Abstract
It has been well documented that human milk contains several immunomodulator components which are important during infant period when the newborn's immune system is still under development. In this study, we aim at examining levels of cytokines, zinc (Zn), and copper (Cu) in milk from mothers of premature and mature infants, and comparing changes during lactation periods consequently. Milk was collected from total of 40 mothers (group M: mothers of mature infants, n = 20; group PM: mothers of premature infants, n = 20) from four lactation stages: colostrum (0–7 days), transitional (7–14 days), mature milk (21 days), and mature milk (2nd month). Levels of cytokines (interleukin [IL]-lβ, IL-2, IL-6, IL-8, tumor necrosis factor-alpha [TNF-α]) were determined by chemiluminesence method, whereas atomic absorption spectrophotometer was used for the determination of Zn and Cu levels. Cytokine levels were determined to be high in colostrum and transient milk from mothers of full-term infants, whereas their levels were reduced drastically in the 21st day and the 2nd month milk (P < .01 , P < .001). Similar trends were observed in milk from mothers of premature infants, but cytokine levels were significantly lower in colostrum compared to colostrum from mothers of mature infants (P < .01). The differences in cytokine levels were continuous in transient milk (P < .05) and mature milk (21 days) (P < .05), whereas there was no statistically significant differences between milk from both groups of mothers in the 2nd month (P > .05). Zn levels in milk from mothers of premature infants were significantly lower compared to the ones from mothers of mature infants (P < .01) and these differences continued through the 2nd month. Although Cu levels were lower in milk from mothers of premature infants, there was no statistically significant difference except colostrum (P > .05). Our results clearly demonstrate that the level of immunomodulating agents such as cytokines and trace elements in milk from mothers of premature infants is less than the level of the same agents in milk from mothers of full-term infants. Although there are commercially available products for infant feeding, human milk is still the best natural nutrient for newborns. Therefore, when premature infants are breastfed, necessary precautions such as supplemantary diets must be considered for possible infections and risks related with immune system deficiency.
INTRODUCTION
Human breast milk provides an ideal nutrient composition for the newborn. The composition of human milk provides the infant with all its nutritional requirements in its early life [1]. It is widely believed that human milk not only contains nutrients necessary for growth and development of newborns, but also contains immunomodulator agents which helps the development of the immune system [2, 3]. The immune system in human milk is composed not only of direct acting antimicrobial agents and antiinflammatory factors, but also of immunoregulators [4]. The transfer of numerous cytokines and growth factors via mother milk may add to an active stimulation of the infant's immune system [5, 6]. Human breast milk contains a variety of substances such as hormones, growth factors, cytokines (Interleukin [IL]-1β, IL-2 IL-6, IL-8, tumor necrosis factor-alpha [TNF-α]), and trace elements (Zinc [Zn], Copper [Cu]) [1, 2].
It has been reported that human milk components protect babies from pathogen bacteria [7]. When human milk was given to premature newborns, incidence of infections were reported to be lower with respect to commercially available formulas [1, 2, 3, 7]. The babies who were fed with human milk develop a natural defence against Bacteroides, Clostridium, and Escherichia coli. Cytokines are among the agents with potential immunological components in milk and they play vital roles in the regulation and behavior of the immune system cells. Cytokines are small soluble glycoproteins that act in autocrine-paracrine fashions by binding to specific cellular receptors, operating in networks, and orchestrating immune system development and functions. These cytokines are present at picogram quantities. However, early milk has an abundance of cytokines at a time when neonatal organ systems are immature, suggesting that these bioactive components of milk might be important in neonatal development [8, 9].
In addition, trace elements such as Zn and Cu found in human milk are very important for newborns growth and development. Those trace elements deficiency may lead to adverse effects on growth velocity, motor development, cell-mediated immunty, and skeletal development in preterm babies who can not receive adequate amount of them [10, 11].
In the present study, we aimed at examining and comparing the levels of some cytokines and trace elements in milk from mothers with mature and premature infants. In addition, changes during the lactation period were also evaluated.
