Abstract
To determine a detailed picture of tuberculosis (TB) epidemiology in Hamburg, Germany, 423 Mycobacterium tuberculosis complex isolates from 77.0% of all patients with culture-confirmed TB diagnosed from 1997 to 1999 in Hamburg were analyzed by IS6110 DNA fingerprinting. IS6110 restriction fragment length polymorphism (RFLP) clusters were assumed to have arisen from recent transmission. Results of contact tracing and additional patient interviews were used for further epidemiological analyses. Of the 423 cases, 398 were included in the cluster analysis, of which 135 (33.9%) were classified into 35 clusters ranging from 2 to 23 patients. Epidemiological links verifying recent transmission could be confirmed for 87 of the 135 clustered patients. Risk factors for recent transmission were calculated by a two-step procedure: first, based on patients with clustered isolates; and second, based on patients with clustered isolates and transmission links. In both analyses, alcohol abuse appeared to be the strongest predictor for recent transmission, followed by a history of previous contact tracing and unemployment. Homelessness, foreign ethnicity, sex, drug addiction, and human immunodeficiency virus positivity were not independent risk factors for clustering in multivariate analyses. Classical contact tracing performed prior to IS6110 RFLP analysis identified only 24 of the 135 clustered patient. In conclusion, recent transmission seems to be frequent in Hamburg and was found to be strongly associated with alcohol abuse. Conventional contact tracing appears to be insufficient for the detection of recent transmission chains. The data presented also indicate that improved TB control strategies, including the use of RFLP for the detection of transmission chains, are needed for TB control in the setting of countries with a low incidence of TB.
During recent years, tuberculosis (TB) epidemiology has successfully been analyzed by applying both classical epidemiological and molecular strain-typing techniques in population-based studies, e.g., in Europe (3, 10, 30) or the United States (1, 24). Surprisingly, and in contrast to assumptions made based on older epidemiological studies (32), several recent studies revealed a high degree of ongoing transmission even in countries with a low incidence of TB (3, 24). Hence, recently transmitted infections that rapidly progress to active TB seem to play a more important role in countries with a low TB incidence than previously thought, also indicating a need for more carefully targeted TB control measures.
DNA fingerprinting using the insertion sequence IS6110 as a probe has become the worldwide standard technique for comparison of Mycobacterium tuberculosis isolates on the strain level in epidemiological studies (5, 27). The epidemiological analysis of TB using IS6110 is based on the observation that the polymorphism of IS6110 restriction fragment length polymorphism (RFLP) patterns among unrelated clinical isolates is high, whereas epidemiologically related M. tuberculosis strains show identical or similar (one band variation) fingerprint patterns (28). Hence, M. tuberculosis strains with identical fingerprint patterns likely indicate recent transmission and a chain of transmission.
In Germany as well as in other Western European countries, the incidence of TB is low compared to that in less developed regions of the world (16, 23). Nevertheless, in 1999, a total of 9,974 cases were reported to the German Federal Statistical Office (25), with an incidence of 12.1 cases per 100,000 inhabitants. Since in large cities, as well as in the immigrant population, TB incidence rates are generally two- to fivefold higher than the national average, TB remains a considerable public health challenge in Western European countries. Moreover, especially in Germany, the incidence of TB may increase in the future due to enhanced spreading of susceptible and/or drug-resistant strains from countries with high prevalences of TB, e.g., from the former Soviet Union (SU) (17, 34).
The highest TB incidence (19.1 cases per 100,000 residents) of all 16 German federal states in 1999 was documented in the city of Hamburg, which has a population of 1.7 million. Hamburg also had the highest proportion of foreign residents, 15.4%, compared with a German national average of 8.9% reported for 31 December 1999 (25). Hamburg is further characterized by its international harbor, the largest in Germany, and by being one of the most popular tourist destinations in Germany.
TB surveillance in Germany has so far been based on case reports to the statistical offices of the German federal states though the public health offices of the district in which patients reside. These reports include information about gender, year of birth, foreign or nonforeign origin, entry date of the report reached at the public health office, listing of body organs involved in the TB disease, microbiological confirmation of M. tuberculosis complex infection by culture, and classification by first onset of the disease or relapse. Only a few studies have been performed in Germany applying modern molecular DNA fingerprint techniques that are able to directly trace routes of transmission (27), e.g., to analyze the epidemiology of resistant M. tuberculosis strains in Germany (17) or to describe small-scale outbreaks of resistant strains (19). In general, there is a lack of information on important issues of TB epidemiology, such as the contribution of recent transmission to the number of annual new cases or the proportion of TB cases attributable to non-German source cases. However, improved epidemiological data are urgently needed to better target TB control efforts and to allow forecasting of future epidemiological trends.
This prospective, population-based study was undertaken to obtain a detailed picture of the TB epidemiology in Hamburg, which is the second largest city in Germany. The study focused on the determination of the rate of TB cases attributable to recent transmission, on possible risk factors associated with recent transmission, and on the identification of pathways of TB transmission in the community. The observations made in Hamburg and conclusions regarding intervention strategies for the future may be of relevance to other urban centers in European countries.
MATERIALS AND METHODS
Data collection.
