Abstract
Mice with null mutations in the E2A gene are highly susceptible to the spontaneous development of thymic lymphomas. To understand better how E2A deficiency may contribute to lymphomagenesis, we have observed the consequences of enforced expression of the E2A gene products E12 and E47 in cell lines derived from lymphomas that arose spontaneously in E2A-deficient mice. E2A-expressing cells are steadily eliminated from lymphoma cultures into which E47 or E12 was introduced. The mechanism underlying the loss of E2A-expressing cells does not involve an arrest in cell-cycle progression. Rather, the E2A proteins activate a programmed cell death pathway in these lymphomas. This E2A-mediated cell death appears to be preceded by a loss of mitochondrial transmembrane potential. These data provide direct evidence that E2A gene products can act as tumor suppressors.
The E2A gene encodes two basic helix–loop–helix (HLH) transcription factors, E12 and E47 (1). These two proteins are classified as Class I HLH proteins, which are characterized by their ability to bind DNA either as homodimers or as heterodimers with tissue-specific Class II HLH proteins (2–5). In addition to the HLH domain and basic region near the C terminus, which are required for dimerization and DNA-binding (1, 3, 6, 7), Class I HLH proteins also typically contain two separate domains in the N-terminal portion of the protein that have been shown to activate transcription (8–10). E12 and E47 are generated through differential splicing to either of two exons that encode the HLH domain. The E12 and E47 HLH exons differ by 20% in their predicted amino acid sequence (1). Thus the E2A gene encodes two proteins that are distinguished only by differences in their dimerization and DNA-binding domains.
E12 and E47 bind to E-box sites present in a wide variety of tissue-specific enhancers (11). In the development of many tissue types, the E2A proteins bind to critical enhancer regions through heterodimerization with various Class II HLH proteins. However, in lymphocytes, E2A proteins bind DNA principally either as a homodimer or, in T cells, as a heterodimer with the Class I HLH protein HEB (5, 12–14). The generation of mice with E2A null mutations has revealed an essential role for this gene in lymphopoiesis. B cell development is completely blocked at the earliest identifiable stages in E2A-deficient mice (15, 16). In addition, these mice have severe defects in T cell maturation (13). The E2A-deficient thymus exhibits a severe reduction in the numbers of CD4+ CD8+ thymocytes, which is apparently the consequence of a partial block in the maturation and proliferation at the earlier CD4− CD8− developmental stage. Furthermore, nearly all E2A-deficient mice develop thymomas that can arise as early as 75 days after birth (13, 17). These lymphomas usually migrate to peripheral lymphoid tissues as well as to nonlymphoid organs such as kidney, liver, and lung. The E2A-deficient lymphomas grow readily when transplanted to nude mice and can often be adapted to in vitro culture conditions. They are characterized by monoclonal T cell antigen-receptor β rearrangements, heterogeneous CD4 and CD8 expression and, in a majority of cases, elevated expression of c-myc and interleukin 2 receptor α chain (CD25).
The spontaneous generation of lymphomas in E2A-deficient mice suggests that this gene encodes proteins with tumor-suppressor activity. We have proposed a model in which various forms of human T cell acute lymphoblastoid leukemias develop because of a deficiency in E2A activity. For example, human T cell acute lymphoblastoid leukemias are frequently characterized by the expression of the Class II HLH genes SCL/tal-1, tal-2, and lyl-1 (18–21). The products of these genes can form heterodimers with E2A proteins that have altered DNA-binding and/or transcriptional activation properties relative to E2A/E2A or E2A/HEB dimers (18–22) and thus may act at least in part by interfering with the normal function of E2A. However, the mechanisms by which E2A expression prevents lymphomagenesis are not known. We have thus developed a system to study the consequences of E2A expression in lymphoma lines derived from E2A-deficient mice. The data derived from these studies indicate that E2A proteins may act as tumor suppressors in developing thymocytes.
MATERIALS AND METHODS
Cell Lines, Plasmids, and Reagents.
