Abstract
Three homeologous genes encoding a sucrose (Suc) transporter (SUT) in hexaploid wheat (Triticum aestivum), TaSUT1A, 1B, and 1D, were expressed in germinating seeds, where their function is unknown. All three TaSUT1 proteins were confirmed to be capable of transporting both Suc and maltose by complementation tests with the SUSY7/ura3 yeast (Saccharomyces cerevisiae) mutant strain. The role of Suc transporters in germinating grain was examined by combining in situ hybridization, immunolocalization, fluorescent dye tracer movement, and metabolite assays. TaSUT1 transcript and SUT protein were detected in cells of the aleurone layer, scutellar epidermis, scutellar ground cells, and sieve element-companion cell complexes located in the scutellum, shoot, and root. Ester loading of the membrane-impermeable fluorescent dye carboxyfluorescein into the scutellum epidermal cells of germinating seeds showed that a symplasmic pathway connects the scutellum to the shoot and root via the phloem. However, the scutellar epidermis provides an apoplasmic barrier to solute movement from endosperm tissue. Measurements of sugars in the root, shoot, endosperm, and scutellum suggest that, following degradation of endosperm starch, the resulting hexoses are converted to Suc in the scutellum. Suc was found to be the major sugar present in the endosperm early in germination, whereas maltose and glucose predominate during the later stage. It is proposed that loading the scutellar phloem in germinating wheat seeds can proceed by symplasmic and apoplasmic pathways, the latter facilitated by SUT activity. In addition, SUTs may function to transport Suc into the scutellum from the endosperm early in germination and later transport maltose.
High fluxes of nutrients occur in seeds when storage products are accumulated or remobilized during development and germination, respectively. Nutrient movement to and from seed storage sites involves transport between symplasmically isolated compartments and hence transport across plasma membranes of neighboring cells. For developing seeds, Suc uptake into filial tissues (cotyledons or endosperm) is mediated by plasma membrane Suc/H+ symporters (Suc transporter [SUT]) localized to their outer cell layers in both monocots (Bagnall et al., 2000; Weschke et al., 2000; Furbank et al., 2001) and dicots (Harrington et al., 1997; Weber et al., 1997; Tegeder et al., 1999; Rosche et al., 2002). For germinating seeds, SUT transcripts have been detected in phloem tissues of castor bean (Ricinus communis; Bick et al., 1998) and rice (Oryza sativa; Matsukura et al., 2000). The role of non-phloem tissues in Suc transport during seed germination is less certain. In germinating castor bean seeds, epidermal transfer cells of cotyledons abutting the endosperm were found to contain abundant SUT mRNAs (Bick et al., 1998). However, the function of these SUTs in sugar retrieval from the endosperm is unclear because starch hydrolysis products are generally known to be Glc and maltose.
In cereal species, rice OsSUT1 (Hirose et al., 1997; Matsukura et al., 2000) and maize (Zea mays) ZmSUT1 (Aoki et al., 1999) are highly expressed in germinating seeds. For rice, expression of OsSUT1 dominates compared with the other four SUT isogenes (Aoki et al., 2003), and antisense OsSUT1 suppression lines exhibit retarded germination (Scofield et al., 2002). These results strongly suggest that expression of the SUT1 ortholog is very important for seed germination in cereal species. However, cellular localization of SUT1 expression in germinating cereal seeds has not been investigated fully. To our knowledge, the only report (Matsukura et al., 2000) is for germinating rice seeds where OsSUT1 mRNA was found to be present in the scutellum phloem.
We previously reported that three closely related SUT genes are expressed in a variety of tissues of hexaploid wheat (Triticum aestivum). These SUTs may play important roles in postphloem transport of Suc in developing seeds (Bagnall et al., 2000; Aoki et al., 2002) and in long-distance transport of Suc through phloem sieve elements of leaves and stems (Aoki et al., 2004). The three genes, TaSUT1A, 1B, and 1D are single-copy homeologous genes, represented in each of the A, B, and D subgenomes of hexaploid wheat, respectively, and the only SUT genes from wheat that have been characterized to date (Aoki et al., 2002). Their cDNA sequences show 96% identity, but are distinguishable from one another by a size polymorphism in the 3′-untranslated region. Their deduced peptide sequences show 98% identity to one another, all with more than 85% similarity to SUT1 proteins from other cereal species. The cereal SUT1 proteins form a cluster in a phylogenetic tree of plant SUT proteins (Aoki et al., 2003).
Here we report expression and localization analyses of TaSUT1, an analysis of the symplasmic transport domains and sugar composition of tissues of germinating wheat seeds. The possible roles of SUT proteins in sugar transport during wheat seed germination are discussed.
RESULTS
Anatomy of Wheat Seeds and Seedlings
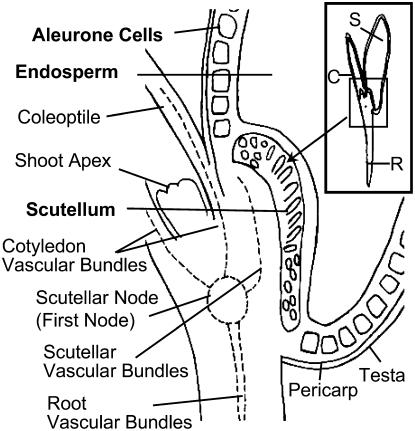
The anatomy of a germinating wheat seed is illustrated in Figure 1. Single-cell layers composed of testa and pericarp form a seed coat that encapsulates the remaining seed tissues. Inward of the seed coat, a single layer of aleurone cells surrounds the starchy endosperm, except for a region adjacent to the embryo. In this position, scutellum cells replace those of aleurone. Scutellum tissues, located between the endosperm and embryonic axis, are delimited from the endosperm by columnar-shaped epidermal cells containing prominent nuclei (compare with Fig. 7C). Two vascular bundles, oriented parallel to the plane of the epidermal surface, are embedded in the ground tissues of the scutellum. The scutellum vascular bundles, fully differentiated at 3 d after imbibition (DAI), fuse with the vascular system of the germinating seedling at the scutellar node that is located immediately below the shoot apex. The shoot apex is enshrouded by the coleoptile serviced by two vascular bundles linked to the scutellar node (the first node). A vascular stele also arises from the scutellar node and extends longitudinally through the radicle.
Figure 1.