The study includes patients with culture-confirmed TB that were reported to each of the seven district public health departments of Hamburg between 1 January 1997 and 31 December 1999. Case data were collected prospectively by trained public health staff using a standardized questionnaire. Information was obtained on gender, date and country of birth, nationality, immigration status (if necessary), number of years of residency in Hamburg or elsewhere in Germany, present address (or whether the patient was homeless), whether the patient was living in a health care institution or any public institution, the nature of the patient's employment, socioeconomic status, any previous known exposure to other persons with TB (especially within the 6 months before development of any symptoms), and the identity of the patient's household contacts and respective close contacts in occupational or crowded settings, if necessary.
To obtain clinical variables, the following data were also included: date of first onset of illness, type of symptoms and cause of diagnosis, first date of case report to a public health office, and associated medical data, such as human immunodeficiency virus (HIV) infection, tuberculin skin test, chest radiography findings, and results of microbiological analyses. Alcoholism was defined as a maladaptive pattern manifested by three or more criteria of the World Health Organization ICD-10 classification (33) occurring at any time in the same 12-month period.
Bacterial strains and drug susceptibility testing.
Mycobacterial strains from 423 patients were analyzed. Primary isolation and culturing of mycobacterial isolates were performed as described elsewhere (15). All isolates were identified as M. tuberculosis complex by using gene probes as instructed by the manufacturer (ACCUProbe, GenProbe, San Diego, Calif.); differentiation was performed as described previously (20). Drug susceptibility was determined by the proportion method on Löwenstein-Jensen medium and/or the modified proportion method in BACTEC 460TB (Becton-Dickinson Microbiology Systems, Cockeysville, Md.).
IS6110-RFLP and spoligotyping analysis.
Extraction of DNA from mycobacterial strains and DNA fingerprinting using IS6110 as a probe was performed according to a standardized protocol as described elsewhere (17, 27). PvuII-digested total DNA of reference strain Mt.14323 was used in each Southern blot experiment as an external size standard and was used for quality control and quality assurance of IS6110 RFLP experiments. The IS6110 fingerprint patterns of mycobacterial strains were analyzed using the Gelcompar software (Windows 95, version 4.2; Applied Maths, Kortrijk, Belgium) as described previously (17, 27). Clusters were defined as groups of patients with M. tuberculosis strains showing identical RFLP patterns (the same number of IS6110 bands at identical positions [position tolerance, 1.3%]).
Isolates with fewer than five bands (n = 24) were excluded from the initial cluster and epidemiological analysis and were subjected to spoligotyping for separate analysis of strain relationship. Spoligotyping of isolates was performed as described by Kamerbeek et al. (14).
Recent transmission.
Clusters were assumed to have arisen from recent transmission, and the clustering rate was used to measure the amount of recent transmission in the study population. Independent risk factors for recent transmission were calculated by a two-step procedure: first, based on patients with clustered isolates, and, second, based on patients with clustered isolates for whom a transmission link was established.
Statistical analysis.
All patients included were classified in two groups: individuals with clustered and nonclustered M. tuberculosis strains. Categorical data were compared by the chi-square test or Fisher's exact test, when expected cell sizes (n) were smaller than five. The Wilcoxon rank sum test was performed to determine whether the distribution of age as a continuous variable differed between the two groups. All tests were performed as two-sided tests. P values below 0.05 were considered statistically significant. Variables that were highly significantly associated with clustering in the univariate analysis were then included in a multivariate logistic regression model to determine independent risk factors (e.g., adjusted for confounding). The two-sided Cochran Armitage test for trend was used to determine correlations between increasing size of a cluster and variables associated with clustering.
RESULTS
Study population.
Between 1 January 1997 and 31 December 1999, 529 cases of pulmonary TB and 20 cases of nonpulmonary TB that were culture positive for M. tuberculosis complex were reported to the public health offices. Culture isolates from 423 patients (77.0%) were available for RFLP fingerprinting, of which 130 were diagnosed in 1997, 149 in 1998, and 144 in 1999.
The male-to-female ratio in the study population was 2.5:1. The patients were from 3 to 97 years old, most of whom (approximately 70%) were between 20 and 60 years of age. Two hundred and forty-seven patients (58.4%) were born in Germany, while 176 (41.6%) were born in 43 different countries, including 47 patients from Afghanistan (26.7%), 31 patients form Turkey (17.6%), 25 patients from Africa (14.2%), 18 patients from countries of the former SU (10.2%), and 17 patients from countries of former Yugoslavia (9.6%). Eighty-one of the non-German patients (46%) were from Asian countries (including 47 patients from Afghanistan as well as 14 patients from Kazakhstan and Uzbekistan).
Species differentiation revealed 418 infections with M. tuberculosis, three infections with Mycobacterium bovis, one infection with Mycobacterium africanum, and one infection with Mycobacterium microti. The latter case, which represents the first reported case of M. microti infection causing pulmonary TB in an HIV-positive patient in Germany, has recently been described in detail in previous work (12).
