Abstract
A plant-based system for continuous production of monoclonal antibodies based on the secretion of immunoglobulin complexes from plant roots into a hydroponic medium (rhizosecretion) was engineered to produce high levels of single-chain and full-size immunoglobulins. Replacing the original signal peptides of monoclonal antibodies with a plant-derived calreticulin signal increased the levels of antibody yield 2-fold. Cosecretion of Bowman-Birk Ser protease inhibitor reduced degradation of the immunoglobulin complexes in the default secretion pathway and further increased antibody production to 36.4 μg/g root dry weight per day for single-chain IgG1 and 21.8 μg/g root dry weight per day for full-size IgG4 antibodies. These results suggest that constitutive cosecretion of a protease inhibitor combined with the use of the plant signal peptide and the antibiotic marker-free transformation system offers a novel strategy to achieve high yields of complex therapeutic proteins secreted from plant roots.
Therapeutic recombinant proteins have been produced in many different hosts, both prokaryotic and eukaryotic (Fischer et al., 1999). Each of them provides a unique set of advantages and can be tailored to the production of a target protein, depending on the specific requirements imposed by the manufacturing process. When the protein of interest originates from a eukaryotic source, the manufacturing method of choice primarily depends on the yield, codon usage, solubility, and set of complex posttranslational modifications required for structural integrity and biological activity of the protein (Higgins and Hames, 1999).
Most first-generation recombinant proteins were well-characterized peptides, such as insulin and other hormones, which functioned as therapeutic agents just as they normally would (Gibbons, 1991). Many second- and third-generation recombinant products, however, are complex monoclonal antibodies (mAbs) that require multiple processing steps to preserve their original bioactivity. Therefore, high costs and limited production capacities remain the major obstacles to many long-term therapies based on mAb treatments (Maloney et al., 1997).
In general, plant-based systems compare favorably with alternative expression platforms, both in terms of quality and cost of complex therapeutic proteins. After a routine transformation protocol was developed for plants, two research groups successfully expressed full-size recombinant antibodies in tobacco (Nicotiana tabacum) leaf tissue (During, 1988; Hiatt et al., 1989). Since then, a variety of antibody fragments and/or full-length mAbs have been produced in plants (Stoger et al., 2002). Biologically active mAbs require a number of assembly steps and posttranslational modifications that are carried out in the endoplasmic reticulum (ER). Once the recombinant protein is directed to the ER, it is generally secreted to the apoplast following the default secretion pathway (Deneke et al., 1990), targeted to the vacuole (Frigerio et al., 2002), or retained in the ER by the addition of the KDEL C-terminal sequence (Conrad and Fiedler, 1998).
Proteases released during plant tissue harvesting, extraction, and downstream protein purification often result in antibody degradation (Ma et al., 1994; Sharp and Doran, 2001). Using the nondestructive secretion process that provides high yields of recombinant proteins over the lifetime of a plant and facilitates downstream purification can circumvent this manufacturing challenge. Two related plant production systems have been designed recently to achieve a nondestructive production process utilizing rhizosecretion (Borisjuk et al., 1999) or guttation (Komarnytsky et al., 2000). The rhizosecretion of a functional murine mAb from the roots of previously transformed tobacco plants, resulting in a mean antibody yield of 12 μg/g root dry weight per day, was demonstrated subsequently (Drake et al., 2003). Here, we describe an optimized antibiotic-free transformation and rhizosecretion system for stable high-yield production of complex proteins based on the pRYG transformation vector (Komarnytsky et al., 2004). The system was engineered to provide enhanced levels of tissue-specific expression of the human single-chain IgG1 and full-length IgG4 immunoglobulin complexes and improve production rates based on the use of plant-derived signal peptides. Additionally, we demonstrate that cosecretion of the Bowman-Birk Ser protease inhibitor (BBI) into the plant growth medium significantly enhances antibody stability and yield.
RESULTS AND DISCUSSION
Speed of development, as well as increasing stability and yield of the target protein, are the most important factors if plants are to become a system for the commercial manufacturing of therapeutic recombinant proteins (Peeters et al., 2001). The utilization of the pRYG transformation vector harboring a cluster of rol genes is a fast and effective method for generating transgenic plants without the introduction of antibiotic resistance (Komarnytsky et al., 2004). This vector rapidly induces a large number of independently transformed adventitious roots, enabling efficient screening of individual root clones, selection of the best producers, and subsequent regeneration of fertile plants from them (Gaume et al., 2003). To estimate the efficiency and production capacity of the system, we attempted a rhizosecretion of both single-chain and full-length human mAbs.