MATERIALS AND METHODS
Study population
Total of 40 lactating mothers (mothers with mature babies [group M, n = 20], mothers with premature babies [group PM (less than 37 weeks gestational age), n = 20]) voluntarily participated in this study. Their parity ranged from 1–2 times. The cigarette smoking mothers were excluded. The mothers have the same socioeconomic conditions. These women were routinly visiting pediatric clinics at the Firat Medical Center, Elazig. All women were healthy, group M aged (23.0 ± 1.0) and group PM (21.0 ± 2.0). Their weights ranged from 50–65 kg and were not taking antiinflammatory medication at the enrollment time. Milk was collected from all mothers at four different time points; 0–7 days (postpartum, colostrum), 7–14 days (transient period), 21st day (mature milk), and 2nd month (mature milk) [12].
In this study milk samples were collected from each mother by manual expression into sterile polypropylene containers on 0–7 days, 7–14 days, 21st day and 2nd month postpartum either at home or at the hospital. Collection was standardized to reduce potential diurnal variability in cytokine measurements. Specimens were obtained within 2 hours of the first feeding in the morning; defined as 8 AM to 11 AM. Mothers were asked not to feed from the breast used for specimen collection for 2 hours before collection. All samples were stored at 4°C and processed within 2 hour of collection. After centrifugation at 690 Xg for 20 minutes, the aqueous phase was collected and stored at −40°C until analyzed for cytokine and trace elements [17]. Samples were assayed within the two weeks of storage. Special directions were forwarded to mothers not to take any medications.
Cytokine levels (IL-lβ, IL-2, IL-6, IL-8, TNF-α) were determined with chemilumminessence method, and BIODPC diagnostic kits (IL-lβ, IL-2, IL-6, IL-8, TNF-α, BIODPC, Istanbul, Turkey, DPC, Calif) with IMMULITE 2000 hormon auto-analyzer (BIODPC, Istanbul, Turkey, DPC, Calif), whereas levels of Zn and Cu were determined by atomic absorption flame spectrophotometer (Schimadzu AA 6701F, Schimadzu Corp, Tokyo, Japan).
Statistical analysis
Values obtained from each group are presented as mean ± SD. Student t test was used for evaluation between two groups of mothers. To analyze changes dependent on time, in this study were utilized ANOVA and post ANOVA tests, Turkey and Scheffe.
RESULTS
Values obtained from measurement in milk from mothers of premature and mature infants are shown in Figures 1a, 1b, and 2.
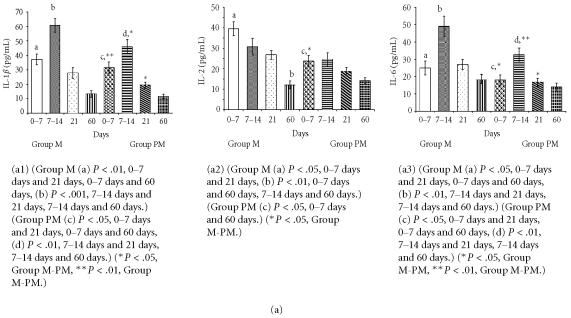
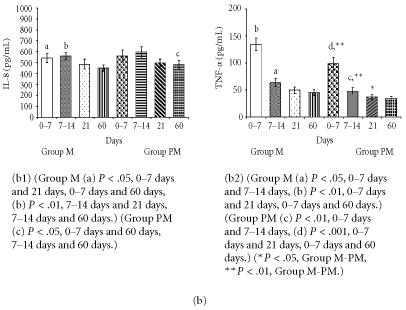
Figure 1.
(a) Comparison of cytokine (IL-1β, IL-2, IL-6) levels in milk from mothers of mature (group M) and premature (group MP) infants. (b) Comparison of cytokine (IL-8, TNF-α) levels in milk from mothers of mature (group M) and premature (group PM) infants.
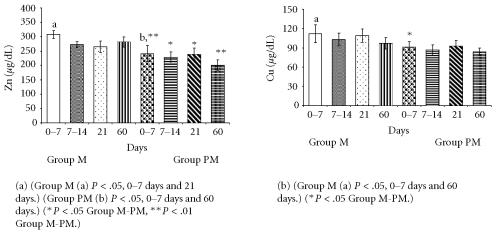
Figure 2.