Four hundred and one isolates (94.7%) were susceptible to all four drugs tested (isoniazid [INH], rifampin [RIF], pyrazinamide, and ethambutol). Five percent were resistant to at least one drug, with 4.0% showing resistance to INH, 0.7% to RIF, 0.7% to pyrazinamide (excluding three M. bovis isolates), and 0.9% to ethambutol. All strains resistant to RIF were also resistant to INH and thus were multidrug resistant (one patient from Germany, one from Spain, and one from Kazakhstan).
IS6110 RFLP analysis.
Of 423 strains, 24 isolates with fewer than five IS6110 RFLP bands were excluded from the initial cluster analysis. The patient with M. microti infection was also excluded from epidemiological analysis, since nothing is known about the transmissibility of M. microti in humans.
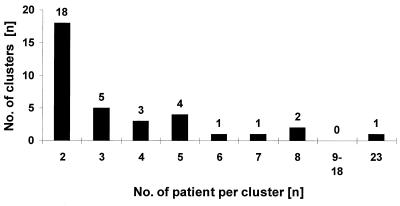
Of the remaining 398 patients, a unique M. tuberculosis RFLP pattern was found among 263 (66.1%) patients (nonclustered cases). One hundred thirty-five cases (33.9%) shared an identical RFLP pattern with one or more other patients (clustered cases) and were classified into 35 clusters ranging in size from 2 to 23 persons (Fig. 1), of which 18 (51.4%) included just two patients.
FIG. 1.
Number of clusters by cluster size. Total number of clustered patients with TB in Hamburg during the study period from 1997 to 1999 was 135.
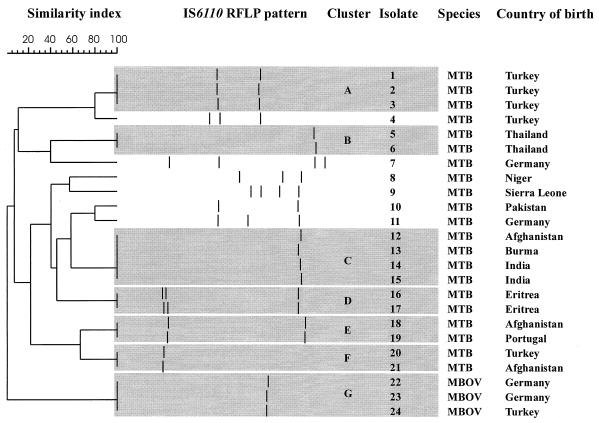
Among the 24 isolates with fewer than five IS6110 bands, only six (25.0%) showed a unique RFLP pattern (Fig. 2). The other 18 isolates (75.0%) clustered into seven groups ranging in size from two to four patients. Of the 24 patients, only four were of German ethnicity (two of whom had M. bovis infections), indicating that M. tuberculosis strains with fewer than five bands were not indigenous isolates but were predominately imported into Germany from foreign countries (Fig. 2). Considering the IS6110 RFLP patterns and the ethnicity of the patients, it becomes obvious that some RFLP patterns correlate with the origin of the patients e.g., cluster A, B, or D. This observation was further confirmed by the fact that the M. tuberculosis RFLP pattern of the two Eritrean patients in cluster D was previously described by Hermans and coworkers (11) as a genotype (E2) typical of M. tuberculosis isolates from Ethiopia, which is adjacent to Eritrea. Additional spoligotyping analysis reduced the number of clustered isolates to 10 of the 24 strains in five spoligotyping groups (41.7%; data not shown), which, however, still represents a high rate of clustering.
FIG. 2.
IS6110 RFLP patterns of strains with fewer than five bands. The positions of the IS6110 band were normalized and displayed as bands. The scale depicts the similarity of patterns calculated as described in Materials and Methods. MTB, M. tuberculosis; and MBOV, M. bovis.
We also identified 15 M. tuberculosis isolates that displayed RFLP and spoligotype patterns (hybridization only to spacers 35 to 43) typical for the Beijing family (data not shown), which has initially been found to represent approximately 80% of all M. tuberculosis strains from the Beijing region, China (29). The majority (n = 13) of the 15 patients were immigrants from several Asian countries (Afghanistan, n = 3; China, n = 1; Indonesia, n = 1; Kazakhstan, n = 4; Korea, n = 1; Nepal, n = 1; Pakistan, n = 1; and Vietnam, n = 1); only two patients were born in Germany.
Epidemiological analysis of clustered cases and efficiency of classical contact tracing.
In addition to the data based on standardized patient questionnaires, intensive epidemiological investigations including additional patient interviews have been performed for clustered patients (135 patients in 35 clusters). Definite transmission links (e.g., household contacts or working contacts) could be verified for 52 patients in 13 of the 35 clusters. In addition, there was strong evidence for epidemiological relationships between 35 members of eight further clusters. Hence, recent transmission could be confirmed for 87 of the 135 clustered patients (64.4%) in 21 clusters (60.0%).
In 4 of the 14 clusters without transmission links, the patients had the same nationality (Afghanistan and Turkey) and were coming from the same or from proximate geographical regions. Even the clinical aspects confirmed that in these cases recent transmission in Germany was not very likely.