Selection of Genetic Elements for Single-Chain IgG1 Production
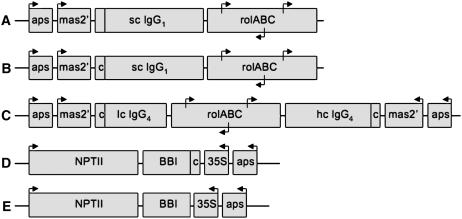
To fully capitalize on rhizosecretion capacity, we have used an amplification-promoting sequence (aps; known to stabilize and enhance expression levels of heterologous genes in plants as described by Borisjuk et al. [2000]) linked to the strong tissue-specific mas2′ promoter (Leung et al., 1991) to localize the target gene expression within the root tissue of the transgenic plant, where rhizosecretion occurs (Fig. 1, A and B). The 5′-untranslated region of a single-chain IgG1 gene was also modified to include the CCACC Kozak motif (Kozak, 1986) immediately upstream of the initiation codon.
Figure 1.
Transformation vectors designed for root-specific expression of a single-chain IgG1, full-length IgG4, and constitutive expression of BBI. A and B, Schematic illustrations of a single-chain (sc) IgG1 expression cassette containing the original signal peptide (A) or cIgG1, c (B). C, Full-length IgG4 expression cassette in which mAb light-chain (lc) and heavy-chain (hc) sequences are separated by a cluster of root proliferation (rol) genes. D, Secreted BBI expression cassette inserted next to the kanamycin-selection marker gene (nptII). E, Cytosolic BBI expression cassette inserted next to the nptII gene. Individual arrows identify the orientation of regulatory genetic elements, such as aps; a root-specific promoter, mas2′; a constitutive CaMV 35S promoter; and three promoters located in the cluster of the rol genes. Terminator sequences are not shown.
Another important element, which is often overlooked in expression studies, is the signal peptide that directs a target protein to the ER and further into the default secretion pathway. Although protein translocation, folding, and assembly are believed to be conserved between plants and animals, considerable variability has been noted in the levels of protein production, depending on the source of the signal peptide used (compare Hiatt et al., 1989; Hein et al., 1991; De Neve et al., 1993). To estimate the effect of different signal peptides on protein accumulation in the plant growth medium, an original single-chain IgG1 expression cassette (Fig. 1A) and its modified variant carrying the plant-derived calreticulin signal peptide (Borisjuk et al., 1998) cloned in frame with the single-chain IgG1 gene (Fig. 1B) were used to construct pRYG-based transformation vectors and to generate transgenic tobacco plants. For further comparison studies, we selected individual transgenic lines that expressed the single-chain IgG1 transcripts at similar levels, as confirmed by northern-blot analysis (data not shown). The antibody concentrations in the plant growth medium were determined by ELISA after excised axenic tobacco shoots were allowed to root, then transferred to fresh medium for 7 d. Production rates as high as 9.7 μg/g root dry weight per day were observed for single-chain IgG1 protein targeted by the original secretion peptide (n = 30). On the contrary, cIgG1-directed secretion of single-chain IgG1 using pRYG(single-chain cIgG1) vector (n = 32) resulted in a 2-fold increase in antibody production rates (P < 0.05; Fig. 2A).
Figure 2.
Production of the single-chain (sc) IgG1 containing either original or modified signal peptide to direct the immunoglobulin complex to the default secretion pathway. A, ELISA quantification of average immunoglobulin production after IgG1 (n = 30) and cIgG1 (n = 32) plants were grown in fresh medium for 7 d (mean ± sem; *, P < 0.05). B, Western-blot analysis of a single-chain IgG1 in hydroponic medium samples under nonreducing conditions. Total soluble proteins in the root supernatants of the transgenic plant lines secreting the original antibody (IgG1) or the modified variant for which the signal peptide sequence was changed to plant-based cIgG1 were separated by PAGE and probed with the peroxidase-conjugated goat anti-human IgG (H + L) antibody.
To further characterize the single-chain IgG1 rhizosecreted into the hydroponic medium, the root supernatant proteins were separated on SDS-PAGE under both reducing and nonreducing conditions and subjected to western-blot analysis. Under reducing conditions, a major protein band of about 45 kD was detected corresponding to the expected molecular mass of the single-chain IgG1 monomer (data not shown). Under nonreducing conditions, two bands of about 85 and 45 kD were detected corresponding to the expected sizes of dimerized single-chain IgG1 and its monomer unit. Additional bands of various molecular masses were also observed, especially in transgenic plants producing higher levels of the modified single-chain IgG1 (Fig. 2B). When compared with immunoglobulin complexes secreted in cell culture (Sharp and Doran, 2001) or from roots of previously transformed plants (Drake et al., 2003), these molecular mass distribution patterns most likely suggest extracellular degradation of the antibody in the apoplast and plant growth medium.
