Abstract
We compared 75 nontypeable (NT) Haemophilus influenzae isolates by pulsed-field gel electrophoresis (PFGE), enterobacterial repetitive intergenic consensus (ERIC)-PCR, and automated ribotyping. PFGE was the most discriminatory of the techniques. ERIC-PCR provides a useful screen but should not replace other techniques as the sole method to group NT H. influenzae strains.
In distinguishing strain similarities and differences among nontypeable (NT) Haemophilus influenzae isolates, phenotypic typing methods such as outer membrane protein analysis, biotyping, and lipooligosaccharide analysis are gradually being replaced by genotypic techniques (6, 8, 15). Pulsed-field gel electrophoresis (PFGE) analysis compares the patterns of genomic DNA digested with a rare cutting restriction enzyme and is considered to be the “gold standard” for typing NT H. influenzae isolates. Enterobacterial repetitive intergenic consensus (ERIC) sequences are conserved regions of DNA dispersed throughout the genomes of gram-negative, enteric bacteria (7). The distribution of ERIC sequences varies between strains, and ERIC-specific primers have been used to produce genetic fingerprints of bacterial genomes (14), including NT H. influenzae strains (13). An automated technique based on traditional ribotyping has also recently been used for the epidemiologic analysis of H. influenzae strains (16).
With the exception of total genome sequencing, genotypic methods are not definitive in identifying all possible strain differences. Rather, these methods group strains according to the presence of common restriction sites (PFGE and ribotyping) or the presence of PCR products of uniform size (ERIC-PCR). The choice of typing method depends on factors including time, cost, reproducibility, and the ability of a method to correctly distinguish between clonal and nonclonal isolates. In order to assess these typing techniques for studies of NT H. influenzae, we compared 75 strains by each of the three methods.
Bacterial strains used in this study included H. influenzae strain Rd (1), 44 middle ear isolates, 28 nasopharyngeal or throat isolates, and 2 Brazilian purpuric fever strains. Strains were collected from sites in Minnesota (3), Ann Arbor, Michigan (11), Battle Creek, Michigan (11), and Bardstown, Kentucky (5), between 1980 and 1999.
PFGE was performed on NT H. influenzae genomic DNA digested with SmaI (Gibco-BRL) as previously described (11). A 1-μl aliquot of crude genomic NT H. influenzae DNA was used for ERIC-PCR in a 50-μl reaction volume containing 25 pmol of ERIC1 primer (5"CACTTAGGGGTCCTCGAATGTA3"), 5mM MgCl2, 2 U of Platinum Taq polymerase (Gibco-BRL), and a 0.2 mM concentration of each deoxynucleoside triphosphate. PCR was initiated with a 2-min incubation at 94°C, followed by 35 cycles of 90°C for 30 s, 60°C for 1 min, and 72°C for 4.5 min. To check for reproducibility, four NT H. influenzae strains were typed using ERIC-PCR on three separate occasions. Ribotyping was performed using the RiboPrinter Microbial Characterization System from Qualicon (Wilmington, Del.), which is an automated typing system that produces a RiboPrint pattern using an Escherichia coli rRNA probe hybridized to restriction enzyme-digested chromosomal DNA. H. influenzae isolates were digested using EcoRI enzyme based on the manufacturer's instructions.
PFGE patterns were compared visually and evaluated using criteria developed by Tenover et al. (12). Briefly, isolates with differences in zero, three or fewer, four to six, or more than six bands were considered identical, related, possibly related, and unrelated, respectively. ERIC-PCR patterns were analyzed using BioNumerics software from Applied Maths (Kortrijk, Belgium). The Dice coefficient was used to create a similarity matrix based on the presence and absence of bands. A dendrogram was created using this matrix based on the unweighted pair-group method with arithmetic averages. Isolates with over 85% similarity were placed in the same ERIC group. Ribotype groups were defined by the RiboPrinter system, which compares the ribopattern of each isolate to others in the database and assigns groups by differences in band number, position, and signal intensity (9).