The largest cluster identified in this study comprises 23 patients, including 12 smear-positive/culture-confirmed and 11 smear-negative/culture-confirmed cases diagnosed between March 1997 and December 1999 (6 cases in 1997, 5 cases in 1998, and 12 cases in 1999). Classical contact tracing was difficult, since the patients' residences are located in four different districts with four responsible public health offices. Moreover, three patients had previous phases of pulmonary TB, so that for these cases relapse was suspected as the cause of the actual disease.
Subsequent patient interviews and analysis of questionnaires revealed that transmission of the strain presumably occurred in a particular bar in the red-light district of Hamburg (18 cases) and in a hostel for men (five cases). A 42-year-old highly infectious white male with pulmonary TB could be identified as the connecting link between these two centers of transmission. Although conventional contact tracing correctly identified the relationship of four patients in the hostel, the fifth patient was allocated to another TB case occurring in the same time period in the hostel. The link of the five patients in the hostel to the other 18 cases has not been initially determined, since the index patient did not name all contact persons correctly in the first interview. Among these, contact tracing investigations performed prior to RFLP analysis revealed transmission links only for two patients who were customers of the bar and named one another as contacts.
However, retrospective analyses established specific transmission links among 13 further patients: five had named one another as contacts and eight had been visiting the bar at the same time period in which the other transmissions occurred. The remaining three patients were tuberculin reactors who presumably had been infected before the start of the study and developed an active pulmonary TB in the study period. Since these patients frequently used the bar, spending most of their free time there, it is reasonable to assume that their infection occurred in the bar milieu.
The difficulties in establishing transmission links in a further cluster comprising five young male patients in an age range from 19 to 35 years again demonstrated the limited efficiency of conventional contact tracing. The patients were born in three different African countries, all coming to Hamburg seeking asylum. TB was diagnosed in a time period of 13 months after their arrival in Hamburg. In contact tracing investigations, the patients named 65 contact persons; however, they did not name one another. They all lived on a ship used for housing of immigrants without direct household contacts. However, in August 1998, one of the patients was arrested because of illegal drug use. Subsequent investigation by the police revealed that all clustered patients were frequently smoking water pipe marihuana in the cabin of the index patient, ideal circumstances for TB transmission.
Regarding the efficiency of classical contact tracing for determination of recent chains of transmission compared with IS6110 RFLP typing, in total, conventional investigation of the patient contacts conducted before any RFLP typing identified only 24 (27.6%) of the 87 clustered patients with confirmed epidemiological links.
Characterization of cluster patients and identification of risk factors associated with recent transmission.
In a first attempt to identify significant differences between the 135 patients in clusters and the 263 patients not in clusters, univariate analysis was performed. The results of the different parameters analyzed are summarized in Table 1. With regard to the distribution of age between the two groups determined by the Wilcoxon rank sum test, there was no significant difference (P = 0.46). Chi-square testing showed that the patients in clusters were likelier than the nonclustered patients to be HIV infected (P = 0.003), to be male (P < 0.001), to be drug abusers (P < 0.001), to be alcoholics (P < 0.001), to be homeless (P < 0.001), to be unemployed (P< 0.001), and to be sputum smear positive (P = 0.001) and had more known contacts with patients who had active TB (P < 0.001). A previous history of being traced for contact with TB patients before the study period was also significantly associated with clustering (P < 0.001).
TABLE 1.
Univariate analysis of variables associated with IS6110 RFLP clustersa
| Characteristic | No. of patients (%) with characteristic from
|
% All patients with characteristic | P | |
|---|---|---|---|---|
| Noncluster group (n = 263) | Cluster group (n = 135) | |||
| Mean age (yr) (±SD) | 47.8 (±19.8) | 44.4 (±15.8) | .46 | |
| AIDS | 11 (4.2) | 13 (9.6) | 6.0 | .003 |
| Resistance to any drug | 26 (9.9) | 2 (1.5) | 7.0 | .002 |
| Multidrug resistance | 2 (0.8) | 1 (0.7) | 0.8 | .98 (NS) |
| Foreign birth | 116 (44.1) | 39 (28.9) | 41.4 | .003 |
| Crowded living conditions | 21 (8.0) | 4 (3.0) | 6.3 | .051 (NS) |
| Male | 172 (65.4) | 112 (83.0) | 71.4 | <.001 |
| Female | 91 (34.6) | 23 (17.0) | 28.6 | <.001 |
| Alcohol abuse | 30 (11.4) | 75 (55.6) | 26.4 | <.001 |
| Intravenous drug abuse | 15 (5.7) | 22 (16.3) | 9.3 | <.001 |
| Homelessness | 10 (3.8) | 20 (14.8) | 7.5 | <.001 |
| Previous history of TB | 20 (7.6) | 17 (12.6) | 9.3 | .11 (NS) |
| Site of TB | ||||
| Pulmonary | 257 (97.7) | 130 (96.3) | 97.2 | .41 (NS) |
| Extrapulmonary | 6 (2.3) | 5 (3.7) | 2.8 | .41 (NS) |
| Both | 1 (0.4) | 1 (0.7) | ||
| Sputum smear positivity | 87 (33.1) | 67 (49.6) | 38.7 | .001 |
| Chest film findings | ||||
| Cavitary disease | 60 (22.8) | 38 (28.1) | 24.6 | .24 (NS) |
| Other (%) | 197 (17.2) | 92 (71.9) | 72.6 | .15 (NS) |
| History of contact tracing | 18 (6.8) | 42 (31.1) | 15.1 | <.001 |
| Symptomatic status | 224 (85.2) | 123 (91.1) | 87.2 | .09 (NS) |
| Unemployment | 39 (14.8) | 75 (55.6) | 28.6 | <.001 |
| Asylum | 35 (13.3) | 16 (11.9) | 12.8 | .68 (NS) |
| Contact with persons with disease | 5 (1.9) | 21 (15.6) | 6.5 | <.001 |
NS, not statistically significant.