Protective Effect of BBI on Antibody Accumulation and Stability
Extracellular degradation significantly reduces the levels of functional immunoglobulin complexes once they are synthesized and assembled (Sharp and Doran, 2001). In addition to being metabolically wasteful, protein degradation fragments contaminate the final product with nonfunctional proteins that are difficult to separate. Although antibody degradation can be partially prevented by continuous recovery on purification columns, this procedure is laborious and expensive. Therefore, there is a need to develop strategies that reduce extracellular degradation of the secreted antibody in the apoplast and in the hydroponic medium. An attempt to use externally supplied bacitracin, a small toxic peptide of microbial origin, to prevent degradation of the immunoglobulin complexes released from the plant cell achieved little success (Sharp and Doran, 1999).
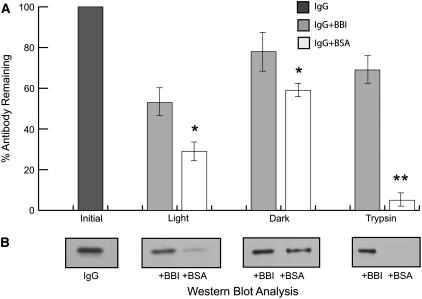
In this study, we hypothesized that codirection of a recombinant protease inhibitor into the default secretion pathway used by the recombinant antibody may partially protect the assembled immunoglobulin complexes at all stages of the secretion process, including the ER, apoplast, and hydroponic medium. Initially, we evaluated the protective effect of soybean (Glycine max) BBI (Birk, 1985) on the antibody degradation in vitro under various physiological conditions. BBI has a strong Ser protease inhibitory activity; the protein is available commercially and the gene encoding this protein can be effectively expressed (Yakoby and Raskin, 2004). Once exogenously supplied to the antibody solution in vitro, BBI provided some stabilization effect to the human IgG1 antibody kept on a rotary shaker at room temperature in the dark for 24 h, as measured by ELISA. A much stronger protective effect was observed when antibody solution was subjected to light or when external protease was supplied to the medium (P < 0.05 or P < 0.01; Fig. 3A). Western-blot analysis of the media samples further confirmed the protective effect (Fig. 3B). BBI has previously been shown to act as a potent, yet selective, tissue radioprotector due to the presence of its chromophore (Dittmann et al., 2005), which can also explain the greater level of antibody protection observed with light exposure. As expected, exogenously supplied equimolar amounts of bovine serum albumin (BSA), which lacks protease inhibitory activity, had a significantly reduced protective effect on the antibody under the same conditions.
Figure 3.
Protective effect of BBI protease inhibitor on antibody stability under various conditions, including dark, light, and protease treatment. A, ELISA quantification of exogenously added IgG1 antibody remaining in the supernatants after 24 h in the presence of 0.1 μm BBI or BSA (mean ± sem; *, P < 0.05; **, P < 0.01 compared with the respective control). Sample collected immediately at the beginning of the experiment (IgG1) was used as a 100% reference point. B, Representative western-blot analysis of the same supernatants under nonreducing conditions probed with peroxidase-conjugated goat anti-human IgG (H + L) antibody.
Putative Interaction between BBI and Immunoglobulin Complexes
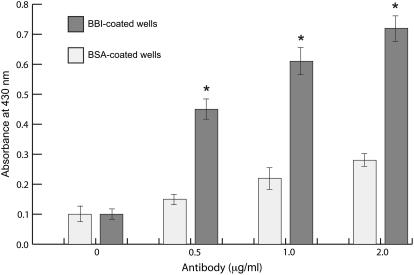
The nature of BBI-induced protection of immunoglobulin complexes is unknown. To test the hypothesis that BBI stabilizes the antibodies by direct binding to the immunoglobulin complexes, as reported for the inter-α-trypsin inhibitor and the IgG complexes in human serum (Salier et al., 1983), the ELISA wells were coated with 1 μg/mL BBI or BSA, subsequently blocked with 5% nonfat dry milk, and incubated with various concentrations of monoclonal human IgG1. Measurable binding between BBI and IgG1 was observed; no such interaction was noted when ELISA wells were coated with BSA (P < 0.05; Fig. 4). Therefore, it is possible that BBI stabilizes antibodies by direct interaction with the IgG molecules. However, the fact that the BBI molecule interacts with the immunoglobulins is only indirect evidence of the putative mechanism behind its protection effect. The observed interaction was weak and did not increase linearly with the increased concentration of the immunoglobulins in the medium, suggesting that an interaction between the molecules alone is not sufficient for explanation of the observed effect. Understanding whether physical interaction between a protease inhibitor and a target heterologous protein is required for protection or whether the presence of a protease inhibitor in the medium interferes with downstream purification of a recombinant protein remains to be elucidated.
Figure 4.
Antibody binding to immobilized BBI protein. IgG1 antibody concentrations were measured by ELISA at OD430 using peroxidase-conjugated goat anti-human IgG (H + L) antibody and the trace metal-buffered substrate (mean ± sem; *, P < 0.05).