Diagram of a germinating wheat seedling at 3 DAI in longitudinal section, illustrating the seedling anatomy and that of associated seed tissues. S, Seed; C, coleoptile; R, radicle.
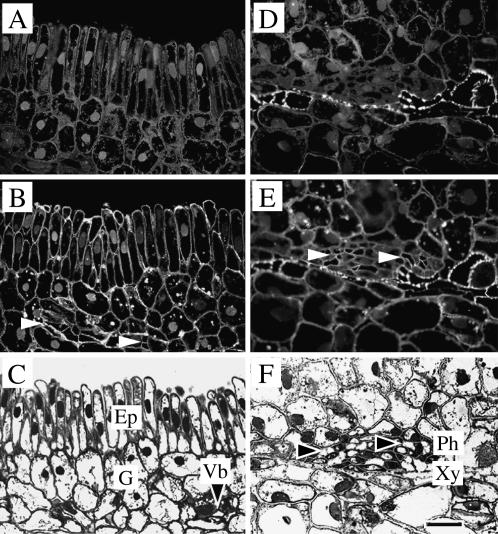
Figure 7.
Cellular localization of TaSUT1 proteins in the scutellum tissue of germinating wheat seeds. Photomicrographs of longitudinal sections through the scutellum region of germinating wheat seeds at 7 DAI showing the ground tissues (A–C) and vascular bundles (D–F). A and D, Immunofluorescent images of scutellar ground tissues (A) and vascular bundles (D) treated with preimmune serum and secondary antibody. Note strong autofluorescence of xylem elements (D). B and E, Immunofluorescent images of scutellar ground tissues (B) and vascular bundles treated with the SUT1 antibody, detected as a fluorescent signal emitted by FITC-conjugated anti-rabbit secondary antibody. Note clear immunofluorescence restricted to the plasma membranes of epidermal cells (B) and sieve elements (B and E, white arrowheads) with more scattered occurrence in the plasma membranes of the ground cells of the scutellum. C and F, Bright-field micrographs of scutellum sections stained with toluidine blue, illustrating the structure of the epidermal and ground (C) and vascular (F) cells. Note sieve elements (black arrowheads) of the scutellum vascular bundle. Ep, Epidermal cells; G, ground cells; Ph, phloem; Vb, vascular bundle; Xy, xylem. Scale bar = 25 μm.
By 3 DAI, the coleoptile has ceased elongation growth and its two vascular bundles are fully differentiated. By 7 DAI, the first leaf is fully expanded. Radicles of 3-DAI seedlings were 2 to 4 cm in length. A typical time course of shoot growth under the growth condition used in this study is shown in Figure 2A.
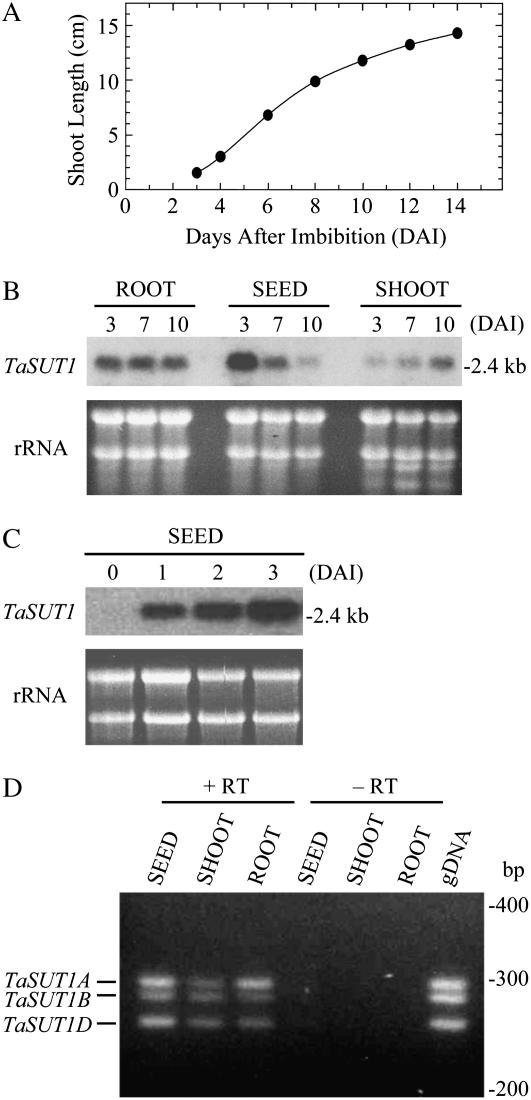
Figure 2.
TaSUT1 expression in wheat seedling tissues. A, Shoot growth. Each value represents the average of 20 seeds. Error bars are included within the black circles. B and C, Northern-blot analysis for TaSUT1 in roots, seeds, and shoots of wheat seedlings harvested at 3, 7, and 10 DAI (B) and in seeds harvested before imbibition and at 1, 2, and 3 DAI (C). Ethidium bromide-stained agarose gel used for the RNA blot is shown below for the standardization of RNA loading (rRNA). D, Ethidium bromide-stained agarose gel of polymorphic RT-PCR for the TaSUT1 gene family. Total RNA was isolated from seeds, shoots, and roots of hexaploid wheat seedlings harvested at 3, 10, and 3 DAI, respectively, and reverse transcribed to single-strand cDNA. Single-strand cDNA (equivalent to 1 ng μL−1 total RNA; +RT), corresponding total RNA (10 ng μL−1; −RT) or hexaploid wheat genomic DNA (10 ng μL−1; gDNA), was used as template in each PCR with 30 cycles. No PCR products were found when total RNA was used, indicating no contamination of genomic DNA (−RT). When genomic DNA from hexaploid wheat was used as template, three DNA fragments were equally amplified at expected sizes, demonstrating the validity of optimization to provide quantitative amplification because the hexaploid DNA is considered to contain equal amounts of the three TaSUT1 templates. Reproducible results were obtained from three independent experiments.
Accumulation Patterns of TaSUT1 mRNAs in Wheat Seedlings
Expression of TaSUT1 was examined by northern-blot analysis using a TaSUT1-specific probe that hybridizes with all three homeologs, TaSUT1A, 1B, and 1D. TaSUT1 mRNAs were found in roots, seeds, and shoots of germinating wheat seedlings (Fig. 2B). In roots, the amount of TaSUT1 mRNAs was constant at 3, 7, and 10 DAI, whereas in shoots it gradually increased over the time course. In seeds, TaSUT1 mRNAs were absent from dry seeds but, on imbibition, accumulated rapidly to 3 DAI (Fig. 2C). Thereafter, transcript levels declined substantially and were barely detectable by 10 DAI (Fig. 2B).