The prevalence of cavitary lung disease and other presentations, such as radiological features as well as the level of crowded living, were similar for patients in and not in IS6110 RFLP clusters. Drug resistance was more common in patients not included in a cluster (P = 0.002). The proportion of foreign-born patients was reduced among the clustered patients compared to nonclustered patients (P = 0.003).
Independent risk factors for recent transmission were determined by logistic regression. Analyses were performed first, for patients with clustered isolates (n = 135) and, second, for patients with clustered isolates and a confirmed transmission link (n = 87). In both analyses, alcohol abuse was the strongest predictor for recent transmission, followed by a history of contact tracing (Table 2). Unemployment also represented a considerable risk of being in a transmission chain (odds ratio, 3.30; P < 0.001), whereas living as a drug addict, being male, and suffering from AIDS or homelessness were not significant independent risk factors in these analyses. Sputum smear positivity was a significant risk factor for clustered patients with confirmed transmission links but not for all clustered patients.
TABLE 2.
Risk factors for recent transmission of M. tuberculosisa
| Risk factor | Results for:
|
||||||
|---|---|---|---|---|---|---|---|
| Patients with clustered isolates (n = 135)
|
Patients with clustered isolates and transmission links (n = 87)
|
||||||
| Odds ratio | 95% confidence interval | P | Odds ratio | 95% confidence interval | P | ||
| Alcohol abuse | 5.11 | 2.77-9.43 | <0.001 | 8.01 | 3.72-7.26 | <0.001 | |
| History of contact tracing | 3.65 | 1.83-7.25 | <0.001 | 4.85 | 2.12-1.07 | <0.001 | |
| Unemployment | 3.30 | 1.69-6.45 | <0.001 | 3.62 | 1.58-8.31 | <0.002 | |
| Intravenous drug abuse | 1.66 | 0.62-4.45 | 0.31 | 2.69 | 0.89-8.11 | 0.08 | |
| Being male | 1.49 | 0.81-2.72 | 0.20 | 1.67 | 0.74-3.79 | 0.22 | |
| Sputum smear positivity | 1.22 | 0.72-2.06 | 0.46 | 2.04 | 1.07-3.91 | 0.03 | |
| AIDS | 0.94 | 0.30-2.99 | 0.92 | 1.02 | 0.27-3.93 | 0.97 | |
| Homelessness | 0.89 | 0.33-2.40 | 0.82 | 0.52 | 0.16-1.69 | 0.28 | |
Independent risk factors for recent transmission were calculated by multiple regression procedures for patients with clustered isolates and for patients with clustered isolates for whom transmission links were established.
Analyzing the trend for increasing cluster size by applying the Cochrane Armitage test for trend revealed a strong positive correlation between increasing size of a cluster and the number of cluster patients matching one of the following variables: alcohol abuse (P < 0.001), a history of contact tracing (P < 0.001), and being male (P = 0.009). In contrast, the number of drug addicts decreased with increasing cluster size (P = 0.047). There was no significant correlation regarding unemployment (P= 0.08), AIDS (P = 0.25), homelessness (P = 0.14), and sputum smear positivity (P= 0.82).
DISCUSSION
In the study presented, a detailed picture of TB epidemiology in Hamburg, Germany, has been attained by combining IS6110 RFLP typing and classical epidemiological methods. The data obtained are based on a study period of 3 years in which approximately 80% of all culture-confirmed TB cases reported to the public health offices in Hamburg were analyzed.
The percentage of clustered isolates of 33.9% observed in our study is comparable with that described for other cities, e.g., Paris (approximately 36% [10]) or New York (approximately 37% [1]); however, it is slightly lower than observed in studies performed in The Netherlands and Denmark (ranging from 47 to 57% [3, 26, 30]). These differences between the clustering rates determined in the nationwide studies in The Netherlands and Denmark may be explained by the shorter study period as well as a smaller study population analyzed in the present study. Differences in the diversity of the patients might also account for differences in clustering rates among M. tuberculosis isolates observed in different study regions. The exceptionally high rate of clustering found in the Denmark study may moreover be partially the effect of an outbreak of two M. tuberculosis strains, which represented approximately 40% of all clustered isolates.
A further reason for differences among clustering rates observed in other investigations may be the strict cluster definition in this study, including only isolates with identical IS6110 RFLP patterns in clusters. This strict definition was chosen, since our previous studies indicated a high stability of IS6110 RFLP patterns in serial patient isolates and in isolates in actual chains of transmission from Germany (18, 21). We further excluded isolates with fewer than five IS6110 bands that showed a lowered IS6110 RFLP in our and in previous studies (30), in order to achieve a maximum predictive value of clustering for recent transmission.