Directing BBI to the Default Secretion Pathway
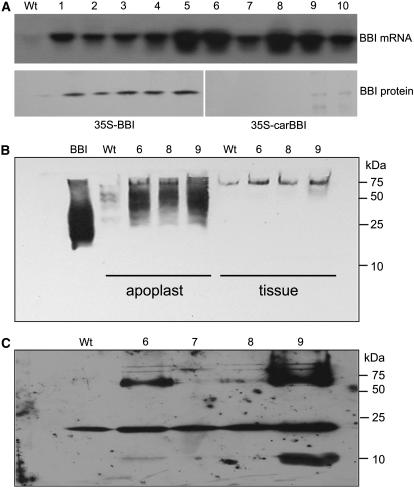
To develop a transgenic tobacco line producing BBI protein directed into the default secretion pathway, the gene encoding for the BBI protease inhibitor (Yakoby and Raskin, 2004) was fused in frame to the plant-derived calreticulin signal peptide (Borisjuk et al., 1998). The resulting expression cassette was ligated into the pBin19-derived transformation vector under the control of the constitutive cauliflower mosaic virus (CaMV) 35S promoter and the aps element (Fig. 1D). An identical cytosolic BBI expression cassette lacking the signal peptide was used to generate transgenic plants that produce, but do not secrete, BBI (Fig. 1E). To confirm successful transformation events, independent transgenic lines (n = 8–10) from each group were analyzed for the presence of BBI mRNA using northern-blot analysis (Fig. 5A, top), as well for accumulation of BBI protein in the cytoplasm after the apoplast liquid was extracted from the plant tissue. Plants engineered to secrete BBI showed negligible amounts of recombinant BBI in the cytoplasm, thus confirming the correct targeting of the recombinant protein (Fig. 5A, bottom). Three transgenic plant lines that expressed high levels of BBI mRNA were grown to maturity, and the presence of functional BBI in the apoplast liquid was determined by protease inhibitory assay after axenic transgenic tobacco shoots were excised, allowed to root, and transferred to fresh medium for 7 d (data not shown) and western-blot analysis (Fig. 5B). The proteins in root exudates were separated by reducing SDS-PAGE gel and analyzed by western blot using rabbit polyclonal antibodies produced against BBI. The molecular mass of a BBI monomer (8 kD) was observed in transgenic lines secreting higher amounts of BBI protein, whereas, under natural conditions, BBI exists as numerous multimers of higher molecular mass (Fig. 5C). No significant amount of BBI was detected in the hydroponic medium of plants engineered to produce a cytosolic version of the protein (data not shown). BBI-secreting transgenic line 9 was used as a master plant line to study the cosecretion of the human single-chain IgG1 and full-length IgG4 antibodies, whereas cytosolic BBI transgenic line 5 was used to generate the control plant coexpressing BBI and immunoglobulins but not secreting BBI (Fig. 5).
Figure 5.
Analysis of transgenic tobacco lines expressing BBI. A, Northern-blot quantification of BBI mRNA expression and western-blot detection of cytosolic (lanes 1–5) or secreted (lanes 6–10) form of BBI in plant tissue of individual tobacco transgenic lines after the apoplast liquid was extracted. B, Western-blot detection of multimeric BBI complexes in the apoplast of the BBI-secreting plants under nonreducing conditions. C, Western-blot detection of the secreted BBI protein in root exudates under reducing conditions using rabbit anti-BBI polyclonal antibodies.
Stable High-Level Secretion of the Immunoglobulin Protein Complexes
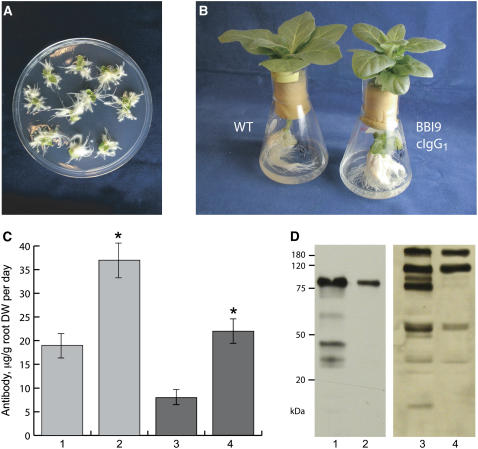
To estimate the effect of cosecretion of BBI and the immunoglobulin complex, the pRYG(single-chain cIgG1) and pRYG(cIgG4) vectors (Fig. 1, B and C) were transformed into the BBI-secreting tobacco line 9 and BBI-producing tobacco line 5 was used as a control. The typical root proliferation response was observed 2 weeks after inoculation, as expected (Fig. 6A); however, the constitutive coexpression of the protease inhibitor incurred some fitness costs on the transgenic tobacco plants as evident from smaller plant size (Fig. 6B) and lessened lifetime reproductive output (data not shown), similar to a previously described study (Zavala et al., 2004). The antibody concentration in the hydroponic medium was determined by ELISA after excised axenic tobacco shoots were allowed to root and transferred to fresh medium for 7 d. In this experiment, the rhizosecretion of the single-chain cIgG1 construct from the roots of BBI-secreting plant lines was 36.4 μg/g root dry weight per day of the antibody (n = 16), whereas full-length IgG4 antibody accumulated in the growth media at the rate of 21.8 μg/g root dry weight per day (n = 18), as determined by ELISA (Fig. 6C). Control plants that did not secrete BBI protein produced 18.9 μg/g root dry weight per day of the single-chain antibody (n = 11) and 8.2 μg/g root dry weight per day of the full-size antibody (n = 9).