To investigate whether the three homeologous TaSUT1 genes are differentially expressed, a semiquantitative reverse transcription (RT)-PCR system for the TaSUT1 gene family was established using a primer combination that spans a polymorphic region in TaSUT1 sequences (Aoki et al., 2002, 2004). Using this diagnostic RT-PCR system, transcript abundance of the three TaSUT1 genes was compared. A typical gel separation of the RT-PCR products is shown in Figure 2D. The three TaSUT1 mRNAs were detected in all seedling tissues tested. However, in 3-DAI roots, TaSUT1A transcript levels were approximately 3 times higher than the other two homeologous transcripts. In 3-DAI seeds, TaSUT1B transcript levels were marginally lower than the other two transcripts, whereas in 10-DAI shoots, the three homeologous genes were expressed equally.
Functional Expression of TaSUT1 in Yeast Cells
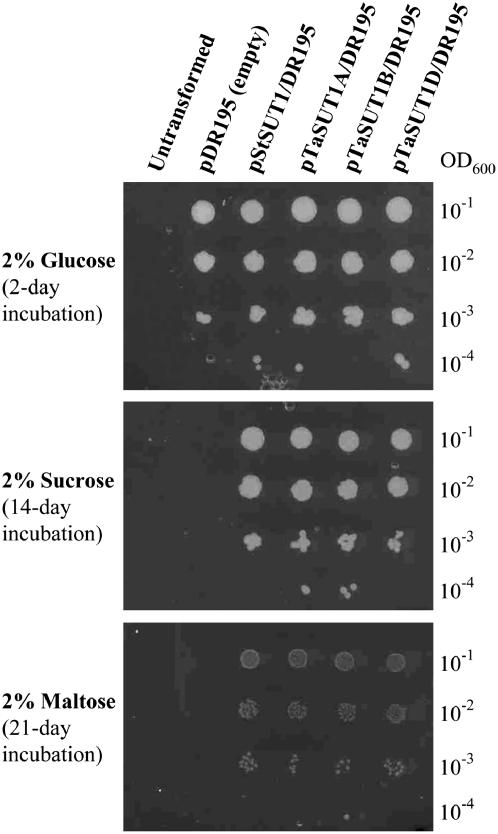
Sugar transport by TaSUT1A, 1B, and 1D proteins was examined by complementation of the SUSY7/ura3 yeast (Saccharomyces cerevisiae) strain, using selective media containing Glc, Suc, or maltose as the sole carbon source. This yeast strain lacks the ability to utilize external Suc and maltose (Riesmeier et al., 1992). The StSUT1-pDR195 construct (Weise et al., 2000) was used as a positive control for the experimental system. Figure 3 shows a representative result. When the pDR195 empty vector was introduced into this strain, transformed cells grew well in the Glc media, but not at all in the Suc or maltose media. Transformed lines harboring StSUT1, TaSUT1A, 1B, and 1D all grew on either Suc or maltose media. However, the growth rates were much slower when maltose was used as the sole carbon source.
Figure 3.
Complementation of the SUSY7/ura3 yeast strain with TaSUT1A, 1B, and 1D. Growth of SUSY7/ura3 lines containing different constructs was examined on agar plates containing 2% Glc, 2% Suc, or 2% maltose as the sole carbon source. Untransformed SUSY7/ura3 cells did not grow at all as uracil was absent in the media. Ten microliters of cell suspension with different cell densities were spotted onto the agar plates and incubated at 30°C.
Cellular Localization of TaSUT1 mRNAs in Germinating Seed Tissues
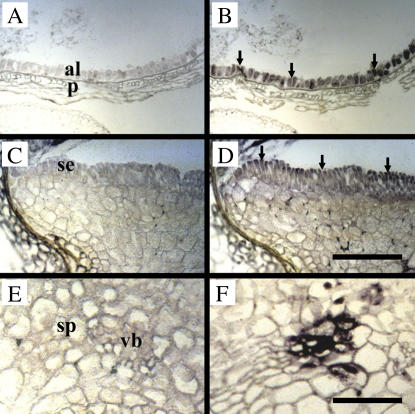
In situ hybridization was carried out to examine cellular localization of TaSUT1 mRNAs in 3-DAI seeds using riboprobes prepared from a full-length TaSUT1B cDNA, which will detect all three homeologous transcripts and thus maximize sensitivity of detection (Fig. 4). A strong signal for TaSUT1 mRNAs was observed in aleurone cells, scutellum epidermal cells, and scutellum vascular cells (Fig. 4, B, D, and F, arrows). Although the signal intensity was weaker, ground cells of the scutellum were also found to contain TaSUT1 mRNAs (Fig. 4D).
Figure 4.
Cellular localization of TaSUT1 mRNAs in germinating wheat seeds. Photomicrographs of longitudinal sections of 3-DAI germinating wheat seeds treated with sense (A, C, and E) and antisense (B, D, and F) TaSUT1 RNA probes. Probe binding was visualized using an alkaline phosphatase reaction yielding a blue-purple product. A and B, Longitudinal sections through the aleurone and pericarp tissues. The TaSUT1 transcript signal is localized to the aleurone cells (arrows). C and D, Longitudinal sections through the scutellum region. The TaSUT1 transcript signal (arrows) is localized to the columnar-shaped epidermal cells and the ground cells of the scutellum. E and F, Longitudinal sections through a portion of the scutellar vascular bundle embedded in ground parenchyma. Note the TaSUT1 transcript signal localized to vascular cells that are probably companion cells. al, Aleurone; p, pericarp; se, scutellum epidermis; sp, scutellum parenchyma; vb, scutellum vascular bundle. Scale bars = 200 μm (A–D) or 100 μm (E and F).