The 1.F9 line was derived from a thymoma that developed spontaneously in an E2A−/− mouse and was adapted to growth in culture after several passages in the presence of thymic stroma. 1.F9 cells were cultured in RPMI 1640 media containing 10% fetal bovine serum and 50 μM 2-mercaptoethanol and supplemented with glutamine, penicillin, and streptomycin. The ΦNX ecotropic packaging line was provided by Garry Nolan (Stanford University, Stanford, CA) and was cultured in DMEM (high glucose) plus 10% fetal bovine serum, glutamine, penicillin, and streptomycin. All cells were cultured at 37°C plus 5% CO2. The retroviral vector LZRSpBMN-linker-IRES-enhanced green fluorescent protein (EGFP) (NotI−) (S-003) was obtained from Hergen Spits (The Netherlands Cancer Institute, Amsterdam, The Netherlands). The basic region mutation of E12 was produced by Gretchen Bain (University of California at San Diego, La Jolla, CA). The anti-human E2A antibody G98–271, the antibody 382.6 (specific for E12, HEB, and E2-2), and goat anti-mouse IgG-fluorescein isothiocyanate (FITC) were obtained from PharMingen. Hexadimethrine bromide (Polybrene) was purchased from Sigma and DOTAP was purchased from Boehringer-Mannheim.
Retroviral Supernatant Production and T Lymphoma Transduction.
cDNA copies of full-length human E47, E12, and a basic region mutant of E12 (E12BM) were inserted into the polylinker region of the retroviral vector S-003 (23) by standard recombinant DNA techniques. The φNX-eco packaging line (24) was transfected with these retroviral constructs by means of calcium phosphate precipitation (25). The transfected cells were switched to 1.F9 culture media 24 hr after transfection and supernatants were harvested after an additional 24 hr and stored at −80°C. For transduction, supernatants were thawed and incubated in either polybrene at 5 μg/ml or DOTAP at 0.01X for at least 10 min at 0°C. The supernatants were then aliquoted into standard 6- or 24-well tissue culture plates containing 1.F9 cells (suspended in 0.1 ml or less of growth media) at a ratio of 0.3–1.0 × 106 cells per ml of supernatant. The plates were then spun at 2,500 rpm for 60–90 min at 30–34°C in a Sorvall RT 6000D centrifuge fitted with an H1000B rotor and microplate carriers (Sorvall). After centrifugation, the majority of the supernatant was removed from the wells and replaced with fresh growth media, after which the cells were cultured under routine conditions.
Flow Cytometric Analysis.
To determine expression of EGFP (CLONTECH), transduced cells were analyzed either fresh or fixed in PBS plus 1% formaldehyde or paraformaldehyde by using a FACScan (Becton-Dickinson Immunocytometry Systems). Live and dead cells were distinguished either by forward and side scatter or by staining dead cells in unfixed samples with 1 μg/ml propidium iodide; these two methods always gave essentially identical results. For E2A expression, transduced cells were fixed and permeabilized in 70% ethanol/30% PBS. The cells were washed once with wash buffer (PBS plus 0.5% Tween-20) and then incubated sequentially for 30 min at room temperature with 1 μg of G98–271 and 0.5 μg of goat anti-mouse IgG-FITC, each in 100 μl wash buffer plus 1% BSA. The cells were washed with wash buffer after each antibody incubation and then resuspended in PBS and analyzed by using a FACScan. BrdUrd/propidium iodide analysis was performed essentially as described by Page and Defranco (26), except that BrdUrd-labeled DNA was detected by using anti-BrdUrd-FITC (Becton-Dickinson Immunocytometry Systems), and 1% BSA was substituted for 1.5% dry milk as a blocking agent. 1,1′,3,3,3′,3′-hexamethylindocarbocyanine iodide [DiIC1(3)] staining was performed as described by Shapiro (27) and was analyzed by FACScan.
Electrophoretic Mobility-Shift Assay (EMSA).
Nuclear extracts were prepared as described previously (12). Binding reactions were performed with 6 μg of extract and an oligonucleotide containing the μE5 E2-box sequence (12). Extracts were preincubated in binding buffer (12) with or without the G98–271 or 382.6 antibodies for 5 min at room temperature and then the probe was added followed by 15 min further incubation at room temperature. The samples were then electrophoresed as described previously (12).
RESULTS
Transduction of E2A Gene Products by Retroviral-Mediated Gene Transfer.