Figure 6.
pRYG-based production of the single-chain IgG1 and full-length IgG4 immunoglobulin complexes in transgenic tobacco plants cosecreting BBI. A, Antibiotic-free selection of transgenic root clones based on the morphological root proliferation response of rol genes. B, Wild-type (WT) and BBI-secreting transgenic tobacco line 9 (BBI9) cultivated in sterile hydroponic medium. BBI9 plant was slightly smaller than its nontransgenic control of the same age; however, its root system was more branched than the roots of a nontransformed plant. C and D, Antibody production rates were estimated for the single-chain IgG1 (lanes 1 and 2) and full-length (lanes 3 and 4) IgG4 immunoglobulins produced by transgenic plant lines expressing the cytosolic (lanes 1 and 3) or secretory (lanes 2 and 4) form of BBI by ELISA quantification after plants were grown in fresh medium for 7 d (mean ± se; *, P < 0.05; C) or western-blot detection under nonreducing conditions after probing with the peroxidase-conjugated goat anti-human IgG (H + L) antibody (D).
To further characterize the immunoglobulin complexes rhizosecreted into the hydroponic medium, the supernatant proteins from representative transgenic lines generated in this experiment were separated on SDS-PAGE under nonreducing conditions and subjected to western-blot analysis. One major band of about 85 kD was detected in the hydroponic medium of the BBI-secreting plants engineered to cosecrete IgG1, which is the expected size for a fully dimerized IgG1 antibody (Fig. 6D). The fully assembled IgG4 mAb was also detected in the root supernatant of BBI-secreting plants; however, two other bands of about 120 and 60 kD were also observed. This suggests that cosecreted BBI was not able to completely protect the full-length antibody, although the proteolytic pattern was greatly reduced as compared to previous studies (Sharp and Doran, 2001; Drake et al., 2003) or control groups of plant expressing, but not secreting, BBI (Fig. 6D). Also, IgG4 accumulated in the plant growth medium at a rate of 21.8 μg/g root dry weight per day, a 2-fold increase in the previously reported yield (Drake et al., 2003).
Unlike previous reports on the secretion of mAbs in plant tissue culture or from the roots of the transformed plants, this study demonstrates an antibiotic-free transformation system that allows efficient introduction of both light and heavy chains of mAb and simultaneous modification of the plant root system to further capitalize on the rhizosecretion ability of rol-induced hairy roots (Gaume et al., 2003). Our study also provides crucial initial evidence for the potential of cosecreted protease inhibitors as effective tools to stabilize and enhance the yield of recombinant secreted proteins. This study also emphasizes the importance of carefully selected genetic elements in achieving stable, high-level expression and secretion of the target transgene. We believe that this approach could have potential applications in further development of plant-based systems for manufacturing complex therapeutic proteins and may provide a tool for efficient in vivo study of multiple protein targets.