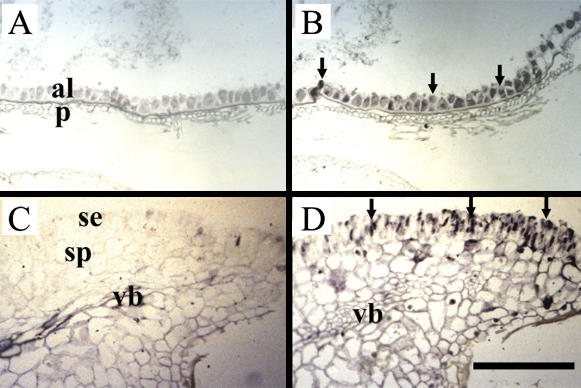
Similar histological sections from 3-DAI seeds were hybridized with riboprobes from a hexose transporter sequence (Fig. 5). Similar to the expression pattern found for TaSUT1 mRNAs, a strong signal for hexose transporter mRNAs was found in aleurone cells and scutellar epidermis and ground cells (Fig. 5, B and D, arrows). However, no signal was detected in the scutellum vascular bundle (Fig. 5D).
Figure 5.
Cellular localization of hexose transporter mRNAs in germinating wheat seeds. Photomicrographs of longitudinal sections of 3-DAI paraffin-embedded germinating wheat seeds treated with sense (A and C) and antisense (B and D) probes from a highly conserved hexose transporter sequence. Probe binding was visualized using an alkaline phosphatase reaction yielding a blue-purple product. A and B, Longitudinal sections through the aleurone and pericarp tissues. Note the signal of the hexose transporter transcript (arrows) is localized to the aleurone cells (arrows). C and D, Longitudinal sections through the scutellum region showing the hexose transcript signal (arrows) localized to the columnar-shaped epidermal cells and the ground cells of the scutellum. Note the absence of signal from the scutellum vascular bundle. al, Aleurone; p, pericarp; se, scutellum epidermis; sp, scutellum parenchyma; vb, scutellum vascular bundle. Scale bar = 200 μm.
Cellular Localization of SUT Proteins in Germinating Seed Tissues
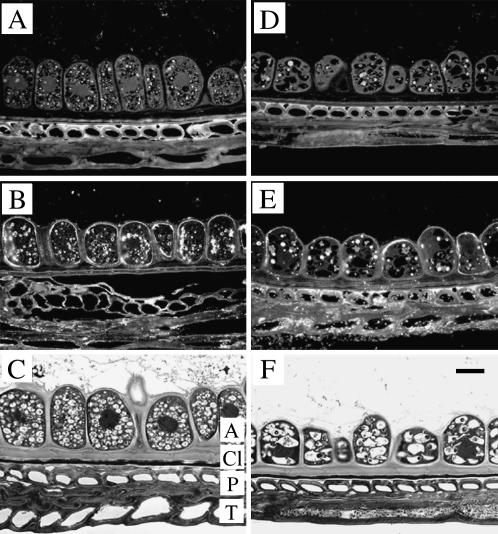
Consistent with a secretory function during germination (see Bewley and Black, 1994), aleurone cells at 3 DAI contained high densities of vesicles and large nuclei (Fig. 6C). By 7 DAI, the aleurone cells exhibited signs of degradation characterized by increased vacuolation and loss of vesicles (Fig. 6F). Plasma membranes of aleurone cells exhibited pronounced immunofluorescent labeling when exposed to the SUT antibody (Fig. 6B).
Figure 6.
Cellular localization of TaSUT1 proteins in the aleurone layer of germinating wheat seeds. A and D, Immunofluorescent images of aleurone sections from 3-DAI (A) and 7-DAI (D) seeds treated with preimmune serum and secondary antibody. Note the strong autofluorescence of protein bodies in the aleurone cells and walls of the sclerenchyma cells located in the pericarp. B and E, Immunofluorescent images of aleurone sections from 3-DAI (B) and 7-DAI (E) seeds treated with the SUT1 antibody, detected as a fluorescent signal emitted by FITC-conjugated anti-rabbit secondary antibody. Note that immunofluorescence is restricted to the plasma membranes of aleurone cells. C and F, Bright-field micrographs stained with toluidine blue illustrating the degradation of aleurone cell structure, including characteristic changes in vacuole composition. A, Aleurone; Cl, cuticle layer; P, pericarp; T, testa. Scale bar = 25 μm.
Plasma membranes of both epidermal and ground cells of the scutellum were also labeled with the SUT1 antibody (Fig. 7B). The scutellum epidermal cells exhibited the greater labeling intensity, particularly so on their outer edges, whereas labeling in the ground cells was scattered (Fig. 7B). Sieve elements, but not vascular parenchyma cells, of the scutellum vascular bundles were found to contain SUT1 epitopes located in their plasma membranes at 3 DAI (Fig. 7, B and E, arrowheads).
Symplasmic Continuity between Cells in the Scutellum
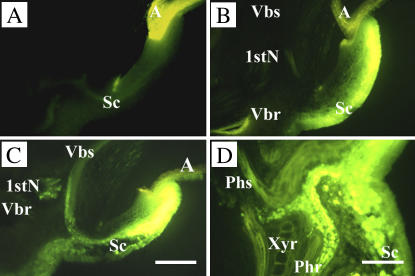
To examine symplasmic connections between epidermal cells and other cells in the scutellum, carboxyfluorescein (CF) diacetate (CFDA) was pulse loaded from the surface of scutellum epidermal cells of 3-DAI seeds, and movement of CF, the membrane-impermeable fluorochrome, was chased thereafter (Fig. 8). After 3-min loading, CF signal was principally confined to the scutellum epidermal cells and one to two layers of underlying scutellum (Fig. 8B). Following a 4-h chase, the loaded CF migrated from the outer cell layers of the scutellum to the scutellum vascular bundle and moved in the phloem through the first node to vascular traces entering the shoot and root (Fig. 8C). At higher magnifications (Fig. 8D), the CF located in the vascular bundles was found to be restricted to the phloem directed to the shoot and root.
Figure 8.
Symplasmic movement of CF in the embryo of 3-DAI wheat seedlings. The surface of the scutellum epidermis was exposed to CFDA solution for 3 min, washed exhaustively, and then examined by fluorescent microscopy. A, Autofluorescence from scutellum and aleurone, detected with the same microscopic setting as used in B to D. B, Distribution of CF in embryonic tissues after 3-min exposure and washout. C and D, Distribution of CF after chasing for 4 h. A, Aleurone; 1stN, first node; Phr and Phs, phloem directed to root and shoot; Sc, scutellum; Vbr and Vbs, vascular trace entering root and shoot; Xyr, xylem directed to root. Scale bar = 50 μm.