We have used a retroviral transduction strategy to study the effects of the expression of E12 and E47 in E2A-deficient lymphoma lines. Human E2A cDNA constructs were subcloned into the retroviral vector S-003 (23). This vector features an internal ribosomal entry site linked to coding sequence for EGFP that allows for translation of both E2A and EGFP from one retroviral transcript. Thus expression of EGFP as measured by flow cytometry can be used to identify retroviral transductants. The S-003 E2A constructs were transfected into the φNX-eco retroviral packaging line (24), and supernatants from these cells were then used to transduce virus into three different E2A-deficient lymphoma lines. One of these lines (1.F9) was derived from a thymoma that arose in an E2A-null mutant mouse, while the other two lines (0531 and 0714) were established from thymomas found in mice homozygous for a mutation that blocks E47 expression but allows low-level expression of E12. The data presented here were obtained by using the 1.F9 line, but similar results were found with the other two lines. We routinely obtained transduction levels of 70–98% with these lymphomas. The transduced cells expressed the retrovirally encoded genes relatively rapidly, because cells expressing EGFP could be detected as early as 8 hr after exposure to virus, while virtually all transduced cells expressed detectable levels of EGFP within 24 hr after transduction (Fig. 1B and data not shown). E2A expression was also measured directly by means of flow cytometry by staining fixed and permeabilized transductants with the anti-human E2A antibody G98–271 (Fig. 1). We found that the kinetics of E2A and EGFP expression were very similar (data not shown). We also transduced the E2A-deficient lymphomas with supernatants containing a virus encoding a form of E12 that had mutations introduced into the basic region that prevented specific DNA binding (7), designated E12BM.
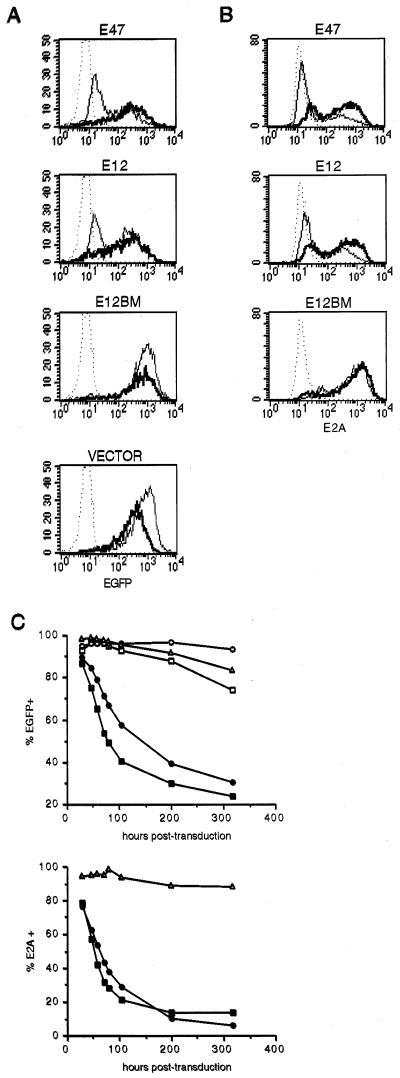
Figure 1.
Flow cytometric analysis of 1.F9 transductants. (A) Histograms representing EGFP fluorescence from cells transduced with viral supernatants containing E47, E12, E12BM, or vector control virus, comparing samples harvested and fixed 24 hr (thick lines) and 87 hr (thin lines) after transduction with that of untransduced cells (dotted lines). All overlaid histograms compare samples taken from the same transduced population, and all data were collected in the same FACScan session. Note that the numbers of events represented in each histogram are not the same. (B) Histograms depicting the expression of E2A in transduced populations as determined by staining fixed and permeabilized cells with mouse anti-human E2A antibody followed by goat-anti-mouse Ig-FITC, comparing samples harvested 24 hr (thick lines) or 72 hr (thin lines) after viral transduction with that of vector control transductants (dotted lines). All samples were stained and analyzed at the same time, and all histograms compare cells harvested from the same transduction. Each histogram represents 5,000 events. EGFP is lost from the cells on permeabilization, thus simplifying the analysis for E2A protein. (C) Time course of EGFP and E2A expression in viral transductants. Cells harvested at various times after transduction with vector control (open squares), antisense E12 (open circles), E47 (filled squares), E12 (filled circles), or E12BM (open triangles) viral supernatants were analyzed for EGFP expression or fixed, permeabilized, and stained for E2A. After flow cytometry data were collected, the percentage of cells with significant EGFP (Upper) and E2A (Lower) was determined and plotted. All data presented in Fig. 1 are representative of at least three independent experiments.