MATERIALS AND METHODS
Vector Construction
Genes encoding for human single-chain IgG1 (pcDNA3) as well as light (pDONRL) and heavy (pDONRH) chains of monoclonal IgG4 were kindly provided by Dr. Subinay Ganguly (Bristol-Myers Squibb). The single-chain IgG1 gene was amplified using the pair of primers that introduced the SalI site and the Kozak motif to the 5′ end of the amplified sequence and BsiEI site to the 3′ end (forward 5′-CCAGTCGACACCAATGGGTGTACT and reverse 5′-TTGCCGGCCGTCGCACTCATTTAC primer, respectively). The resulting PCR product was isolated from gel using the Qiaquick PCR purification kit (Qiagen) and cloned into the pCR2.1 plasmid using the TOPO TA cloning kit (Invitrogen). From there, the SalI-BsiEI fragment was cloned into the SalI-SacII sites of the pLit-aps-mas-β-glucuronidase (GUS) plasmid (Komarnytsky et al., 2004), effectively replacing the original GUS sequence and placing the single-chain IgG1 gene under the control of the aps element, mas2′ promoter, and nos terminator. At the final cloning step, the KpnI-BsiWI (blunted) fragment containing the entire expression cassette was cloned into the KpnI-EcoRI (blunted) sites of the pRYG plasmid to produce the pRYG(single-chain IgG1) transformation vector (Fig. 1A). When specified, a native immunoglobulin signal peptide was replaced with the plant-based calreticulin signal sequence, PCR amplified from a previously isolated cDNA clone (Borisjuk et al., 1998) using 5′-GTCGACGATCTCACAACAGTGG and 5′-CACGTGCATTGCTACCTCAGCGGA primers. The reverse primer contained a PmlI restriction site that was later used for in-frame fusion of the signal peptide to the IgG1 coding sequence. The modified sequence for the single-chain IgG1 gene was cloned into the pRYG transformation vector following the exact strategy outlined above for the original sequence, resulting in the pRYG(single-chain cIgG1) vector (Fig. 1B). Similar cloning steps have been repeated for replacing the signal peptides of light and heavy chains of full-length IgG4. At the last cloning step, both expression cassettes were inserted into the same transformation vector using the KpnI-EcoRI (blunted) sites of the pRYG plasmid to clone the light chain, and the XbaI (blunted) site of the pRYG plasmid to construct the final transformation vector pRYG(cIgG4), carrying both expression cassettes separated by the rol genes (Fig. 1C).
To generate a transformation vector for cosecretion of BBI, the NruI (blunted)-XhoI fragment containing the previously constructed bbi gene was cloned in frame to cIgG1 using NcoI (blunted) and XhoI sites, therefore replacing the green fluorescent protein (GFP) coding region downstream of the CaMV 35S promoter in the pNB-car-GFP plasmid (Borisjuk et al., 2000). The resulting plasmid was then restricted with XbaI and HindIII to insert a CaMV 35S transcriptional terminator downstream of the bbi sequence. Finally, the complete expression cassette was cloned into the HindIII site of the pBin19-aps plasmid (Borisjuk et al., 2000) to construct the final transformation vector pBin-carBBI (Fig. 1D). pBin-BBI, a vector that expresses a cytosolic form of BBI protein, was constructed using the above strategy once cIgG1 was excised from the pNB-car-GFP plasmid prior to cloning (Fig. 1E).
Plant Transformation
The transgenic lines of tobacco (Nicotiana tabacum) expressing the secreted version of BBI were generated following the standard Agrobacterium-mediated transformation protocol using kanamycin-based selection (Horsch et al., 1985). Plant transformation and antibiotic-free selection for individual transgenic lines expressing either IgG1 or IgG4 were performed essentially as described earlier for the pRYG-based transformation system (Komarnytsky et al., 2004).
Northern Blots and RT-PCR
Total RNA was isolated from plants as described elsewhere (Chomczynski and Sacchi, 1987). Ten micrograms of total RNA were loaded and subjected to electrophoresis on a 1% denaturing agarose gel containing formaldehyde before capillary blotting onto a Hybond N+ nylon membrane (Amersham). Hybridizations with 32P-labeled DNA probes were performed according to published procedures (Sambrook et al., 1989).
Apoplast Liquid Collection and Tissue Extraction
Leaf apoplast liquid was collected after vacuum infiltration (Terry and Bonner, 1980) with an ice-cold buffer (50 mm Tris-HCl, 10 mm EDTA, pH 8.0). The remaining plant tissue was frozen in liquid nitrogen, homogenized, and extracted with the same buffer to recover the total soluble proteins present in the tissue after apoplast removal. All samples were used immediately or stored at −20°C.
Western Blots and ELISA
Total protein in the sample was determined by Bradford dye-binding assay (Bio-Rad). For PAGE protein analysis, protein samples were separated in Tris-Gly gels under reducing/nonreducing conditions. For western-blot analysis of immunoglobulin complexes, proteins separated by PAGE (20 μg) were transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% nonfat dry milk and incubated with the goat anti-human IgG (H + L) conjugated to horseradish peroxidase (HRP; Pierce) for immunoglobulin detection. BBI was observed by treating the membranes with custom-raised rabbit anti-BBI polyclonal antibodies (Pocono Rabbit Farm and Lab) followed with murine anti-rabbit IgG-HRP conjugate (Promega). Protein bands were visualized by exposure to x-ray film (Kodak) after treatment with ECL chemiluminescence substrate (Amersham).