For these reasons, it is likely that this study actually underestimates the real amount of recent transmission in Hamburg. However, the data presented indicate that a reasonable proportion of annual new TB cases in Hamburg is attributable to recent infection, which may be considered a problem of the present TB control program.
Despite our strict cluster definition and the intensive epidemiological investigations performed, transmission links have been determined for only 64% of the clustered patients; for nearly one-third of the patients with clustered isolates, no epidemiological relationship has been established. A possible reason for this result might be that a portion of clustered patients are infected with strains prevalent in a given area and by chance developed an active pulmonary TB during the study period. Furthermore, importation of genetically more homogenous M. tuberculosis strains from areas of the world in which TB is endemic, as shown for a subset of clustered patients in our study, may also account for a portion of strains in IS6110 RFLP clusters without any obvious epidemiological link. These data in part question the assumption that every RFLP cluster is indicative of a recent epidemiological link. Consistent with this suggestion, other studies (6, 9) indicated that clustering may also be observed between individuals without any recent transmission link or common contacts by infection with circulating strains within a community spreading over a long time. An active TB disease of these patients observed during a study period may then occur by chance through reactivation during the observation period (6, 9). The proportion of such clusters, not representing recent transmission, depends on the number and stability of different strains in a given area and on the number of genetically homogenous strains imported from regions of the world where M. tuberculosis strains with lower IS6110 polymorphism are prevalent. Thus, clustering rates should be interpreted carefully. Proportions of recent transmission incurred from clustering rates might vary in different studies.
In our study several factors associated with clustering were identified in univariate analysis, including alcohol abuse, HIV infection, and being male, homeless, or unemployed. Multivariate analyses indicated that alcohol abuse was the strongest risk factor associated with recent transmission in Hamburg. This is somewhat surprising, since in several international reports published so far (1, 4, 10), alcohol abuse has not been identified as an independent predictor for recent transmission. Further independent risk factors for being in an IS6110 cluster are a history of contact tracing and unemployment, whereas being male, being homeless, testing positive for sputum smear, living as a drug addict, or suffering from AIDS was not significantly associated with clustering in multivariate analysis.
The finding that HIV infection is not associated with recent transmission is in accordance with other studies performed in Europe, e.g., in The Netherlands (30) and in Paris (10) but is contrary to studies performed in the United States (1, 4, 7, 13, 24).
In contrast to the studies performed in The Netherlands (30) and in Paris (10), homelessness, although highly significant in univariate analysis, did not prove to be an independent risk factor for recent transmission in multivariate analysis. This might be due to the relatively low total number of homeless people (n = 30; 7.5%) recruited in our study population.
In our study population clustering was not associated with younger patients, representing a major difference with other studies (1, 26). This finding might be due to the specific epidemiological situation in Hamburg, which to a great extent is characterized by alcoholism and the social behavior associated with it. Even in the largest cluster determined, frequent visiting of a particular bar was identified as the most obvious transmission link. The increasing size of this cluster in 1999 indicates that the “bar milieu” in a red-light district might be one major setting for transmission of TB in Hamburg.
The predominance of unexpected risk factors (e.g., alcohol abuse) in this study might have important implications for inner-city control of TB in the future. According to the stone-in-the-pond-principle (8, 31), expansion contact tracing in Germany is usually performed by screening close contacts, starting in concentric circles from the infectious index case. The question, however, is, how effectively this intervention identifies exposed contacts in a particular case. Moreover, the effectiveness of traditional contact tracing obviously is largely dependent on individual willingness to cooperate. Denial of alcohol abuse (or other substance abuse) and the high prevalence of comorbid psychiatric disorders among alcoholics (22) may result in misinformation and in examinations such as tuberculin skin testing and chest radiography of persons wrongly suspected as contacts.
The present approach of contract tracing seems to be insufficient to uncover transmission pathways in urban areas like Hamburg. Only approximately 27.6% (n = 24 of 87) of clustered patients with confirmed transmission links were identified correctly by the conventional contact tracing in our study. A similar observation was made in Amsterdam by van Deutekom and coworkers (26), where only 5.6% of cases with identical M. tuberculosis IS6110 RFLP patterns were identified by conventional contact tracing (26). Improved strategies for contact tracing and more targeted case finding thus might be needed.
IS6110 RFLP typing may more precisely define target groups for health examinations and more precisely show how contact tracing strategies could be adapted to population subgroups with a high potential of exposure to M. tuberculosis transmission. Recent observations made in San Francisco (13) suggest that an appropriate intensification of TB control measures directed towards high-risk groups may result in a decrease of TB transmission.
Our results also confirm previous observations that IS6110 RFLP patterns might be used to trace the global spread of distinct families of genetically highly related M. tuberculosis strains (11), as shown for the isolates from Eritrean patients or strains of the Beijing family, and the spread of imported strains in the community. Interestingly, strains of the Beijing family, which initially were described to be predominant in China and Asia (2, 29) were also found in our study population. Most patients with strains of the Beijing family were immigrants from several Asian countries; only two were of German nationality. Our previous data have shown that strains of the Beijing genotype were also present among drug-resistant M. tuberculosis isolates from German patients (17), mainly isolated from immigrants from countries of the former SU. Since strains of the Beijing family have been assumed to have a selective advantage leading to their prevalence in different countries in Asia (29), it might be also possible that this genotype will spread in the German community in the future. However, our data indicate that patients with Beijing-type strains in Germany likely acquired their infection in their respective homelands. Indications for transmission of strains of the Beijing genotype from immigrants to German patients have been found only in few cases.