Sugar Composition of Germinating Seedlings
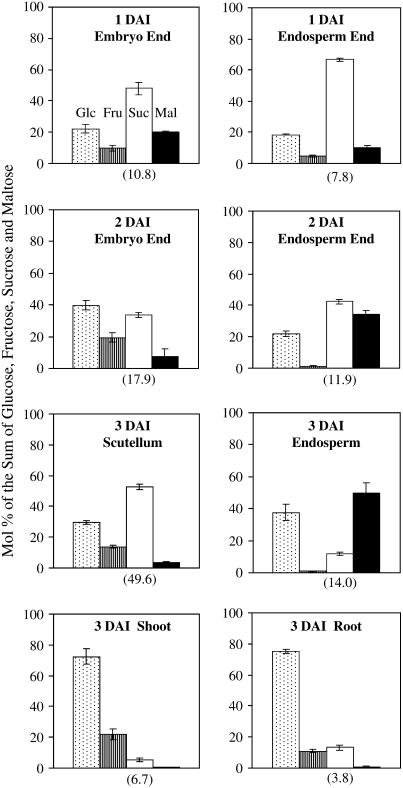
To ascertain the major sugar fluxes occurring during germination, the sugar composition was analyzed in different tissues dissected from 1-, 2-, and 3-DAI wheat seedlings (Fig. 9). In embryo-rich or scutellar tissues, Suc and Glc were the major sugar species. The concentration of Suc, on a fresh-weight basis, was much higher in the 3-DAI scutellum than in the other tissues examined. In the endosperm, Suc comprised a major portion of total sugars at 1 DAI. However, by 3 DAI, the major sugars present in the endosperm became Glc and maltose, products of starch degradation. Glc was found to be the dominant sugar in rapidly elongating shoots and roots.
Figure 9.
Changes in sugar composition in wheat seedlings during early germination. Data are represented as mol % of the sum of Glc, Fru, Suc, and maltose (Mal); the means and sds calculated from three biological replicates, each of which contains six seedlings. The mean values of Suc concentration (μmol g−1 fresh weight) in the tissues are shown in parentheses below the Suc columns.
DISCUSSION
In germinating cereal seeds, GA3, released from the developing embryos, induces synthesis of hydrolytic enzymes in scutellum and aleurone cells and their subsequent secretion into the starchy endosperm. Secreted amylases catalyze the hydrolysis of endospermic starch, stored as amylose and amylopectin, to Glc and maltose (Bewley and Black, 1994). These sugars, along with other hydrolysis products, support the heterotrophic growth of the germinating seedling. The role of sugar transporters in the germination process is uncertain and, in this context, cellular localization of Suc symporter proteins provides some useful insights.
Localization of hexose transporter mRNAs in the scutellar epidermis (Fig. 5) supports the widely held view that Glc produced from starch degradation in the endosperm is taken up by the scutellum, where it is converted to Suc for further translocation (Edelman et al., 1959; Bewley and Black, 1994). For scutella isolated from germinating maize seeds, influx of hexoses appears to occur by passive diffusion (compare with Matsukura et al., 2000; Sherson et al., 2000). In contrast, Suc uptake by isolated maize scutella exhibits behavior consistent with Suc/H+ symport (Humphreys, 1985).
An unexpected result from this study was the discovery that plasma membranes of aleurone and scutellar epidermal cells contained high densities of SUTs (Figs. 4, 6, and 7). Expression of these genes occurs coincident with the onset of imbibition and persists until these tissues begin to degrade (Fig. 2). Early in development, Suc stored in the aleurone layer, and also produced from oil catabolism, is believed to be actively secreted into the endosperm and taken up by the embryo as an early energy source before starch breakdown becomes the dominant source of carbohydrate (see Chrispeels et al., 1973, and refs. therein). Consistent with this early work, Suc is found to be the dominant sugar in the endosperm at 1 DAI (Fig. 9). Because the scutellar epidermis appears to present an apoplasmic barrier for solute movement from the endosperm to the embryo (Fig. 8), it is likely that SUTs expressed in the scutellar epidermis function to move this Suc into the embryo during this period. This is also consistent with TaSUT1 transcript abundance in the seed early in the germination process (Fig. 2).
Once starch degradation is proceeding rapidly, Glc and maltose become the major sugars in the endosperm (e.g. 3 DAI in Fig. 9). Suc symporters located in the epidermis and ground tissues of the scutellum may also function to retrieve maltose from the endosperm. SUT proteins in several dicot species (Lemoine, 2000; Chandran et al., 2003) and barley (Hordeum vulgare; Sivitz et al., 2005) have been shown to be capable of maltose transport. Similarly, we found that TaSUT1 proteins can transport maltose with lower affinities compared to Suc (Fig. 3). The membrane-transported maltose, together with Glc, is rapidly converted to Suc, which accumulates in the scutellar tissues (Fig. 9). This is consistent with the early metabolite measurements and localization of Suc phosphate synthase made by Edelman et al. (1959). Suc, synthesized in the scutellum, could be loaded into the scutellar sieve elements by Suc symporters (Figs. 4 and 7) following Suc release to the scutellar apoplasm (Humphreys, 1985), or move into the sieve elements symplasmically. The fluorescent dye tracer experiments shown here (Fig. 8) indicate that the entire shoot and root is symplasmically connected with the scutellum, favoring the second option, with SUTs acting to retrieve Suc lost from sieve elements to the phloem apoplasm. The absence of Suc symporters in the surrounding vascular parenchyma (Figs. 4 and 7) allows for the possibility that Suc is loaded directly into the scutellar sieve elements from the phloem apoplasm, a finding that may also apply to phloem loading in wheat leaves (Aoki et al., 2004). A similar distribution of Suc symporter protein has been reported for cotyledons of germinating castor bean seeds (Bick et al., 1998). However, the requirement for Suc synthesis in the scutellum of germinating wheat seeds dictates that sugars first must be taken up by scutellum ground tissues. This situation contrasts with the operation of parallel apo- and symplasmic pathways for phloem loading in germinating castor bean seeds in which Suc is supplied directly from the endosperm (Orlich et al., 1998).