The concentration of the IgG1 and IgG4 immunoglobulin complexes in the plant growth medium were determined using a sandwich ELISA. In short, 96-well ELISA plates were coated with 1 μg/mL goat anti-human IgG (H + L; Rockland). The plates were blocked with PTB buffer (phosphate-buffered saline, 1% BSA, 0.05% Tween 20) and incubated with the samples or various concentrations of the reference standard (human IgG1-κ; Sigma). The plates were then incubated with the goat anti-human IgG (H + L) conjugated to HRP (Pierce) and stained by adding trace metal-buffered substrate (Amersham). The plates were monitored in a microplate reader (HTS-7000; Perkin-Elmer) at OD650 and, once the reaction was stopped by 2 m H2SO4, were subsequently read at OD450.
Protease Inhibitory Activity Assays
Inhibitory activity against trypsin and chymotrypsin was visualized by staining of plant growth medium samples spotted on the surface of the polyvinylidene difluoride membrane (normalized for total soluble protein content). After soaking in 100 μg/mL enzyme in 50 mm Tris-HCl, pH 8.0, for 10 min at 37°C, the freshly prepared reaction mixture containing 0.75 mm N-acetyl-dl-phenyl-alanine-β-naphthylester, 1 mm O-dianisidine tetrazotized dye, 10% N,N-dimethylformamide in 50 mm Tris-HCl, pH 8.0, was applied to the surface of the membranes and the clear zone indicative of the inhibitory activity was recorded. All chemicals were purchased from Sigma, unless noted otherwise.
Antibody Stability
Monoclonal human IgG1 (Sigma) was added to 10 mL of phosphate-buffered saline in triplicate 100-mL shake flasks at the concentration of 1 μg/mL. The flasks were subsequently incubated at 25°C on the orbital shaker (120 rpm) under dark/light conditions as specified by individual treatments. When necessary, BBI or BSA (negative control) was added to the flask in the final concentration of 0.1 μm at the same time, whereas trypsin was supplied at the final concentration of 1 μg/mL. Samples were taken immediately after antibody addition and after 24 h to be analyzed by ELISA and western blotting.
Antibody Binding Assay
To evaluate the potential binding activity of the immunoglobulin complexes to BBI, 96-well ELISA plates were coated with 1 μg/mL BBI or BSA (negative control). The plates were then blocked with 5% nonfat dry milk and incubated with various concentrations of monoclonal human IgG1 (Sigma). The plates were subsequently incubated with the goat anti-human IgG (H + L) conjugated to HRP and stained following the procedure described above for the ELISA assay.
Acknowledgments
We thank Dr. Stanton Gelvin (Purdue University) for the mas2′ promoter and Dr. Thomas Schmulling (Freie Universitat Berlin) for the cluster of the rol genes. We are grateful to Ivan Jenkins for his technical assistance in the greenhouse.
This work was supported in part by grants from Phytomedics, Inc. (Dayton, NJ), Rutgers, the State University of New Jersey, and N.J. Agricultural Experiment Station, and in part by Vaadia-BARD (postdoctoral award no. FI–302–2000 to N.Y.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Slavko Komarnytsky (komar@aesop.rutgers.edu).
References
- Birk Y (1985) The Bowman-Birk inhibitor: trypsin- and chymotrypsin-inhibitor from soybeans. Int J Pept Protein Res 25: 113–131 [DOI] [PubMed] [Google Scholar]
- Borisjuk N, Borisjuk L, Komarnytsky S, Timeva S, Hemleben V, Gleba Y, Raskin I (2000) Tobacco ribosomal DNA spacer element stimulates amplification and expression of heterologous genes. Nat Biotechnol 18: 1303–1306 [DOI] [PubMed] [Google Scholar]
- Borisjuk N, Borisjuk L, Logendra S, Petersen F, Gleba Y, Raskin I (1999) Production of recombinant proteins in plant root exudates. Nat Biotechnol 17: 466–469 [DOI] [PubMed] [Google Scholar]
- Borisjuk N, Sitailo L, Adler K, Malysheva L, Tewes A, Borisjuk L, Manteuffel R (1998) Calreticulin expression in plant cells: developmental regulation, tissue specificity and intracellular distribution. Planta 206: 504–514 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Conrad U, Fiedler U (1998) Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immuno-modulation of physiological functions and pathogen activity. Plant Mol Biol 38: 101–109 [PubMed] [Google Scholar]
- Deneke J, Botterman J, Deblaere R (1990) Protein secretion in plant cells can occur via a default pathway. Plant Cell 2: 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Neve M, De Loose M, Jacobs A, Van Houdt H, Kaluza B, Weidle U, Van Montagu M, Depicker A (1993) Assembly of an antibody and its derived antibody fragment in Nicotiana and Arabidopsis. Transgenic Res 2: 227–237 [DOI] [PubMed] [Google Scholar]