In conclusion, our study demonstrates that recent transmission (defined by the degree of clustering) accounts for a considerable number of annual new TB cases in Hamburg and is strongly associated with alcohol abuse and social behavior associated with it. Conventional contact tracing appeared to be insufficient in these cases. Routine DNA fingerprinting will be required to observe the dynamics of M. tuberculosis transmission or the prevalence of distinct strain types. DNA fingerprinting should therefore become an integral component of surveillance strategies aimed at control of TB in metropolitan areas such as the city of Hamburg. TB control measures may be improved by contact tracing and case-finding strategies that are more carefully targeted to high-risk groups.
Acknowledgments
We thank I. Radzio, B. Schlüter, P. Vock, and A. Zyzik, Borstel, Germany, for excellent technical assistance. We are grateful to S. Schwander (Department of Medicine, UMD-New Jersey Medical School, Newark, N.J.) for critical reading of the manuscript.
Parts of this work were supported by the Robert Koch-Institut, Berlin, Germany.
REFERENCES
- 1.Alland, D., G. E. Kalkut, A. R. Moss, R. A. McAdam, J. A. Hahn, W. Bosworth, E. Druckner, and B. R. Bloom. 1994. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N. Engl. J. Med. 330:1710-1716. [DOI] [PubMed] [Google Scholar]
- 2.Anh, D. D., M. W. Borgdorff, L. N. Van, N. T. Lan, T. van Gorkom, K. Kremer, and D. van Soolingen. 2000. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg. Infect. Dis. 6:302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, J., Z. Yang, S. Poulsen, and Å. B. Anderson. 1998. Results from 5 years of nationwide DNA fingerprinting of Mycobacterium tuberculosis complex isolates in a country with a low incidence of M. tuberculosis infection. J. Clin. Microbiol. 36:305-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, W. Z., J. Koehler, E. H. Hiyam, P. C. Hopewell, A. L. Reingold, C. B. Agasino, M. D. Cave, S. Rane, Z. Yang, C. M. Crane, and P. M. Small. 1998. Dissemination of Mycobacterium tuberculosis across the San Francisco Bay Area. J. Infect. Dis. 177:1104-1107. [DOI] [PubMed] [Google Scholar]
- 5.Cave, M. D., K. D. Eisenach, P. F. McDermott, J. H. Bates, and J. T. Crawford. 1991. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol. Cell. Probes 5:73-80. [DOI] [PubMed] [Google Scholar]
- 6.Dale, J. W., R. M. Nor, S. Ramayah, T. H. Tang, and Z. F. Zainuddin. 1999. Molecular epidemiology of tuberculosis in Malaysia. J. Clin. Microbiol. 37:1265-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daley, C. L., P. M. Small, G. F. Schecter, G. K. Schoolnik, R. A. McAdam, W. R. Jacobs, and P. C. Hopewell. 1992. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. N. Engl. J. Med. 326:231-235. [DOI] [PubMed] [Google Scholar]
- 8.Deutsches Zentralkomitee zur Bekämpfung der Tuberkulose. 1996. Richtlinien für die Umgebungsuntersuchungen bei Tuberkulose. Gesundheitswesen 58:657-665. [PubMed] [Google Scholar]
- 9.Glynn, J. R., J. Bauer, A. S. de Boer, M. W. Borgdorff, P. E. Fine, P. Godfrey-Faussett, and E. Vynnycky. 1999. Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. European Concerted Action on Molecular Epidemiology and Control of Tuberculosis. Int. J. Tuberc. Lung Dis. 3:1055-1060. [PubMed] [Google Scholar]
- 10.Gutierrez, M. C., V. Vincent, D. Aubert, J. Bizet, O. Gaillot, L. Lebrun, C. Le Pendeven, M. P. Le Pennec, D. Mathieu, C. Offredo, B. Pangon, and C. Pierre-Audigier. 1998. Molecular fingerprinting of Mycobacterium tuberculosis and risk factors for tuberculosis transmission in Paris, France, and surrounding area. J. Clin. Microbiol. 36:486-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermans, P. W. M., F. Messadi, H. Guebrexabher, D. van Soolingen, P. E. W. de Haas, H. Heersma, H. de Neeling, A. Ayoub, F. Portaels, D. Frommel, M. Zribi, and J. D. A. van Embden. 1995. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and the Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J. Infect. Dis. 171:1504-1513. [DOI] [PubMed] [Google Scholar]
- 12.Horstkotte, M. A., I. Sobottka, C. K. Schewe, P. Schäfer, R. Laufs, S. Rüsch-Gerdes, and S. Niemann. 2001. Mycobacterium microti llama-type infection presenting as pulmonary tuberculosis in a human immunodeficiency virus-positive patient. J. Clin. Microbiol. 39:406-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasmer, R. M., J. A. Hahn, P. M. Small, C. L. Daley, M. A. Behr, A. R. Moss, J. M. Creasman, G. F. Schecter, E. A. Paz, and P. C. Hopewell. 1999. A molecular epidemiologic analysis of tuberculosis trends in San Francisco, 1991-1997. Arch. Intern. Med. 130:971-978. [DOI] [PubMed] [Google Scholar]
- 14.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. D. A. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology. A guide for the Level III laboratory. Centers for Disease Control, U.S. Department of Health and Human Services, Atlanta, Ga.