Ectopic Expression of E47 and E12 Inhibits Growth of E2A-Deficient Lymphomas.
We first examined the consequences of expression of E2A proteins in our lymphoma lines by measuring the percentage of EGFP+ and E2A+ cells at several time points after transduction with E47 and E12 retroviral supernatants. The percentage of cells expressing EGFP and E2A in populations transduced with E47 or E12 dropped dramatically during the first few days of culture (Fig. 1). In contrast, the percentage of EGFP+ and E2A+ cells in lymphoma populations transduced with E12BM stayed nearly constant for over a week after exposure to virus (Fig. 1C). Similarly, lymphomas transduced with empty vector or antisense-oriented constructs also maintained stable expression of EGFP for several days (Fig. 1C). The decline in the percentage of cells expressing EGFP and E2A during the first 3 to 4 days of culture after transduction was consistently more rapid for E47 transductants than for E12-transduced populations.
After 80–100 hr of culture, the decline in %EGFP+ within the populations transduced with E12 and E47 virus became much more gradual, such that after 300 hr of culture, 20–30% of the transduced cells still expressed EGFP (Fig. 1C). However, intracellular staining revealed that nearly all of the remaining E12 transductants did not express E2A (Fig. 1C). A distinct population of E47 transductants did remain E2A+ after 300 hr of culture as determined by flow cytometry. To determine whether this population contained E2A DNA-binding activity, we prepared nuclear extracts from cells transduced with E47, E12, E12BM and vector-only viral supernatants and harvested after culturing for 19 and 335 hr. These extracts were then tested for DNA-binding activity to the E2 box-containing μE5 probe (12) by EMSA. As expected, no human E2A DNA-binding activity could be detected in extracts from E12BM or vector transductants (Fig. 2). Extracts from lymphomas transduced with E47 and E12 and harvested after 19 hr of culture exhibited strong human E2A DNA-binding activity, confirmed by super-shifting of the complexes with anti-human E2A antibody (Fig. 2). In contrast, extracts from E12- and E47-transduced populations harvested 335 hr after transduction exhibited no E2A-specific activity (Fig. 2). Furthermore, we were also unable to detect E2A DNA-binding activity in long-term cultures of E47 transductants that were enriched for E2A expression by flow cytometric sorting for EGFP+ cells (data not shown). Thus although long-term cultures of E47 transductants retained some E2A expression, the remaining transduced protein could not bind DNA specifically. In addition, Western blot analyses of extracts from long-term cultures of E47 transductants indicated that the only form of E47 detected in these extracts had a significantly lower molecular weight than that of wild-type E2A (data not shown). Thus extended culture of E47-transduced lymphomas resulted in the selection of a population that expressed a truncated form of E47 that could no longer bind DNA. Taken together, these results demonstrated that wild-type E2A expression was completely lost from transduced cultures.
Figure 2.
EMSA analysis of nuclear extracts prepared from viral transductants. Nuclear extracts prepared from 1.F9 cells harvested 19 hr or 335 hr after viral transduction with control vector, E47, E12, and E12BM viral supernatants were tested for specific binding to a probe containing a μE5 binding site. Extracts were preincubated in binding buffer containing either no antibody (−), the G98–271 antibody (+), which supershifts human E2A:DNA complexes, or the 382.6 antibody (∗), which blocks the binding of the probe to HEB, E12, or E2–2. Lane 1 represents probe without nuclear extract.
Ectopic Expression of E47 and E12 in E2A-Deficient Lymphomas Does Not Perturb Cell-Cycle Progression.
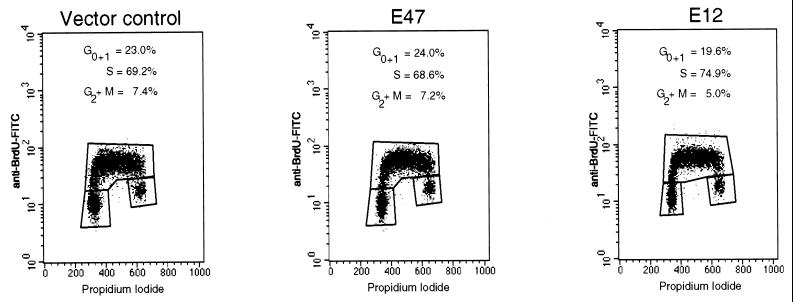
The disappearance of E12- and E47-expressing cells from transduced populations suggests that E2A transductants are likely subject either to cell-cycle arrest or to an increased rate of death. To determine whether ectopic expression of either E12 or E47 leads to a block in cell-cycle progression, we characterized the cell-cycle profiles of E12 and E47 transductants pulsed with BrdUrd 24 hr after transduction by measuring DNA content and BrdUrd incorporation. Cells transduced with E47 or E12 exhibited no significant perturbations in the percentage of G1, S, or G2+M populations relative to control populations (Fig. 3). Examination of DNA content at later time points after transduction also revealed no significant changes in the cell-cycle profile of E2A transductants (data not shown). Thus E2A proteins do not induce cell-cycle arrest in E2A-deficient lymphomas.