During the heterotrophic phase of germination, seedling growth depends entirely on reserves mobilized from the endosperm and transported to the developing shoot and root systems in the seedling vasculature. Vascular differentiation appears to proceed rapidly in the scutellum prior to 3 DAI. SUT proteins are localized to sieve elements of the coleoptile vascular bundles where the membrane protein presumably functions to load and retrieve Suc in transit to and, when the organ reaches photosynthetic independence, from the coleoptile (X.-D. Wang, J.W. Patrick, and C.E. Offler, unpublished data). A similar cellular distribution of symporters was observed in the three size classes of vascular bundles located in the fully expanded lamina of leaves (Aoki et al., 2004). Because OsSUT1 transcript in vascular bundles of rice leaves is restricted to companion cells (Matsukura et al., 2000), localization of SUT protein to the sieve elements suggests that the transcript or protein must be trafficked from companion cells to adjacent sieve elements (Kühn et al., 1997).
Detection of TaSUT1 in the aleurone layer presents an interesting paradox. Aleurone tissue is highly active at 3 DAI (compare with Fig. 6C) and energy requiring, so these symporters could function in recovering Suc or maltose (as in the scutellum) from the endosperm to support metabolism. Sugar flows into aleurone cells could be amplified by hexose transporters retrieving Glc from the endosperm (Fig. 5; Matsukura et al., 2000; Sherson et al., 2000). However, as described above, aleurone is a net exporter of Suc during germination and Suc levels stored by the time of desiccation can reach concentrations of more than 4% dry weight (see Chrispeels et al., 1973, and refs. therein). TaSUT1 is a plasma membrane Suc/H+ symporter, the energetics of which favor uptake into the cell (Bush, 1993; Lalonde et al., 2004). It is unlikely that SUT proteins are present in the aleurone layer predesiccation because, although transcript was detected in the aleurone layer throughout seed filling (Bagnall et al., 2000), protein was only detectable in aleurone and subaleurone tissue adjacent to the endosperm cavity. It seems likely that expression of SUTs in aleurone during germination is yet another example of this class of transporter being present in tissues exporting sugars, such as wheat nucellus (Bagnall et al., 2000) and maternal seed coats of legumes (Harrington et al., 1997). The possibility of SUT proteins acting as Suc effluxers has been raised a number of times (M'Batchi and Delrot, 1988; Bagnall et al., 2000; Meyer et al., 2000; Borstlap and Schuurmans, 2004; Carpaneto et al., 2005) and seems the only explanation in this case of cereal aleurone.
Whereas TaSUT1 is the only SUT to be cloned and characterized from wheat to date, it is likely that a gene family of at least five members is present by analogy to the SUT sequences present in the rice genome (Aoki et al., 2003). Cloning and localization of expression of the three homeologous transcripts from each of the five members of the gene family is a daunting and technically challenging task. In this study, for reasons of sensitivity, we have chosen to use an in situ hybridization probe that will detect all three homeologs of TaSUT1 and, consequently, may not be specific for this class of SUT. However, in a previous study (Aoki et al., 2002), we showed that the same probe detected only the three TaSUT1 homeologs in genomic Southern blots from hexaploid wheat and a single gene in the proposed progenitor species of the three genomes. This evidence suggests that the probes are likely specific to the TaSUT1 transcript.
The issue of unequivocal identification of the TaSUT1 protein in the localization work reported here is also worthy of discussion. The antibody used here for immunolocalization has previously been shown to recognize a 60-kD polypeptide from microsomal preparations of wheat and rice tissues (Furbank et al., 2001; Aoki et al., 2004). Because the deduced sizes of the mature SUT polypeptides do not differ substantially in rice (Aoki et al., 2003) and the entire SUT gene family has not been cloned in wheat, it cannot be determined whether this antibody is specific for TaSUT1. However, it is clear that this antibody strongly labeled SUT proteins in several tissues of germinating wheat seeds, corresponding to the regions labeled by the in situ probes. In a variety of wheat vegetative tissues, this antibody also labeled sieve elements of vascular bundles (Aoki et al., 2004) adjacent to companion cells where SUT1 transcript was detected.
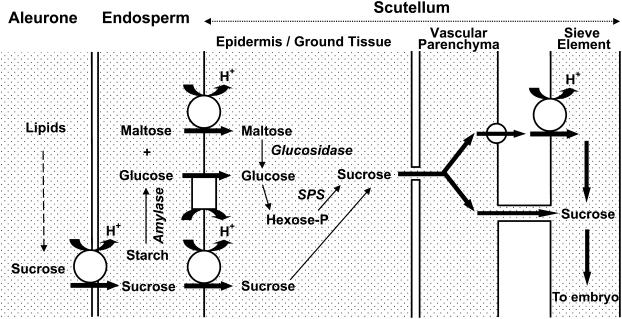
In conclusion, we propose the following speculative model for the cellular route of carbohydrate transport from the endosperm to scutellar sieve elements in germinating wheat seeds (Fig. 10). Early in germination, before starch degradation is the dominant source of carbohydrate, Suc moves out of the aleurone cells into the endosperm to be actively accumulated by the scutellum via TaSUT1. Once starch hydrolysis is fully under way in the endosperm, maltose and Glc become the dominant sugars in this tissue (see Fig. 9). The TaSUT1 and hexose transporters function to load maltose and Glc, respectively, into the scutellum tissues (mainly in the epidermal cells). In the scutellum cells, maltose is hydrolyzed by an α glucosidase and, together with Glc, converted to Suc via the hexose phosphate pool and Suc phosphate synthase. The synthesized Suc is transported symplasmically to the scutellar phloem where loading into the sieve elements may follow either apo- or symplasmic pathways operating in parallel. The apoplasmic pathway would also necessitate Suc release to the apoplasm from scutellar ground cells, presumably by a hitherto unidentified Suc effluxer.
Figure 10.
Schematic model of the cellular route of sugar transport from the endosperm to the sieve element in germinating wheat seeds, highlighting the potential location of sugar transporters and symplasmic transport. TaSUT1 is represented by a larger circle, Glc transporters by a rectangle, and a putative Suc effluxer by a smaller circle. SPS, Suc phosphate synthase; hexose-P, hexose phosphate.
MATERIALS AND METHODS
Plant Materials
Seeds of hexaploid wheat (Triticum aestivum L. cv Hartog) were soaked in sodium hypochlorite for 5 min, washed several times with water, imbibed in water for 2 h, sown on moist filter paper pads in petri dishes, and incubated at 26°C in the dark.