- 16.Loddenkemper, R., B. Hauer, D. Sagebiehl, and M. Forβbohm. 1999. Tuberkuloseepidemiologie in Deutschland und der Welt mit Schwerpunkt Osteuropa. Bundesgesundheitsbl. Gesundheitsforsch. Gesundheitsschutz 9:683-693. [Google Scholar]
- 17.Niemann, S., S. Rüsch-Gerdes, and E. Richter. 1997. IS6110 fingerprinting of drug-resistant Mycobacterium tuberculosis strains isolated in Germany during 1995. J. Clin. Microbiol. 35:3015-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niemann, S., E. Richter, and S. Rüsch-Gerdes. 1999. Stability of Mycobacterium tuberculosis IS6110 restriction fragment length polymorphism patterns and spoligotypes determined by analyzing serial isolates from patients with drug-resistant tuberculosis. J. Clin. Microbiol. 37:409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemann, S., E. Richter, S. Rüsch-Gerdes, H. Thielen, and H. Heykes-Uden. 1999. Outbreak of rifampin and streptomycin-resistant tuberculosis among homeless in Germany. Int. J. Tuberc. Lung Dis. 3:1146-1147. [PubMed] [Google Scholar]
- 20.Niemann, S., E. Richter, and S. Rüsch-Gerdes. 2000. Differentiation among members of the Mycobacterium tuberculosis complex by molecular and biochemical features: evidence for two pyrazinamide-susceptible subtypes of M. bovis. J. Clin. Microbiol. 38:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niemann, S., S. Rüsch-Gerdes, E. Richter, H. Thielen, H. Heykes-Uden, and R. Diel. 2000. Stability of IS6110 restriction fragment length polymorphism patterns of Mycobacterium tuberculosis strains in actual chains of transmission. J. Clin. Microbiol. 38:2563-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regier, D. A., M. E. Farmer, D. S. Rae, B. Z. Locke, S. J. Keith, L. L. Judd, and F. K. Goodwin. 1990. Comorbidity of mental disorders with alcohol and other drug abuse. JAMA 264:2511-2518. [PubMed] [Google Scholar]
- 23.Rieder, H. L., J. M. Watson, M. C. Raviglione, M. Forssbohm, G. B. Migliori, V. Schwoebel, A. G. Leitch, and J. P. Zellweger. 1996. Surveillance of tuberculosis in Europe. Working group of the World Health Organization (W. H. O.) and the European Region of the International Union Against Tuberculosis and Lung Disease (IUATLD) for uniform reporting on tuberculosis cases. Eur. Respir. J. 9:1097-1104. [DOI] [PubMed]
- 24.Small, P. M., P. C. Hopewell, S. P. Samir, A. Paz, J. Parsonnet, D. C. Ruston, G. F. Schecter, C. L. Daley, and G. K. Schoolnik. 1994. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 25.Statistisches Bundesamt. 2000. Meldepflichtige Krankheiten, Fachserie 12. Statistisches Bundesamt, Wiesbaden, Germany.
- 26.Van Deutekom, H., J. J. Gerritsen, D. Van Soolingen, E. J. Van Ameijden, J. D. A. van Embden, and R. A. Coutinho. 1997. A molecular epidemiological approach to studying the transmission of tuberculosis in Amsterdam. Clin. Infect. Dis. 25:1071-1077. [DOI] [PubMed] [Google Scholar]
- 27.Van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Soolingen, D., and P. W. M. Hermans. 1995. Epidemiology of tuberculosis by DNA fingerprinting. Eur. Respir. J. 8(Suppl. 20):649s-656s. [PubMed] [Google Scholar]
- 29.Van Soolingen, D., L. Qian, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. A. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Soolingen, D., M. W. Borgdorff, P. E. de Haas, M. M. Sebek, J. Veen, M. Dessens, K. Kremer, and J. D. A. van Embden. 1999. Molecular epidemiology of tuberculosis in the Netherlands: a nationwide study from 1993 through 1997. J. Infect. Dis. 3:726-736. [DOI] [PubMed] [Google Scholar]
- 31.Veen, J. 1992. Microepidemics of tuberculosis: the stone-in-the-pond principle. Tuber. Lung. Dis. 73:73-76. [DOI] [PubMed] [Google Scholar]
- 32.Vynnycky, E., and P. E. Fine. 1997. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol. Infect. 119:183-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. 1992. The ICD-10 classification of mental and behavioral disorders. World Health Organization, Geneva, Switzerland.
- 34.World Health Organization. 2001. W. H. O. report 2001: global tuberculosis control. World Health Organization, Geneva, Switzerland.