Abstract
The roots curl in naphthylphthalamic acid1 (rcn1) mutant of Arabidopsis (Arabidopsis thaliana) has altered auxin transport, gravitropism, and ethylene response, providing an opportunity to analyze the interplay between ethylene and auxin in control of seedling growth. Roots of rcn1 seedlings were previously shown to have altered auxin transport, growth, and gravitropism, while rcn1 hypocotyl elongation exhibited enhanced ethylene response. We have characterized auxin transport and gravitropism phenotypes of rcn1 hypocotyls and have explored the roles of auxin and ethylene in controlling these phenotypes. As in roots, auxin transport is increased in etiolated rcn1 hypocotyls. Hypocotyl gravity response is accelerated, although overall elongation is reduced, in etiolated rcn1 hypocotyls. Etiolated, but not light grown, rcn1 seedlings also overproduce ethylene, and mutations conferring ethylene insensitivity restore normal hypocotyl elongation to rcn1. Auxin transport is unaffected by treatment with the ethylene precursor 1-aminocyclopropane carboxylic acid in etiolated hypocotyls of wild-type and rcn1 seedlings. Surprisingly, the ethylene insensitive2-1 (ein2-1) and ein2-5 mutations dramatically reduce gravitropic bending in hypocotyls. However, the ethylene resistant1-3 (etr1-3) mutation does not significantly affect hypocotyl gravity response. Furthermore, neither the etr1 nor the ein2 mutation abrogates the accelerated gravitropism observed in rcn1 hypocotyls, indicating that both wild-type gravity response and enhanced gravity response in rcn1 do not require an intact ethylene-signaling pathway. We therefore conclude that the RCN1 protein affects overall hypocotyl elongation via negative regulation of ethylene synthesis in etiolated seedlings, and that RCN1 and EIN2 modulate hypocotyl gravitropism and ethylene responses through independent pathways.
Polar auxin transport in higher plants is a directional and regulated process that controls a variety of important growth and developmental processes, including gravity response, root and shoot elongation, embryo and vascular development, and branching of roots and shoots. Auxin is transported from cell to cell, and in shoots indole-3-acetic acid (IAA) moves unidirectionally from the apex to the base (for review, see Friml, 2003). Changes in direction of IAA transport when seedlings are reoriented relative to the gravity vector are implicated in growth promotion on the lower side of shoot tissues (Blancaflor and Masson, 2003). Several proteins that control auxin movement have been identified in Arabidopsis (Arabidopsis thaliana; Blakeslee et al., 2005). The auxin insensitive1 (AUX1) protein is believed to facilitate auxin influx into cells (Marchant et al., 1999; Swarup et al., 2004). Auxin efflux from cells likely requires a complex of proteins, containing PIN proteins, multidrug resistance-like/P-glycoproteins (MDR/PGP), and a regulatory auxin transport inhibitor-binding protein (Noh et al., 2001; Blakeslee et al., 2005). Expression of PGPs in heterologous systems is sufficient to alter IAA movement across the membrane, suggesting that these proteins may be IAA carriers (Geisler et al., 2005; Terasaka et al., 2005). The PIN proteins recently have been shown to mediate auxin efflux (Petrasek et al., 2006), and increasing evidence suggests that they modulate the activity of MDR/PGP proteins and perform specialized auxin transport activities in discrete tissue locations (for review, see Friml, 2003; Blakeslee et al., 2005; Paponov et al., 2005).
Although our understanding of carrier protein function has improved, the mechanisms regulating auxin transport during plant growth and development remain enigmatic (for review, see Muday and DeLong, 2001; Friml, 2003). The existence of gene families encoding auxin transport proteins suggests that altered expression of some family members may alter the amount or polarity of auxin transport (Friml, 2003). Indeed the observation of gravity-induced PIN3 relocalization (Friml et al., 2002), auxin-regulated PIN gene expression (Peer et al., 2004), and auxin regulation of PIN protein cycling between the endosome and plasma membrane (Paciorek et al., 2005) indicate that transport protein expression is dynamic. Endogenous small molecules, such as flavonoids, may control the activity and/or synthesis of auxin transport proteins (Brown et al., 2001; Buer and Muday, 2004; Peer et al., 2004). Auxin transport has also been shown to be regulated by ethylene in a number of species and tissues by mechanisms that are still unknown (Morgan and Gausman, 1966; Suttle, 1988; Lee et al., 1990).
