lagha et al., 2005). Second, Romeo and colleagues (1996) discovered a pronounced suppression of plasma ALLO that coincided with augmented anxiety and depression ratings in alcoholic subjects undergoing acute withdrawal. In a subsequent study, pretreatment with either indomethacin or fluoxetine, two known regulators of ALLO biosynthesis, partially restored ALLO concentrations and concurrently decreased anxiety and depression scores in acutely withdrawn alcoholic patients (Romeo et al., 2000). In a similar manner, alcoholic women undergoing therapy exhibited a restoration in the levels of ALLO and the pregnane neurosteroids pregnanolone and isopregnanolone; changes that were accompanied with an increased psychosomatic stability of the patients (Hill et al., 2005a; 2005b). These observations in humans support the suggestion that manipulation of neurosteroid levels and their metabolism may represent a fruitful avenue for the development of adjuvant therapies in the treatment of multiple aspects of alcoholism.
The current findings in mice indicate an important contribution of endogenous pregnane neurosteroids in modulating the initiation, maintenance and termination of ethanol consumption episodes. Further evaluation of ALLO and related pregnane neurosteroids and their effects on ethanol self-administration should yield a more complete understanding of the regulatory role that these endogenous modulators play under normal and deleterious (i.e., ethanol dependence) physiological conditions.
Acknowledgments
The current study was supported by grants AA015234, AA10760, AA12439, DA07262, the Department of Veterans Affairs and the N.L. Tartar Research Fund.
References
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384:R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effect of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999;289:405–411. [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Cronise K, Finn DA, Metten P, Crabbe JC. Scheduled access to ethanol results in motor impairment and tolerance in female C57BL/6J mice. Pharmacol Biochem Behav. 2005;81:943–953. doi: 10.1016/j.pbb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Engel SR, Purdy RH, Grant KA. Characterization of discriminative stimulus effects of the neuroactive steroid pregnanolone. J Pharmacol Exp Ther. 2001;297:489–495. [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004a;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Long SL, Tanchuck MA, Crabbe JC. Interaction of chronic ethanol exposure and finasteride: sex and strain differences. Pharmacol Biochem Behav. 2004b;78:435–443. doi: 10.1016/j.pbb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Lotrich F, Gallaher EJ. Genetic differences in behavioral sensitivity to a neuroactive steroid. J Pharmacol Exp Ther. 1997;280:820–828. [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004c;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABAA receptor gene expression by allopregnanolone and ethanol. Eur J Pharmacol. 2004;500:413–425. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res. 2002;26:635–643. [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL, Finn DA. Allopregnanolone does not influence ethanol-induced conditioned place preference in DBA/2J mice. Psychopharmacology (Berl) 2004;176:50–56. doi: 10.1007/s00213-004-1862-2. [DOI] [PubMed] [Google Scholar]
- Gallaher EJ, Jones GE, Belknap JK, Crabbe JC. Identification of genetic markers for initial sensitivity and rapid tolerance to ethanol-induced ataxia using quantitative trait locus analysis in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1996;277:604–612. [PubMed] [Google Scholar]
- Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Gorin RE, Crabbe JC, Tanchuck MA, Long SL, Finn DA. Effects of finasteride on chronic and acute ethanol withdrawal severity in the WSP and WSR selected lines. Alcohol Clin Exp Res. 2005;29:939–948. doi: 10.1097/01.alc.0000167742.11566.01. [DOI] [PubMed] [Google Scholar]
- Gorin RE, Finn DA. The effect of finasteride on blood ethanol concentration and plasma hormone levels during acute ethanol withdrawal in C57BL/6 and DBA/2 mice. Alcohol Clin Exp Res. 2005;29:56A. doi: 10.1097/01.alc.0000167742.11566.01. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH. Ethanol-like discriminative stimulus effects of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology (Berl) 1996;124 doi: 10.1007/BF02247439. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L. Role of estradiol in alcohol intake and alcohol-related behaviors. J Stud Alcohol. 1996;57:162–170. doi: 10.15288/jsa.1996.57.162. [DOI] [PubMed] [Google Scholar]
- Hill M, Popov P, Havlikova H, Kancheva L, Vrbikova J, Kancheva R, Pouzar V, Cerny I, Starka L. Altered profiles of serum neuroactive steroids in premenopausal women treated for alcohol addiction. Steroids. 2005a;70:515–524. doi: 10.1016/j.steroids.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hill M, Popov P, Havlikova H, Kancheva L, Vrbikova J, Meloun M, Kancheva R, Cibula D, Pouzar V, Cerny I, Starka L. Reinstatement of serum pregnanolone isomers and progesterone during alcohol detoxification therapy in premenopausal women. Alcohol Clin Exp Res. 2005b;29:1010–1017. doi: 10.1097/01.alc.0000167953.97205.0a. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt's forced swim test: modulation by 3 alpha-hydroxy-5 alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3alpha-hydroxy-5alpha-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology (Berl) 2005;180:267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Juarez J, Barrios De Tomasi E, Virgen M. Effects of estradiol treatment on voluntary and forced alcohol consumption in male rats. Pharmacol Biochem Behav. 2002;71:259–268. doi: 10.1016/s0091-3057(01)00662-1. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, Matthews DB, Morrow AL. Ethanol-induced elevation of 3 alpha-hydroxy-5 alpha-pregnan-20-one does not modulate motor incoordination in rats. Alcohol Clin Exp Res. 2004;28:1249–1256. doi: 10.1097/01.alc.0000134232.44210.06. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O'Buckley T, Morrow AL. Neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res. 2003;980:255–265. doi: 10.1016/s0006-8993(03)02978-0. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Foster GV, Hoehn-Saric R, Svikis DS, Hipsley PA. Family history of alcoholism in women with generalized anxiety disorder who have premenstrual syndrome: patient reports of premenstrual alcohol consumption and symptoms of anxiety. Alcohol Clin Exp Res. 1994;18:664–670. doi: 10.1111/j.1530-0277.1994.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF. Selective antagonism of 5alpha-reduced neurosteroid effects at GABAA receptors. Mol Pharmacol. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Fleming R, Penland S. Ethanol and neurosteroid interactions in the brain. Int Rev Neurobiol. 2001;46:349–377. doi: 10.1016/s0074-7742(01)46068-5. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies . Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academies Press; Washington, DC: 2003. [Google Scholar]
- Nie H, Janak PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168:222–228. doi: 10.1007/s00213-003-1468-0. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Alomary AA, Vallee M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol. 2004;484:241–247. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharmacol. 1996;19 doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Romeo E, Pompili E, di Michele F, Pace M, Rupprecht R, Bernardi G, Pasini A. Effects of fluoxetine, indomethacine and placebo on 3 alpha, 5 alpha tetrahydroprogesterone (THP) plasma levels in uncomplicated alcohol withdrawal. World J Biol Psychiatry. 2000;1:101–104. doi: 10.3109/15622970009150572. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J Neurosci. 1998;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28:1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology (Berl) 2004;172:352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]