Figure 3.
Cell-cycle analysis of viral transductants. 1.F9 cells were transduced with vector control, E47, or E12 viral supernatants, and then cultured for 24 hr. The transductants were then cultured for 20 min in the presence of 20 μM BrdUrd, harvested, fixed, and permeabilized with ethanol, acid denatured and stained with anti-BrdUrd-FITC followed by propidium iodide. Shown are graphs of propidium iodide (DNA content) vs. anti-BrdUrd-FITC (identifying S-phase cells) for each transduced population, which were used to determine the percentages of cells in each phase of the cell cycle printed in each graph. All populations analyzed had transduction levels of at least 70% as determined by flow cytometric analysis for either EGFP or E2A.
Enforced Expression of E47 or E12 Induces Death in E2A-Deficient Lymphomas.
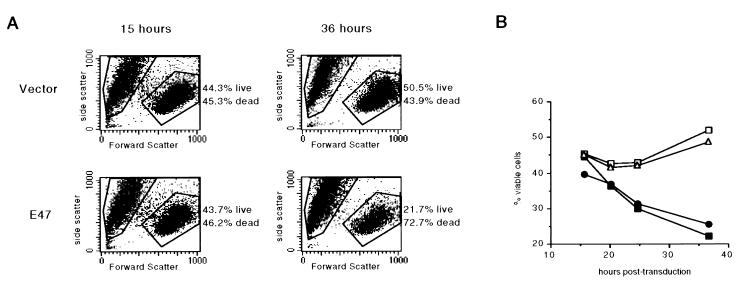
To determine whether the transduction of either E12 or E47 promotes death in E2A-deficient lymphomas, we monitored the viability of the transduced cultures by flow cytometry and microscopy. All transduced lymphoma populations exhibited a moderate amount of cell death that occurred primarily within the first 15 hr of culture. Between 15 and 36 hr, however, we observed a large decrease in the viability of cultures transduced with E12 or E47 retroviral supernatants, with a concomitant increase in the number of dead cells (Fig. 4). The dead cells were distinguished by means of flow cytometry by both a reduction in forward scatter and permeability to propidium iodide. During this time, the percentage of viable cells in cultures transduced with vector control or E12BM supernatants increased (Fig. 4). The total number of live cells recovered from E2A-transduced populations after 36 hr of culture was approximately 40% of that found in control-transduced cultures (data not shown), which was in close agreement with the relative decrease in the percentage of viable cells. Thus the growth disadvantage caused by ectopic expression of E47 or E12 in E2A-deficient lymphomas is directly related to the activation of a programmed cell death pathway.
Figure 4.
Loss of viability in populations transduced with E47 or E12 viral supernatants. (A) Forward by side-scatter plots of 1.F9 cells transduced with vector control (Upper) or E47 (Lower) viral supernatants and harvested 15 hr (Left) or 36 hr (Right) after transduction. Percentages of cells within the “live gate” and “dead gate” are indicated to the right of each graph. (B) Plot indicating the percentages of propidium-iodide impermeable 1.F9 cells harvested at various times after transduction with vector control (open squares), E47 (filled squares), E12 (filled circles), or E12BM (open triangles) viral supernatants. All transduced populations were at least 83% EGFP+ by 24 hr after transduction. Data are representative of at least six independent experiments.
E2A-Deficient Lymphoma Populations Transduced with E47 or E12 Have Increased Percentages of Cells with Low Membrane Potential.