Changes in localization and/or activity of auxin transport proteins due to protein phosphorylation also may regulate auxin transport (for review, see DeLong et al., 2002). Inhibitor studies have implicated protein kinases in regulating auxin transport and its sensitivity to auxin transport inhibitors (Bernasconi, 1996; Delbarre et al., 1998). Genetic evidence for phosphorylation control of auxin transport comes from studies of the pinoid (pid) and roots curl in naphthylphthalamic acid1 (rcn1) mutants, which have defects in genes encoding a protein kinase and a protein phosphatase regulatory subunit, respectively (for review, see DeLong et al., 2002). The PID gene encodes a member of the AGC family of Ser/Thr kinases (Christensen et al., 2000), and pid mutants exhibit altered auxin transport in inflorescences and a floral development defect resembling that of the pin-formed1 (pin1) mutant (Bennett et al., 1995 Christensen et al., 2000; Benjamins et al., 2001). In inflorescences, pid loss of function and PID overexpression have opposite effects on the polar targeting of the PIN1 auxin efflux facilitator protein (Friml et al., 2004), consistent with the hypothesis that reversible protein phosphorylation by PID may act at the level of protein targeting (for review, see for review, see Muday and Murphy, 2002).
Analysis of the rcn1 mutant has shown that protein phosphatase 2A (PP2A) activity is required for regulation of root auxin transport and gravitropic curvature. The RCN1 gene encodes a regulatory α-subunit of PP2A, and the rcn1 mutant has reduced PP2A activity in vivo and in vitro (Garbers et al., 1996; Deruère et al., 1999). Roots of rcn1 seedlings have elevated basipetal auxin transport and exhibit a significant delay in gravitropism (Rashotte et al., 2001). It is clear that reduced PP2A activity causes the phenotypes observed in rcn1 roots and hypocotyls, because these effects can be mimicked by treating wild-type seedlings with low doses of protein phosphatase inhibitors (Deruère et al., 1999; Rashotte et al., 2001; Larsen and Cancel, 2003; Shin et al., 2005). However, the mechanistic basis for rcn1 effects on polar transport in roots has not been determined; localization of the PIN2/AGR1/ethylene insensitive root1 (EIR1) protein appears to be normal in rcn1 root tips (Shin et al., 2005), and neither PIN2/AGR1/EIR1 nor AUX1 is required for the rcn1 transport phenotype (Rashotte et al., 2001). Auxin transport in rcn1 hypocotyls has not been quantitatively assessed in previous experiments. Although rcn1 roots exhibit reduced elongation in both light- and dark-grown seedlings, hypocotyl elongation is reduced in etiolated, but not light-grown, rcn1 seedlings. Similarly, phosphatase inhibitor sensitivity is increased in roots of both light- and dark-grown rcn1 seedlings but in hypocotyls of etiolated seedlings only (Deruère et al., 1999). The regulation of auxin transport is also known to change under different light conditions in normal seedlings. In hypocotyls grown in low light, IAA transport and elongation are inhibited by naphthylphthalamic acid (NPA), while in dark-grown hypocotyls, transport and elongation are insensitive to NPA (Jensen et al., 1998; Rashotte et al., 2003). The connection between RCN1 action and light-regulated auxin transport has not yet been examined.
Interestingly, the rcn1-2 allele was identified in a screen for increased ethylene response in etiolated seedlings and was originally designated enhanced ethylene response1 (eer1; Larsen and Chang, 2001; Larsen and Cancel, 2003). Enhanced ethylene response in rcn1 is a hypocotyl-specific phenotype and is accompanied by ethylene overproduction (Larsen and Chang, 2001). The rcn1-1 and rcn1-2/eer1 alleles show identical phenotypes in several assays (Larsen and Cancel, 2003; A. DeLong, unpublished data). In contrast to the hypocotyl phenotype, rcn1 roots exhibit reduced ethylene response; rcn1 roots are slightly resistant to 1-aminocyclopropane carboxylic acid (ACC), and rcn1 suppresses the constitutive triple response root phenotype (Larsen and Chang, 2001; A. DeLong, unpublished data). It is possible that PP2A regulates ethylene and auxin pathways independently, but mechanistic connections between the auxin transport and ethylene response phenotypes in the rcn1 mutant have not been eliminated.