Comparison of trace elements (Zn, Cu) levels in milk from mothers of mature (group M) and premature (group PM) infants.
Meaningful changes in cytokine levels (IL-lβ, IL-2, IL-6, IL-8, TNF-α) were observed in milk from mothers of preterm babies as seen in Figures 1a and 1b. Nevertheless, the differences between the two groups diminished at the end of the 2nd month of lactation for some parameters.
The cytokine and mineral levels in the milk of mothers with term babies were higher than in the milk of mothers with premature newborn. Moreover, in both groups milk cytokine and mineral levels were high in colostrum and decreased over time.
The levels IL-1β and IL-6 only reached the highest point in 7–14 days milk of mothers of premature and mature infants (Figure 1a).
All studied cytokines and trace elements were ascertained at lower level except IL-8 for milk of mothers of premature infants. It was observed that the change manners in levels of cytokine and trace elements are similar in the milk of mothers of mature and premature infants with respect to milk collecting phase.
IL-2 and TNF-α levels were higher in clostrum, but reduced in later stages of lactation (Figure 1b). Zn and Cu levels were lower in milk from mothers of preterm infants compared to milk of mothers of mature babies (Figure 2).
DISCUSSION
Several different cytokines and chemokines have been discovered in human milk and the list is growing very rapidly. Cytokines operate in networks and produce a cascade of effects that contribute to the orchestration, development, and functions of the immune system. The production of cytokines is delayed in neonates and infants, and cytokines in human milk may be able to interact with mucosal tissues in the upper parts of the respiratory and alimentary tracts providing antiinflammatory and immunomodulatory effects upon the recipient infants [5, 6]. Human milk protects babies from pathogenic organisms and/or reduces risks of infections since it contains several immunomodulator agents and trace elements such as Zn and Cu [1, 7]. Several studies clearly demonstrated that, in addition to hormones and growth factors, human milk also contains several biologically active substances such as cytokines [12, 13]. Nevertheless, there is currently a limited number of studies related with the content of human milk during postpartum period [5]. Changes in the composition of human milk during postpartum lactation period is especially important for premature newborns.
Meki et al [5] reported that the gestational age at delivery may not have any effect on the breast milk levels of cytokines. In our study, levels of some cytokines (IL-1β, IL-6, TNF-α) were found statistically different between two groups of milk except from 60th day mature milk. The levels of IL-2 and Cu were found statistically different only in colostrum. No significant differences between the two groups were found in the levels of IL-8 but statistically significant differences were found in the levels of Zn.
Srivastava et al [12] determined IL-lβ at high levels in human milk, but they did not examine IL-lβ levels during lactation period. Hawkes et al [13] reported that IL-lβ levels were higher in milk from mothers of mature newborns compared to milk from mothers of premature ones. In this study, Il-lβ levels declined at the end of the 2nd month the differences between two groups disappeared after the 2nd month.
We could not find any statistically significant differences in IL-2 levels between the two groups of milk except for colostrum. IL-2 levels gradually decreased in both groups within two months.
IL-6 is often used as a marker for systemic activation of proinflammatory cytokines. The large amount of IL-6 is secreted into whey with the corporation of TNF-α and IL-1β [14, 15]. IL-6 in human milk may affect the immune system of the mammary gland and of the recipient in other ways [5]. IL-6 levels in milk from mothers of mature babies were increased, especially in the two weeks of postpartum period. Although IL-6 levels decreased at the end of the 2nd month, they were still high. On the other hand, IL-6 levels in milk from mothers of premature babies were lower, especially in the 2nd week after birth, compared to IL-6 levels in milk from mothers of mature babies [16]. Eglinton et al [17] and Rudloff et al [18] reported the presence of IL-6 (151 ± 89 pg/mL) in colostrum milk of the 1st week, but their values were low with respect to other reported studies [12, 13]. On the other hand, increased IL-6 levels in human milk were reported by Hawkes et al [13] and Rudloff et al [18] which are in agreement with our findings. Meki et al [5] found that the level of IL-6 in colostrum (978.80 ± 86.80 pg/mL) was significantly higher than both transitional (162.90 ± 29.67 pg/mL) and mature (86.92 ± 2.47 pg/mL) milk. Similarly, Saito et al [14] reported high levels of IL-6 in colostrum. Differences in methods used for determination of IL-6 levels partly can explain the discrepancy among studies. But, the main point is the increase of IL-6 in human milk, especially within the first two weeks after birth.