- Dittmann K, Toulany M, Classen J, Heinrich V, Milas L, Rodemann HP (2005) Selective radioprotection of normal tissues by Bowman-Birk inhibitor (BBI) in mice. Strahlenther Onkol 181: 191–196 [DOI] [PubMed] [Google Scholar]
- During K (1988) Wundinduzierbare Expression und Sekretion von T4 Lysozym und monoklonalen Antikorpern in Nicotiana tabacum. Doctoral dissertation, University of Koln, Germany
- Drake PM, Chargelegue DM, Vine ND, van Dolleweerd CJ, Obregon P, Ma JK (2003) Rhizosecretion of a monoclonal antibody protein complex from transgenic tobacco roots. Plant Mol Biol 52: 233–241 [DOI] [PubMed] [Google Scholar]
- Fischer R, Drossard J, Emans N, Commandeur U, Hellwig S (1999) Towards molecular farming in the future: moving from diagnostic protein and antibody production in microbes to plants. Biotechnol Appl Biochem 30: 101–108 [PubMed] [Google Scholar]
- Frigerio L, Vine ND, Pedrazzini E, Hein MB, Wang F, Ma JK, Vitale A (2002) Assembly, secretion, and vacuolar delivery of a hybrid immunoglobulin in plants. Plant Physiol 123: 1483–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein MB, Tang Y, McLeod DA, Janda KD, Hiatt A (1991) Evaluation of immunoglobulins from plant cells. Biotechnol Prog 7: 455–461 [DOI] [PubMed] [Google Scholar]
- Hiatt A, Cafferkey R, Bowdish K (1989) Production of antibodies in transgenic plants. Nature 342: 76–78 [DOI] [PubMed] [Google Scholar]
- Higgins SJ, Hames BD (1999) Protein Expression. Oxford University Press, New York
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231 [DOI] [PubMed] [Google Scholar]
- Gaume A, Komarnytsky S, Borisjuk N, Raskin I (2003) Rhizosecretion of recombinant proteins from plant hairy roots. Plant Cell Rep 21: 1188–1193 [DOI] [PubMed] [Google Scholar]
- Gibbons A (1991) Biotech pipeline: bottleneck ahead. Science 254: 369–370 [DOI] [PubMed] [Google Scholar]
- Komarnytsky S, Borisjuk NV, Borisjuk LG, Alam MZ, Raskin I (2000) Production of recombinant proteins in tobacco guttation fluid. Plant Physiol 124: 927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarnytsky S, Gaume A, Garvey A, Borisjuk N, Raskin I (2004) A quick and efficient system for antibiotic-free expression of heterologous genes in tobacco roots. Plant Cell Rep 22: 765–773 [DOI] [PubMed] [Google Scholar]
- Kozak M (1986) Point mutations define a sequence flanking the AUG initiator that modulates translation by eukaryotic ribosomes. Cell 44: 283–297 [DOI] [PubMed] [Google Scholar]
- Leung J, Fukuda H, Wing D, Schell J, Masterson R (1991) Functional analysis of cis-elements, auxin response and early developmental profiles of the mannopine synthase bidirectional promoter. Mol Gen Genet 230: 463–474 [DOI] [PubMed] [Google Scholar]
- Ma JK, Lehner T, Stabila P, Fux CI, Hiatt A (1994) Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur J Immunol 24: 131–138 [DOI] [PubMed] [Google Scholar]
- Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, et al (1997) IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90: 2188–2195 [PubMed] [Google Scholar]
- Peeters K, De Wilde C, De Jaeger G, Angenon G, Depicker A (2001) Production of antibodies and antibody fragments in plants. Vaccine 19: 2756–2761 [DOI] [PubMed] [Google Scholar]
- Salier JP, Sesboue R, Hochstrasser K, Schonberger O, Martin JP (1983) Isolation and characterization of an inter-alpha-trypsin inhibitor IgG complex from human serum. Biochim Biophys Acta 742: 206–214 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sharp JM, Doran PM (1999) Effect of bacitracin on growth and monoclonal antibody production by tobacco hairy roots and cell suspensions. Biotechnol Bioprocess Eng 4: 253–258 [Google Scholar]
- Sharp JM, Doran PM (2001) Characterization of monoclonal antibody fragments produced by plant cells. Biotechnol Bioengin 73: 338–346 [DOI] [PubMed] [Google Scholar]
- Stoger E, Sack M, Fischer R, Christou P (2002) Plantibodies: applications, advantages and bottlenecks. Curr Opin Biotechnol 13: 161–166 [DOI] [PubMed] [Google Scholar]
- Terry ME, Bonner BA (1980) An examination of centrifugation as a method of extracting an extracellular solution from peas, and its use for study of indoleacetic acid-induced growth. Plant Physiol 66: 321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoby N, Raskin I (2004) A simple method to determine trypsin and chymotrypsin inhibitory activity. J Biochem Biophys Methods 59: 241–251 [DOI] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT (2004) Constitutive and inducible trypsin protease inhibitor production incurs large fitness costs in Nicotiana attenuata. Proc Natl Acad Sci USA 101: 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]