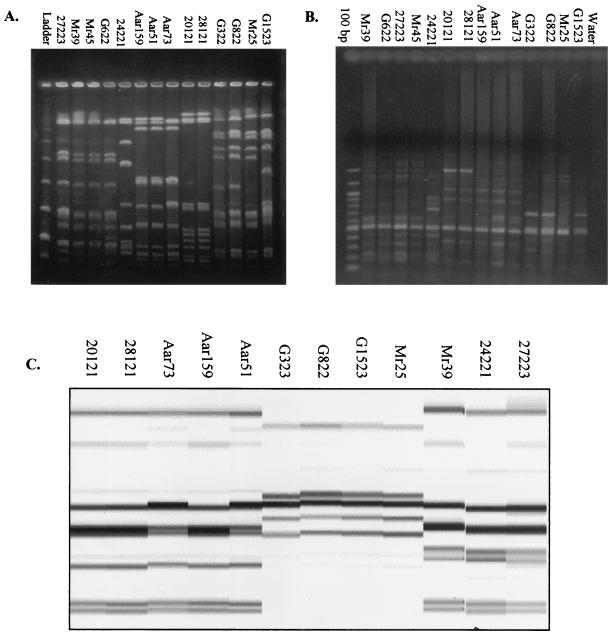
The distribution of the number of isolates within each typing group is shown in Table 1. PFGE produced the largest number of groups, 53. The ERIC-PCR patterns for the four NT H. influenzae isolates selected for reproducibility testing were consistent in each of the three experiments (data not shown). Agreement among the three typing methods is shown in Table 2. Examples of isolates typed by each method are shown in Fig. 1.
TABLE 1.
Number of groups possessing 1, 2 or 3, 4 or 5, and ≥6 NT H. influenzae isolates as determined by PFGE, ERIC-PCR, and AR
| Test used | No. of groups with:
|
Total no. of groups | |||
|---|---|---|---|---|---|
| ≥6 isolates | 4 or 5 isolates | 2 or 3 isolates | 1 isolate | ||
| PFGE | 0 | 3 | 12 | 38 | 53 |
| ERIC-PCR | 3 | 3 | 15 | 10 | 31 |
| Ribotyping | 0 | 5 | 11 | 29 | 45 |
TABLE 2.
Comparison of strain groupings by PFGE, AR, and ERIC-PCR
| Testing combination used | No. (%) of isolates founda
|
Total no. of isolates | ||
|---|---|---|---|---|
| In agreement | Different by A but same by B | Different by B but same by A | ||
| PFGE (A) and ERIC-PCR (B) | 41 (54.7) | 28 (37.3) | 6 (8.0) | 75 |
| AR (A) and ERIC-PCR (B) | 43 (57.3) | 17 (22.6) | 15 (20.0) | 75 |
| PFGE (A) and AR (B) | 68 (90.6) | 7 (9.3) | 0 | 75 |
A and B are different testing methods, as indicated in column 1. Isolates were found to be different when discrepant results were obtained and to be the same when consistent results were obtained by each method.
FIG. 1.
Selected H. influenzae isolate patterns by PFGE (A), ERIC-PCR (B), and AR (C).
Forty-one out of 75 (54.7%) isolates were placed into the same groups by each of the three methods. Three sets of two samples each contained NT H. influenzae isolates from different sites within the same individual and were identified as identical by each of the typing methods, indicating that each method was able to correctly place closely linked strains in the same group. Although we found a high level of diversity within NT H. influenzae strains, several unrelated strains were grouped together by all three methods.
Applications of bacterial strain typing systems include outbreak investigation, surveillance, and the identification of transmission patterns (2). Depending on the specific needs of the investigators, PFGE, ERIC-PCR and automated ribotyping (AR) can be used to successfully discriminate between strains of NT H. influenzae. As reported by Saito et al., we found that PFGE was very useful for discriminating between H. influenzae strains (10), but it is the most time consuming of the three methods (3 days to type an H. influenzae isolate by PFGE). AR takes 8 h per isolate and was in agreement with PFGE 91% of the time. In findings similar to ours, Wang et al. grouped H. influenzae isolates, albeit predominantly type b strains, into the same ribogroup, namely, those strains that were identified as unrelated by PFGE (16). The main drawback of the RiboPrinter system is that the reagents and instrument are very expensive. ERIC-PCR provided a quick (6 h to type an H. influenzae isolate) and inexpensive method to group NT H. influenzae strains, and thus, in certain types of studies, may provide a useful first screen to investigate diversity in NT H. influenzae populations. In our hands, ERIC-PCR was the least discriminating of the three techniques and sometimes produced small, unstable fragments (4).
In considering the relative value of a typing method for H. influenzae strains, subtle differences in the genome of strains may identify differences that are not epidemiologically significant. PFGE and AR both involve restriction endonuclease analysis, and thus, these patterns may be affected by the loss or gain of a single restriction site. As true ERIC sequences are not present in the fully sequenced H. influenzae strain Rd (1), it is likely that ERIC fingerprints reflect random hybridization of the primers. Direct sequencing of ERIC-PCR fragments generated in our laboratory indicates that H. influenzae ERIC-PCR fragments do not resemble the genuine ERIC sequences (data not shown). Gomez-De-Leon et al. have been able to correlate ERIC-PCR patterns with H. influenzae virulence (4). Conceivably, the ERIC primers may hybridize to relatively conserved regions that are linked to increased virulence. Markers identified by PFGE, AR, or ERIC-PCR may not have any direct biological meaning. Typing methods should therefore be used in conjunction with additional epidemiologic and/or molecular data to make meaningful inferences.