High levels of IL-8 may play significant role in the movement of maternal neutrophils, monocytes, and lympocytes to the milk and perhaps subsequently across the neonatal bowel wall. Such trafficing contributes to mucosal defense and the development of the immune system of the newborn. We did not observe any statistically significant changes in IL-8 levels in our study. Milk from 14 days of both groups had slightly increased in IL-8 levels, but those increases diminished quickly. Meki et al [5] found that IL-8 levels in colostrum (585.70 ± 30.75 pg/mL) were significantly higher than in both the transitional (308.10 ± 35.47 pg/mL) and the mature (200.30 ± 25.01 pg/mL) milk. On the other hand, Michei et al [19] reported the same dynamic changes of IL-8 levels. Meki et al [5] found that IL-8 level in preterm milk (386.10 ± 50.30 pg/mL) was apparently lower, but statistically insignificant in comparison with full-term milk (446.90 ± 32.11 pg/mL) at the same periods of lactation. Similarly, Srivasta et al [12] and Michei et al [19] did not find differences for IL-8 levels. Montagne et al [20] reported that the dynamics of major immunologic and nutrient components in early lactation had similar pattern in both preterm and mature human milk. IL-8 is the only cytokine that was higher in milk from mothers of premature babies, but it is not significant. IL-8 is one chemoattractant cytokine and acts as a proinflammatory mediator [19]. IL-8 was significantly elevated in mature milk of allergic mothers. The presence of IL-8 in breast milk might be responsible for the traffic of leukocytes from the maternal circulation to the breast milk [5].
TNF-α existence in human milk stimulates IL-6 production via mononuclear leucocytes, inactivates macrophages, and its level is increased especially within the 1st week to the delivery [18]. TNF-α is a proinflammatory cytokine. It is a physiologically significant regulator of mammary gland development, stimulating growth and branching morphogenesis of mammary epithelial cells, and modulating functional differentiation [21]. The soluble receptors for TNF-α exert antiinflammatory effects in some systems. Interaction of inflammatory cells with vascular endothelium and of proinflammatory cytokines with their cellular receptors occurs early in inflammation, and blockade either result in antiinflammatory effects. Milk TNF-α is secreted by milk macrophages and by mammary epithelium [5]. TNF-α in colostrum and milk besides its proinflammatory action as one of the major host defense components against trauma and infection bridging the defective TNF-α production by the neonate. It produces these effects without causing inflammation, probably due to the presence of their specific receptors in human milk. In our study, the levels of TNF-α in colostrum were significantly higher than both transitional and mature milk, respectively. TNF-α levels were lower in colostrum from mothers of premature babies with respect to colostrum of mothers with mature babies. But during the lactation period of two months, the differences between two groups disapperead. Meki et al [5] found high levels of TNF-α in colostrum (402.80 ± 29.65 pg/mL). High level of TNF-α in colostrum has decreased in transitional milk (135.50 ± 8.26 pg/mL), and then reincreased in mature milk (178.30 ± 14.41 pg/mL). Munoz et al [22] found low levels of TNF-α during first two levels after birth whereas Rudloff et al [18] reported high levels of TNF-α values seem to be in agreement with the data that Meki et al [5] reported in study. When different periods of lactation are considered, there are meaningful differences in TNF-α levels between colostridial and other milk [23].