Because rcn1 alleles were isolated in independent screens for altered IAA transport and altered ethylene response, a critical question is whether the reduced protein phosphatase activity in rcn1 affects these two processes independently or acts on convergent pathways regulating ethylene response and auxin transport. A number of studies have examined the relationship between ethylene and auxin (Vandenbussche et al., 2005; for review, see Swarup et al., 2002). Ethylene levels have been shown to regulate IAA transport in several species (Morgan and Gausman, 1966; Suttle, 1988; Lee et al., 1990), but this relationship has not been tested in Arabidopsis hypocotyls and it is possible that increased ethylene levels alter auxin transport in rcn1. Alternatively, increased basipetal auxin transport in rcn1 could alter the ethylene response. In this regard, the ethylene insensitivity of roots of two mutants linked to auxin transport, aux1 and eir1 (Pickett et al., 1990; Bennett et al., 1996; Luschnig et al., 1998), is noteworthy, as it suggests that ethylene inhibition of root growth either requires normal auxin distribution (Rahman et al., 2001) or may be mediated via inhibition of auxin transport (Suttle, 1988). Consistent with this idea, a recent report indicates that growth inhibition of ethylene-treated Arabidopsis roots is mediated by induction of IAA biosynthesis via activation of the rate-limiting enzyme of Trp-dependent IAA biosynthesis (Stepanova et al., 2005).
Another possibility is that elevated auxin transport in rcn1 seedlings induces ethylene synthesis. Auxin is a positive regulator of ethylene biosynthesis in many plants, including Arabidopsis (Yang and Hoffman, 1984; Woeste et al., 1999; Harper et al., 2000). The rate-limiting step in ethylene synthesis is catalyzed by ACC synthase, which is encoded by the ACS gene family, with 12 members in Arabidopsis (Yamagami et al., 2003). Several of these genes are strongly auxin inducible in dark-grown seedlings/plants (Abel et al., 1995; Yamagami et al., 2003). In light-grown tissues, the induction by auxin is more subtle (Wang et al., 2005) or not detected (Rashotte et al., 2005). These data suggest that ethylene synthesis may be modulated by mutations that alter auxin distribution, especially in dark-grown seedlings. Recently, one ACC synthase gene was shown to be asymmetrically induced across a gravity-stimulated snapdragon (Antirrhinum majus) flower spike, suggesting that asymmetric synthesis of ethylene may be part of gravitropic response in some tissues (Woltering et al., 2005).
The goal of this study was to dissect auxin and ethylene interactions in hypocotyls of the rcn1 mutant. We asked whether hypocotyls of rcn1 exhibit alterations in auxin transport and dependent physiological processes, such as gravitropic bending. We also asked whether the altered auxin transport and gravitropic phenotypes require ethylene-signaling functions. Because some rcn1 phenotypes are observed only in etiolated seedlings, we assayed for light modulation of phenotypes including auxin transport, ethylene synthesis, and phosphatase regulation. Our results identify a role of protein phosphorylation in regulation of hypocotyl auxin transport and gravity response and provide evidence for a separate RCN1-regulated circuit that controls ethylene synthesis in etiolated hypocotyls.