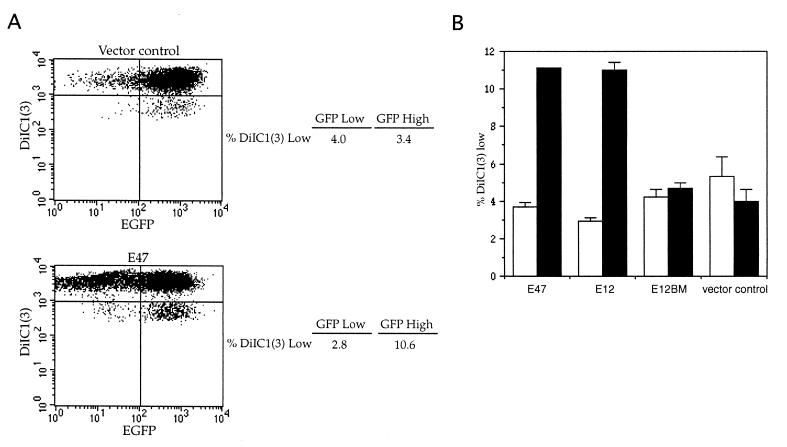
Cells undergoing apoptosis are characterized by a decrease in inner mitochondrial transmembrane potential (Δψm) that can be revealed by a reduction in the fluorescence induced by indocarbocyanines such as DiIC1(3) (28, 29). We measured the relative membrane potential of lymphomas transduced with E2A and control viruses by staining with DiIC1(3) 24–32 hr after transduction and then determining the percentage of cells that were viable on the basis of forward and side scatter but exhibited decreased DiIC1(3) fluorescence. Lymphoma populations that were transduced with E12 or E47 viruses and expressed high levels of EGFP had at least a 2-fold higher percentage of viable cells with low membrane potential than did vector control or E12BM transductants (Fig. 5) or untransduced cells (data not shown). In contrast, the percentage of cells with decreased membrane potential within the fraction of the E2A-transduced populations that expressed little or no EGFP was similar to the background levels observed in the control transductants, providing further evidence that the decrease in membrane potential was specifically correlated with the expression of E2A proteins (Fig. 5). These data suggest that the cell-death pathway induced by E47 and E12 in E2A-deficient lymphomas may involve an impairment of mitochondrial function.
Figure 5.
Loss of membrane potential in E2A transductants. (A) Plots of EGFP vs. DiIC1(3) fluorescence of live 1.F9 cells analyzed 32 hr after transduction with vector control (Upper) or E47 (Lower) viral supernatants. Cells were electronically gated for viability on the basis of forward and side scatter. The percentage of DiIC1(3) low cells within EGFP high- and low-expressing populations is shown to the right of each graph. (B) Bar graph showing the percentage of DiIC1(3) low cells among viable 1.F9 cells transduced with E47, E12, E12BM, or vector control viral supernatants. Open bars refer to cells expressing little or no EGFP, while filled bars represent cells with high levels of EGFP. Cells were stained with DiIC1(3) and analyzed 32 hr after transduction. Live cells were selected as in A. Percentages shown are the means of triplicate samples. Data are representative of at least three independent experiments.
DISCUSSION
A role for E2A proteins in tumor suppression was first suggested by the observation that most E2A-deficient mice develop spontaneous thymic lymphomas (13, 17). To determine the mechanism(s) by which E2A prevents these malignancies, we introduced E47 and E12 into cell lines derived from E2A-deficient lymphomas. We found that E2A expression was not tolerated by these lines, because cells expressing either E12 or E47 disappeared from these cultures at a steady rate until the only cells remaining were those that expressed either truncated protein or no E2A at all. The ability of E2A to prevent the growth of established cell lines indicates that the high rate of lymphoma incidence in E2A-deficient mice is not simply the consequence of the observed abnormalities in thymocyte development. Although these defects in T cell maturation in E2A knockout mice may also contribute to lymphoma development, it is clear that even the growth of in vitro lines, which presumably have incurred a number of tumorigenic mutational events, can be blocked by ectopic expression of E47 or E12. These data thus imply that E2A can act to suppress oncogenesis even at late stages of tumor development.