In this study we also determinded levels of two trace elements, Zn and Cu, whose presence in milk is vitally important, especially for premature newborns. Zn (241 ± 28.1, 228 ± 18.9, 239 ± 20.0, and 201 ± 17.5 μg/dL, resp) and Cu (91.0 ± 8.6, 87.0 ± 7.8, 93.0 ± 8.7, and 84.0 ± 5.3 μg/dL, resp) levels in milk from mothers of premature babies were significantly lower than the ones from mothers of mature newborns (Zn 308 ± 30.4, 272 ± 19.8, 265 ± 20.3, 281 ± 18.0 μg/dL and Cu 112 ± 13.8, 103 ± 9.8, 109 ± 10.7, 97 ± 8.8 μg/dL, resp). Cu levels in milk from mothers of premature babies were no significant difference than the ones from mothers of mature newborns except from colostrum. Nevertheless, at the end of the 2nd month of lactation, there was reduction in their levels in both groups of milk and no significant difference between the two groups. Sharda et al [10] (premature Zn 72.6 ± 1.6 μmol/L and Cu 7.7 ± 0.3 μmol/L, mature Zn 76.9 ± 1.2 μmol/L and Cu 8.0 ± 0.6 μmol/L) emphasize in their study that those babies should be supported with external Zn and Cu. Our results also point out that Zn and Cu levels are lower in milk from mothers of preterm babies and these deficiencies will be supplemented for normal growth and development of preterm babies.
The immune system in human milk plays roles in protecting not only the mature, healthy newborn, but also premature infant who is more prone to infections and damage caused by inflammatory processes. The human milk-fed preterm infant may experience improved health, such as, lower rate of infection, necrotizing enterocolitis, better gastrointestinal function, and neurodevelopment [24, 25]. The effects of milk cytokines on the maturation and functions of the epithelium, mucosal, or submucosal leukocytes and other specialized cells and structures in the alimentary and respiratory system should be particularly targeted for investigation [8].
The physiological changes of cytokine profile at different periods of lactation may reflect the changes in the immune system of the breast and alteration in the needs of the newborn for these cytokines. These dynamic changes in colostrum, transitional, and mature milk may be due to changes in the immune system of milk during lactation periods, which may be assaociated with changes in the immune system of the recipient infants.
References
- 1.Bernt KM, Walker WA. Human milk as a carrier of biochemical messages. Acta Paediatr Suppl. 1999;88(430):27–41. doi: 10.1111/j.1651-2227.1999.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 2.Dewey KG, Heinig MJ, Nommsen-Rivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr. 1995;126(5 pt 1):696–702. doi: 10.1016/s0022-3476(95)70395-0. [DOI] [PubMed] [Google Scholar]
- 3.Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr. 1995;126(2):191–197. doi: 10.1016/s0022-3476(95)70544-9. [DOI] [PubMed] [Google Scholar]
- 4.Goldman AS. The immune system of human milk: Antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J. 1993;12(8):664–671. doi: 10.1097/00006454-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Meki A-R MA, Saleem TA, Al-Ghazali MH, Sayed AA. Interleukins-6, -8 and -10 and tumor necrosis factor-alpha and it soluble receptor I in human milk at different periods of lactation. Nutrition Research. 2003;23:845–855. [Google Scholar]
- 6.Garofalo RP, Goldman AS. Expression of functional immunomodulatory and anti-inflammatory factors in human milk. Clin Perinatol. 1999;26(2):361–377. [PubMed] [Google Scholar]
- 7.Hylander MA, Strobino DM, Dhanireddy R. Human milk feedings and infection among very low birth weight infants. Pediatrics. 1998;102(3):E38. doi: 10.1542/peds.102.3.e38. [DOI] [PubMed] [Google Scholar]