Acknowledgments
This work was supported by an award from the National Institute of Allergy and Infectious Diseases (RO1-AI25630 to J.G.) and the C. S. Mott Children's Hospital Fund for Research (J.G.).
REFERENCES
- 1.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, and J. M. Merrick. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 2.Foxman, B., and B. Riley. 2001. Molecular epidemiology: focus on infection. Am. J. Epidemiol. 153:1135-1141. [DOI] [PubMed] [Google Scholar]
- 3.Gilsdorf, J. R., H. Y. Chang, K. W. McCrea, and L. O. Bakaletz. 1992. Comparison of hemagglutinating pili of Haemophilus influenzae type b with similar structures of nontypeable H. influenzae. Infect. Immun. 60:374-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-De-Leon, P., J. I. Santos, J. Caballero, D. Gomez, L. E. Espinosa, I. Moreno, D. Piñero, and A. Cravioto. 2000. Genomic variability of Haemophilus influenzae isolated from Mexican children determined by using enterobacterial repetitive intergenic consensus sequences and PCR. J. Clin. Microbiol. 38:2504-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krasan, G. P., D. Cutter, S. L. Block, and J. W. St. Geme III. 1999. Adhesin expression in matched nasopharyngeal and middle ear isolates of nontypeable Haemophilus influenzae from children with acute otitis media. Infect. Immun. 67:449-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long, S. S., M. J. Teter, and P. H. Gilligan. 1983. Biotype of Haemophilus influenzae: correlation with virulence and ampicillin resistance. J. Infect. Dis. 147:800-806. [DOI] [PubMed] [Google Scholar]
- 7.Lupski, J. R., and G. M. Weinstock. 1992. Short, interspersed repetitive DNA sequences in prokaryotic genomes. J. Bacteriol. 174:4525-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy, T. F., J. M. Bernstein, D. M. Dryja, A. A. Campagnari, and M. A. Apicella. 1987. Outer membrane protein and lipooligosaccharide analysis of paired nasopharyngeal and middle ear isolates in otitis media due to nontypable Haemophilus influenzae: pathogenic and epidemiological observations. J. Infect. Dis. 156:723-731. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller, M. A., C. Wendt, R. J. Hollis, R. P. Wenzel, S. J. Fritschel, J. J. Neubauer, and L. A. Herwaldt. 1996. Comparative evaluation of an automated ribotyping system versus pulsed-field gel electrophoresis for epidemiological typing of clinical isolates of Escherichia coli and Pseudomonas aeruginosa from patients with gram-negative bacteremia. Diagn. Microbiol. Infect. Dis. 25:1-8. [DOI] [PubMed] [Google Scholar]
- 10.Saito, M., A. Umeda, and S.-I. Yoshida. 1999. Subtyping of Haemophilus influenzae strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 37:2142-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St. Sauver, J., C. F. Marrs, B. Foxman, P. Somsel, R. Madera, and J. R. Gilsdorf. 2000. Risk factors for otitis media and carriage of multiple strains of Haemophilus influenzae and Streptococcus pneumoniae. Emerg. Infect. Dis. 6:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Belkum, A., B. Duim, A. Regelink, L. Moller, W. Quint, and L. van Alphen. 1994. Genomic DNA fingerprinting of clinical Haemophilus influenzae isolates by polymerase chain reaction amplification: comparison with major outer-membrane protein and restriction fragment length polymorphism analysis. J. Med. Microbiol. 41:63-68. [DOI] [PubMed] [Google Scholar]
- 14.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villasenor-Sierra, A., and J. I. Santos. 1999. Outer membrane protein profiles of paired nasopharyngeal and middle ear isolates of nontypable Haemophilus influenzae from Mexican children with acute otitis media. Clin. Infect. Dis. 28:267-273. [DOI] [PubMed] [Google Scholar]
- 16.Wang, C.-C., L. K. Siu, M.-K. Chen, Y. L. Yu, F. M. Lin, M. Ho, and M.-L. Chu. 2001. Use of automated riboprinter and pulsed-field gel electrophoresis for epidemiologic studies of invasive Haemophilus influenzae in Taiwan. J. Med. Microbiol. 50:277-283. [DOI] [PubMed] [Google Scholar]