Our data also further support the theory that inactivation of E2A function may play an important role in human T cell leukemia. Most human T cell acute lymphoblastoid leukemias are characterized by high expression of Class II HLH proteins such as SCL 1/tal-1 or lyl-1. These proteins heterodimerize with E2A proteins, but the resulting dimers have an altered DNA-binding specificity and, in the case of tal-1, reduced transcriptional activation potential as well (18–22). Although there is evidence suggesting that these Class II HLH proteins function as oncogenes by allowing for the formation of DNA-binding complexes together with E2A proteins, LMO 2 and other factors (30, 31), it is possible that the inhibition of normal E2A function is also an important contributing factor to leukemogenesis.
We have found that E2A acts on our lymphoma lines by inducing cell death rather than by causing cell-cycle arrest. These data contrast with the findings of Peverali et al., who reported that E2A could act to block G1 progression when overexpressed in NIH 3T3 cells (32). Obviously these apparently conflicting data could be attributed to differences between the cell lines used in these studies. It will be interesting to see whether there are factors that can be identified in either 3T3 cells or E2A-deficient lymphoma lines that contribute to either cell-cycle arrest or cytotoxicity mediated by E2A expression.
The observation that ectopic expression of E47 and E12 proteins can kill T lymphoma lines raises the question as to how this relates to the function of E2A in normal thymocyte development. The levels of E2A proteins in our retrovirally transduced lines are much higher than those detected in protein extracts prepared from whole thymus (data not shown). However, immunohistochemical analysis has revealed that E2A expression in the thymus is extremely heterogeneous (33); thus it is possible that at certain developmental stages some thymocytes express E2A levels comparable to those of the transduced lymphomas. Although the phenotype of E2A-deficient thymocytes does not suggest any obvious impairment of apoptosis (13, 17), there may be apoptotic defects that are masked by the other effects that E2A deficiency has on thymocyte development. Alternatively, it could be argued that cytotoxic levels of E2A are induced only in thymocytes that have incurred potentially tumorigenic genetic damage; such a hypothesis would propose that E2A is regulated in a manner somewhat analogous to that of p53. Thus the relationship of the tumor suppressor activity of E2A to its role in thymocyte development remains an open question.
We have also observed that the enforced expression of E47 or E12 in E2A-deficient lymphomas results in an increase in the percentage of cells with low membrane potential. These cells are likely to represent an intermediate in the cell-death pathway induced by E2A proteins. The loss of Δψm has been identified as an initiating event of the apoptotic process (28). Impairment of mitochondrial function typically coincides with the release of cytochrome c into the cytosol (34), which in turn results in the activation of critical components of the apoptotic machinery (35). We are currently in the process of determining whether the death pathway induced by E2A proteins shares any of these features. Because we have shown that the DNA-binding domain is necessary for the cytotoxic activity of E2A proteins, we presume that mitochondrial impairment and cell death are induced by transcriptional targets of E47 and E12. It will now be critical to determine the genes regulated by E12 and E47 in E2A-deficient lymphomas to understand how these proteins function as tumor suppressors.
Acknowledgments
We thank Gretchen Bain for construction of the E12 basic region mutation, Garry Nolan for providing the φNX ecotropic packaging line, Hergen Spits for providing the S-003 retroviral vector, Melanie Quong and Craig Walsh for helpful suggestions, and Ruth Schwartz for reading the manuscript. This work was supported by the National Institutes of Health, the Council for Tobacco Research, and the Edward Mallinckrodt, Jr. Foundation.