- 8.Oddy WH, Halonen M, Martinez FD, et al. TGF-β in human milk is associated with wheeze in infancy. J Allergy Clin Immunol. 2003;112(4):723–728. doi: 10.1016/s0091-6749(03)01941-9. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JM, Ferguson SJ, Loane P, Kell M, Millar S, Gillmore WS. Cytokines in human breast milk. Br J Biomed Sci. 1997;54(2):85–87. [PubMed] [Google Scholar]
- 10.Sharda B, Adhikari R, Ajmera M, Gambhir R, Singh PP. Zinc and copper in preterm neonates: relationship with breast milk. Indian J Pediatr. 1999;66(5):685–695. doi: 10.1007/BF02726255. [DOI] [PubMed] [Google Scholar]
- 11.Heinen F, Matern D, Pringsheim W, Leititis JU, Brandis M. Zinc deficiency in an exclusively breast-fed preterm infant. Eur J Pediatr. 1995;154(1):71–75. doi: 10.1007/BF01972977. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava MD, Srivastava A, Brouhard B, Saneto R, Groh-Wargo S, Kubit J. Cytokines in human milk. Res Commun Mol Pathol Pharmacol. 1996;93(3):263–287. [PubMed] [Google Scholar]
- 13.Hawkes JS, Bryan DL, James MJ, Gibson RA. Cytokines (IL-1β, IL-6, TNF-α, TGF-β1, and TGF-β2) and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res. 1999;46(2):194–199. doi: 10.1203/00006450-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Saito S, Maruyama M, Kato Y, Moriyama I, Ichijo M. Detection of IL-6 in human milk and its involvement in IgA production. J Reprod Immunol. 1991;20(3):267–276. doi: 10.1016/0165-0378(91)90051-q. [DOI] [PubMed] [Google Scholar]
- 15.Rudloff HE, Schmalstieg FC, Jr, Palkowetz KH, Paszkiewicz EJ, Goldman AS. Interleukin-6 in human milk. J Reprod Immunol. 1993;23(1):13–20. doi: 10.1016/0165-0378(93)90023-b. [DOI] [PubMed] [Google Scholar]
- 16.Sone S, Tsutsumi H, Takeuchi R, et al. Enhanced cytokine production by milk macrophages following infection with respiratory syncytial virus. J Leukoc Biol. 1997;61(5):630–636. doi: 10.1002/jlb.61.5.630. [DOI] [PubMed] [Google Scholar]
- 17.Eglinton BA, Roberton DM, Cummins AG. Phenotype of T cells, their soluble receptor levels, and cytokine profile of human breast milk. Immunol Cell Biol. 1994;72(4):306–313. doi: 10.1038/icb.1994.46. [DOI] [PubMed] [Google Scholar]
- 18.Rudloff HE, Schmalstieg FC, Jr, Mushtaha AA, Palkowetz KH, Liu SK, Goldman AS. Tumor necrosis factor-alpha in human milk. Pediatr Res. 1992;31(1):29–33. doi: 10.1203/00006450-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Michie CA, Tantscher E, Schall T, Rot A. Physiological secretion of chemokines in human breast milk. Eur Cytokine Netw. 1998;9(2):123–129. [PubMed] [Google Scholar]
- 20.Montagne P, Cuilliere ML, Mole C, Bene MC, Faure G. Immunological and nutritional composition of human milk in relation to prematurity and mother's parity during the first 2 weeks of lactation. J Pediatr Gastroenterol Nutr. 1999;29(1):75–80. doi: 10.1097/00005176-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Shea-Eaton WK, Lee PP, Ip MM. Regulation of milk protein gene expression in normal mammary epithelial cells by tumor necrosis factor. Endocrinology. 2001;142(6):2558–2568. doi: 10.1210/endo.142.6.8199. [DOI] [PubMed] [Google Scholar]
- 22.Munoz C, Endres S, van der Meer J, Schlesinger L, Arevalo M, Dinarello C. Interleukin-1β in human colostrum. Res Immunol. 1990;141(6):505–513. doi: 10.1016/0923-2494(90)90019-u. [DOI] [PubMed] [Google Scholar]
- 23.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1β production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63(1):116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 24.Schanler RJ, Atkinson SA. Effects of nutrients in human milk on the recipient premature infant. J Mammary Gland Biol Neoplasia. 1999;4(3):297–307. doi: 10.1023/a:1018754014330. [DOI] [PubMed] [Google Scholar]
- 25.Montagne PM, Tregoat VS, Cuilliere ML, Bene MC, Faure GC. Measurement of nine human milk proteins by nephelometric immunoassays: Application to the determination of mature milk protein profile. Clin Biochem. 2000;33(3):181–186. doi: 10.1016/s0009-9120(00)00059-x. [DOI] [PubMed] [Google Scholar]