Northern Hybridization and RT-PCR
For RNA extraction, seedlings were harvested at designated time points, separated into roots, seeds, and shoots, and frozen in liquid nitrogen and stored at −80°C until use. Northern-blot analysis was carried out as described in Aoki et al. (2002). A 380-bp 3′-flanking sequence of TaSUT1B (GenBank accession no. AF408843), equivalent to 1,647 to 2,021 bp, was used as a probe. To distinguish the presence of three TaSUT1 transcripts, RT-PCR was carried out under semiquantitative conditions, as described in Aoki et al. (2004). A primer combination was designed to span the polymorphic region of the 3′-untranslated region of the TaSUT1 gene family; forward primer, 5′-AACATCGCCATCGTGATACC-3′ and reverse primer, 5′-CGCCTATGCGAAAATTTGG-3′. The primer combination produces DNA fragments of 294, 280, and 255 bp for TaSUT1A, 1B, and 1D, respectively.
In Situ Hybridization
Seedlings were harvested at 3 DAI. Tissue segments of the germinating seeds were fixed and embedded in paraffin Paraplast, as described in Aoki et al. (2004). Digoxigenin (DIG)-labeled sense and antisense riboprobes were synthesized by in vitro transcription, according to the manufacturer's instructions (DIG RNA labeling kit; Boehringer), from the entire TaSUT1B cDNA in Bluescript SK− (Strategene) or from a highly conserved sequence present in all known hexose transporters (from 140–541 bp of LeHT3; GenBank accession no. AJ132225). The sense and antisense probes were chemically hydrolyzed to the required length of about 150 to 200 bp.
Histological sections, 7-μm thick, were pretreated, hybridized, and washed as described in Aoki et al. (2004). Riboprobe hybridization was detected with DIG antibody conjugated with alkaline phosphatase.
Immunolabeling Experiments
Three uniform replicate seedlings were harvested at 3 and 7 DAI. The roots and shoots of the seedlings were surgically removed from the seed tissues. The seeds were cut transversely in half and halves containing the remainder of the germinating seedling were collected for histological processing.
Tissue samples were fixed and embedded into LR White (ProSciTech), as described in Aoki et al. (2004). Four serial sections, each 0.5 μm in thickness, were cut with glass knives and collected onto microscope slides coated with 1% gelatin. Sets of four serial sections were collected sequentially and repeatedly on six microscope slides, which served as duplicate slides to be treated with antiserum and preimmune control serum or were stained with toluidine blue. Immunolabeling was carried out as described in Aoki et al. (2004) using an anti-OsSUT1 polyclonal antiserum (Furbank et al., 2001; Aoki et al., 2004) raised against oligopeptides corresponding to residues 106 to 124, 278 to 296, and 519 to 537 of the OsSUT1 peptide sequence. These peptides show 84%, 68%, and 79% identity, respectively, to the equivalent regions of the wheat TaSUT1 protein and detected a polypeptide of approximately 60 kD in microsomal preparations of a variety of wheat tissues (Aoki et al., 2004). Labeling was detected using a fluorescent 5(6)-isothiocyanate (FITC)-conjugated secondary antibody.
CF Feeding Experiment
The endosperm was removed carefully from germinating seeds of 3-DAI seedlings and a solution of membrane-permeable 5(6)-CFDA was applied directly to the scutellar epidermis. After 3-min feeding of CFDA, nonloaded CFDA was removed by exhaustive washing of the fed point. Loaded CFDA is cleaved by cytosolic esterases to produce CF, the low-molecular-weight membrane-impermeable fluorescent probe. The CF-fed seeds were hand sectioned immediately or 4 h after the washout to examine the distribution of CF by fluorescence microscopy.
Functional Expression in the SUSY7/ura3 Yeast Strain
The cDNA clones of TaSUT1A, 1B, and 1D in Bluescript SK− were subcloned individually as a BamHI fragment into the yeast (Saccharomyces cerevisiae) expression vector pDR195 (Weise et al., 2000). The SUSY7/ura3 yeast cells (Barker et al., 2000) were separately transformed with the three TaSUT1-pDR195 constructs, the StSUT1-pDR195 construct, or the pDR195 empty vector, and selected in the absence of uracil (Weise et al., 2000). Single colonies of transformants on selection plates were grown in 2% Glc liquid media, containing 1.7 g L−1 yeast nitrogen base without amino acid, 5 g L−1 ammonium sulfate, 20 mg−1 Trp, adjusted pH at 5.0 with HCl (Weise et al., 2000). Cells were incubated at 30°C with shaking, to an OD600 of 1.0 to 1.2, then collected, washed twice with water, and resuspended in water to give an OD600 of 1.0. For the complementation test, cell suspensions were diluted to an OD600 of 10−1 to 10−4, and spotted onto a 2% agar plate of media containing Glc, Suc, or maltose as the sole carbon source. The plates were incubated at 30°C.
Determination of Soluble Carbohydrates in Germinating Seedlings
Wheat seedlings were harvested at 24, 48, and 72 h after imbibition. The 24- and 48-h germinating seeds in which the shoots and roots were too small to be collected separately were transversely cut into two parts, one-third containing the embryo (embryo end) and the rest of the seeds (endosperm end). The 72-h seedlings were separated into shoots, roots, and seeds, and the seeds were further dissected into the scutellum and the rest of the seeds. The scutellar tissues were rinsed in water to remove endosperm cells. All samples were frozen in liquid nitrogen and stored at −80°C until use.
Soluble sugars were extracted in 80% ethanol. Frozen tissues (up to 1 g fresh weight) were plunged into 5 mL of boiling 80% ethanol, boiled for 5 min, then extracted by grinding in a mortar and a pestle into a total of 15 mL 80% ethanol. The ethanol extracts were dried down with a vacuum evaporator and resuspended in 1.0 to 1.5 mL of water.
Glc, Fru, Suc, and maltose were separated and quantified by a high-performance anion-exchange chromatograph (DX-300; Dionex) with a Carbopac PA100 column and a pulse amperometric detector, eluted with a sodium acetate gradient in 150 mm NaOH.
Acknowledgments
We thank Dr. Wolf B. Frommer (Carnegie Institution of Washington) for providing the yeast strain SUSY7/ura3, the yeast shuttle vector pDR195, and the StSUT1-pDR195 construct, and Dr. Colin L.D. Jenkins (Commonwealth Scientific and Industrial Research Organization Plant Industry, Australia) for determining sugar content in seedlings.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Robert T. Furbank (robert.furbank@csiro.au).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.082719.