ABBREVIATIONS
- HLH
helix–loop–helix transcription factor
- FITC
fluorescein isothiocyanate
- DiIC1(3)
1,1′,3,3,3′,3′-hexamethylindocarbocyanine iodide
- EGFP
enhanced green fluorescent protein
- EMSA
electrophoretic mobility-shift assay
- FACS
fluorescence-activated cell sorter
References
- 1.Murre C, McCaw P S, Baltimore D. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty T, Brennan T J, Li L, Edmondson D, Olson E. Mol Cell Biol. 1991;11:3633–3641. doi: 10.1128/mcb.11.7.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lassar A B, Buskin J N, Lockshon D, Davis R L, Apone S, Hauschka S D, Weintraub H. Cell. 1989;58:823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- 4.Murre C, McCaw P S, Vaessin H, Caudy M, Jan L Y, Jan Y N, Cabrera C V, Buskin J N, Hauschka S D, Lassar A B, et al. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 5.Shen C P, Kadesch T. Mol Cell Biol. 1995;15:4518–4524. doi: 10.1128/mcb.15.8.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis R L, Cheng P F, Lassar A B, Weintraub H. Cell. 1990;60:733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- 7.Voronova A, Baltimore D. Proc Natl Acad Sci USA. 1990;87:4722–4726. doi: 10.1073/pnas.87.12.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronheim A, Shiran R, Rosen A, Walker M D. Proc Natl Acad Sci USA. 1993;90:8063–8067. doi: 10.1073/pnas.90.17.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massari M, Jennings P, Murre C. Mol Cell Biol. 1996;16:121–129. doi: 10.1128/mcb.16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quong M W, Massari M E, Zwart R, Murre C. Mol Cell Biol. 1993;13:792–800. doi: 10.1128/mcb.13.2.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murre C, Bain G, van Dijk M A, Engel I, Furnari B A, Massari M E, Matthews J R, Quong M W, Rivera R R, Stuiver M H. Biochim Biophys Acta. 1994;1218:129–135. doi: 10.1016/0167-4781(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 12.Bain G, Gruenwald S, Murre C. Mol Cell Biol. 1993;13:3522–3529. doi: 10.1128/mcb.13.6.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bain G, Engel I, Maandag E C R, te Riele H, Voland J R, Sharp L L, Chun J, Huey B, Pinkel D, Murre C. Mol Cell Biol. 1997;17:4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murre C, Voronova A, Baltimore D. Mol Cell Biol. 1991;11:1156–1160. doi: 10.1128/mcb.11.2.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang Y, Soriano P, Weintraub H. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 16.Bain G, Maandag E, Izon D, Amsen D, Kruisbeek A, Weintraub B, Krop I, Schlissel M, Feeney A, van Roon M, et al. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 17.Yan W, Young A Z, Soares V C, Kelley R, Benezra R, Zhuang Y. Mol Cell Biol. 1997;17:7317–7327. doi: 10.1128/mcb.17.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voronova A F, Lee F. Proc Natl Acad Sci USA. 1994;91:5952–5956. doi: 10.1073/pnas.91.13.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu H-L, Wadman I, Tsan J T, Baer R. Proc Natl Acad Sci USA. 1994;91:5947–5951. doi: 10.1073/pnas.91.13.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto A, Cui X, Naumovski L, Cleary M. Mol Cell Biol. 1996;16:2394–2401. doi: 10.1128/mcb.16.5.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu H L, Huang L, Tsan J T, Frank W, Wright W E, Hu J S, Kingston R E, Baer R. Mol Cell Biol. 1994;14:1256–1265. doi: 10.1128/mcb.14.2.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S T, Sun X-H. J Biol Chem. 1998;273:7030–7037. doi: 10.1074/jbc.273.12.7030. [DOI] [PubMed] [Google Scholar]
- 23.Heemskerk M H M, Blom B, Nolan G, Stegmann A P A, Bakker A Q, Weijer K, Res P C M, Spits H. J Exp Med. 1997;186:1597–1602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsella T M, Nolan G P. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 25.Pear, W. S., Scott, M. L. & Nolan, G. P. (1996) Mol. Med.: Gene Ther. Prot. 41–57.
- 26.Page D M, DeFranco A L. J Immunol. 1988;140:3717–3726. [PubMed] [Google Scholar]
- 27.Shapiro H M. Methods Cell Biol. 1994;41:121–133. doi: 10.1016/s0091-679x(08)61713-6. [DOI] [PubMed] [Google Scholar]
- 28.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J L, Petit P X, Kroemer G. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrenberg B, Montana V, Wei M D, Wuskell J P, Loew L M. Biophys J. 1988;53:785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grutz G G, Bucher K, Lavenir I, Larson T, Larson R, Rabbitts T H. EMBO J. 1998;17:4594–4605. doi: 10.1093/emboj/17.16.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadman I A, Osada H, Grutz G G, Agulnick A D, Westphal H, Forster A, Rabbitts T H. EMBO J. 1997;16:3145–3157. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pevarali F, Ramquist T, Saffrich R, Pepperkok R, Barone M, Philipson L. EMBO J. 1994;13:4291–4301. doi: 10.1002/j.1460-2075.1994.tb06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutherford M N, LeBrun D P. Am J Pathol. 1998;153:165–173. doi: 10.1016/S0002-9440(10)65557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantrow S P, Piantadosi C A. Biochem Biophys Res Commun. 1997;232:669–671. doi: 10.1006/bbrc.1997.6353. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]