References
- Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT (2003) The sucrose transporter gene family in rice. Plant Cell Physiol 44: 223–232 [DOI] [PubMed] [Google Scholar]
- Aoki N, Hirose T, Takahashi S, Ono K, Ishimaru K, Ohsugi R (1999) Molecular cloning and expression analysis of a gene for a sucrose transporter in maize (Zea mays L.). Plant Cell Physiol 40: 1072–1078 [DOI] [PubMed] [Google Scholar]
- Aoki N, Scofield GN, Wang X-D, Patrick JW, Offler CE, Furbank RT (2004) Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta 219: 176–184 [DOI] [PubMed] [Google Scholar]
- Aoki N, Whitfeld P, Hoeren F, Scofield G, Newell K, Patrick J, Offler C, Clarke B, Rahman S, Furbank RT (2002) Three sucrose transporter genes are expressed in the developing grain of hexaploid wheat. Plant Mol Biol 50: 453–462 [DOI] [PubMed] [Google Scholar]
- Bagnall N, Wang X-D, Scofield GN, Furbank RT, Offler CE, Patrick JW (2000) Sucrose transport-related genes are expressed in both maternal and filial tissues of developing wheat grains. Aust J Plant Physiol 27: 1009–1020 [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB (2000) SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M (1994) Seeds: Physiology of Development and Germination, Ed 2. Plenum Press, New York
- Bick J-A, Neelam A, Smith E, Nelson SJ, Hall JL, Williams LE (1998) Expression analysis of a sucrose carrier in the germinating seedling of Ricinus communis. Plant Mol Biol 38: 425–435 [DOI] [PubMed] [Google Scholar]
- Borstlap AC, Schuurmans JAMJ (2004) Sucrose transport into plasma membrane vesicles from tobacco leaves by H+ symport or counter exchange does not display a linear component. J Membr Biol 198: 31–42 [DOI] [PubMed] [Google Scholar]
- Bush DR (1993) Proton-coupled sugar and amino acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol 44: 513–542 [Google Scholar]
- Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R (2005) Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem 280: 21437–21443 [DOI] [PubMed] [Google Scholar]
- Chandran D, Reinders A, Ward JM (2003) Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. J Biol Chem 278: 44320–44325 [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Tenner AJ, Johnson KD (1973) Synthesis and release of sucrose by the aleurone layer of barley: regulation by gibberellic acid. Planta 113: 35–46 [DOI] [PubMed] [Google Scholar]
- Edelman J, Shibko SI, Keys AJ (1959) The role of the scutellum of cereal seedlings in the synthesis and transport of sucrose. J Exp Bot 10: 178–189 [Google Scholar]
- Furbank RT, Scofield GN, Hirose T, Wang X-D, Patrick JW, Offler CE (2001) Cellular localisation and function of a sucrose transporter OsSUT1 in developing rice grains. Aust J Plant Physiol 28: 1187–1196 [Google Scholar]
- Harrington GN, Franceschi VR, Offler CE, Patrick JW, Tegeder M, Frommer WB, Harper JF, Hitz WD (1997) Cell specific expression of three genes involved in plasma membrane sucrose transport in developing Vicia faba seed. Protoplasma 197: 160–173 [Google Scholar]
- Hirose T, Imaizumi N, Scofield GN, Furbank RT, Ohsugi R (1997) cDNA cloning and tissue specific expression of a gene for sucrose transporter from rice (Oryza sativa L.). Plant Cell Physiol 38: 1389–1396 [DOI] [PubMed] [Google Scholar]
- Humphreys TE (1985) The influence of external pH on sucrose uptake and release in the maize scutellum. In RL Heath, J Preiss, eds, Regulation of Carbon Partitioning in Photosynthetic Tissue. American Society of Plant Physiologists, Rockville, MD, pp 215–230
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298–1300 [DOI] [PubMed] [Google Scholar]
- Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55: 341–372 [DOI] [PubMed] [Google Scholar]
- Lemoine R (2000) Sucrose transporters in plants: update on function and structure. Biochim Biophys Acta 1465: 246–262 [DOI] [PubMed] [Google Scholar]
- Matsukura C, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J (2000) Sugar uptake and transport in rice embryo: expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol 124: 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- M'Batchi B, Delrot S (1988) Stimulation of sugar exit from leaf tissues of Vicia faba L. Planta 174: 340–348 [DOI] [PubMed] [Google Scholar]
- Meyer S, Melzer M, Truernit E, Hümmer C, Besenbeck R, Stadler R, Sauer N (2000) AtSUC3, a gene encoding a new Arabidopsis sucrose transporter, is expressed in cells adjacent to the vascular tissue and in a carpel cell layer. Plant J 24: 869–882 [DOI] [PubMed] [Google Scholar]
- Orlich G, Hofbrückl M, Schulz A (1998) A symplasmic flow of sucrose contributes to phloem loading in Ricinus cotyledons. Planta 206: 108–116 [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11: 4705–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosche E, Blackmore D, Tegeder M, Richardson T, Schroeder H, Higgins TJV, Frommer WB, Offler CE, Patrick JW (2002) Seed-specific overexpression of a potato sucrose transporter increases sucrose uptake and growth rates of developing pea cotyledons. Plant J 30: 165–175 [DOI] [PubMed] [Google Scholar]
- Scofield GN, Hirose T, Gaudron JA, Upadhyaya NM, Ohsugi R, Furbank RT (2002) Antisense suppression of the rice sucrose transporter gene, OsSUT1, leads to impaired grain filling and germination but does not affect photosynthesis. Funct Plant Biol 29: 815–826 [DOI] [PubMed] [Google Scholar]
- Sherson SM, Hemmann G, Wallace G, Forbes S, Germain V, Stadler R, Bechtold N, Sauer N, Smith SM (2000) Monosaccharide/proton symporter AtSTP1 plays a major role in uptake and response of Arabidopsis seeds and seedlings to sugars. Plant J 24: 849–857 [DOI] [PubMed] [Google Scholar]
- Sivitz AB, Reinders A, Ward JM (2005) Analysis of the transport activity of barley sucrose transporter HvSUT1. Plant Cell Physiol 46: 1666–1673 [DOI] [PubMed] [Google Scholar]
- Tegeder M, Wang X-D, Frommer WB, Offler CE, Patrick JW (1999) Sucrose transport into developing seeds of Pisum sativum L. Plant J 18: 151–161 [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Sauer N, Wobus U (1997) A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell 9: 895–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer WB, Ward JM (2000) A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H, Wobus U (2000) Sucrose transport into barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J 21: 455–467 [DOI] [PubMed] [Google Scholar]