RESULTS
Hypocotyl Phenotypes of rcn1 Are Dark Dependent
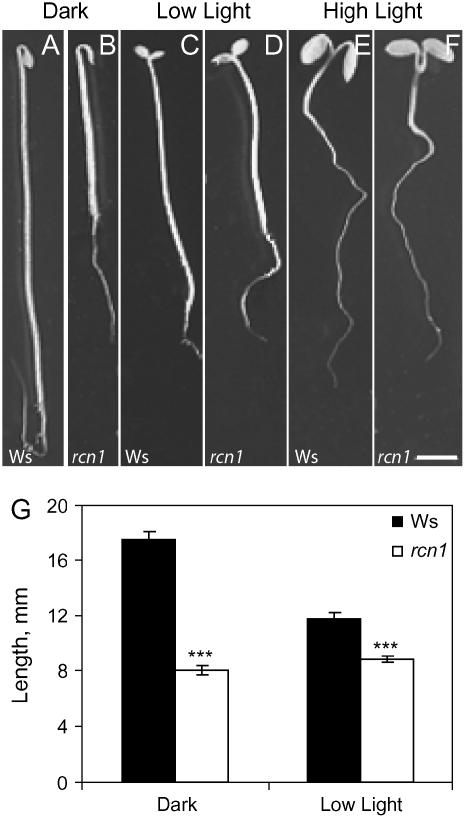
The effect of the rcn1 mutation on hypocotyl phenotype was examined in seedlings grown in the dark, in low light (8 μmol m−2 s−1), and in high light (100 μmol m−2 s−1; Fig. 1). As reported previously, etiolated hypocotyls of the rcn1 mutant exhibit profound phenotypic differences compared to wild-type seedlings, including thicker and shorter hypocotyls with a partial apical hook opening (Garbers et al., 1996; Deruère et al., 1999; Larsen and Chang, 2001). Dark-grown wild-type hypocotyls are approximately twice as long as rcn1 hypocotyls (Fig. 1G), and the average diameter of etiolated rcn1 hypocotyls (0.41 ± 0.01 mm) is significantly greater than that of the wild type (0.35 ± 0.01 mm; P < 0.0001 by Student's t test). In contrast, the rcn1 mutant phenotype is reduced when plants are grown in low light (8 μmol m−2 s−1). This dose of light is sufficient to cause hook opening and cotyledon expansion and greening, but still allows partial hypocotyl elongation in both wild type and rcn1 (Fig. 1, C and D). Wild-type hypocotyls are significantly shorter under low light than in the dark (P < 0.00001), whereas rcn1 length is unchanged (P > 0.05). Thus, hypocotyl elongation in rcn1 is only slightly less than wild type when plants are grown in low light. Hypocotyl lengths of seedlings grown in high light (100 μmol m−2 s−1) are nearly identical (Fig. 1, E and F), as reported previously (Deruère et al., 1999). These results are consistent with the hypothesis that RCN1-containing PP2A promotes hypocotyl elongation in the dark but has little effect on hypocotyl elongation in light-grown plants.
Figure 1.
The rcn1 mutant phenotype is attenuated in light-grown seedlings. Photographs show 5-d-old wild-type (A, C, and E) and rcn1 mutant (B, D, and F) seedlings that were grown in darkness (A and B), low light (8 μmol m−2 s−1; C and D), or high light (100 μmol m−2 s−1; E and F). G, The lengths of 5-d-old hypocotyls grown in the dark and low light were quantified by analysis of digital images of hypocotyls. The values shown are averages (±se) for 20 seedlings from two separate experiments, and length for Ws and rcn1 were compared by Student's t test. ***, P < 0.0005. Scale bar = 2 mm.
We asked whether there were changes in RCN1 gene expression and phosphatase activity under these conditions (and in low light) that might offer an explanation for the dark-dependent growth phenotype described above. Previous experiments using a β-glucuronidase (GUS) reporter fusion and in situ hybridization analysis showed that RCN1 expression in 3-d-old etiolated hypocotyls is highest in a basal region of the hypocotyl in which cells are most rapidly elongating (Deruère et al., 1999). In contrast, RCN1 expression is fairly uniform in 3-d-old light-grown seedlings. Using the same pRCN1:GUS reporter lines, we observe a similar correlation with cell elongation patterns in 5-d-old dark- and high light-grown seedlings (Supplemental Fig. 1). In seedlings grown in low light, the only detectable GUS activity is in the middle of the hypocotyl, while the cotyledons and hypocotyl base and top are devoid of expression. These expression patterns indicate complex developmental differences in plants grown under these conditions, but provide no immediate explanation for the dark dependence of the rcn1 phenotype.
Similarly, PP2A activity differences in rcn1 hypocotyls under these light conditions do not account for the light modulation of the rcn1 phenotype. In an earlier experiment using phosphohistone as a substrate, we observed that PP2A activity is reduced in 3-d-old light- and dark-grown rcn1 hypocotyls (Deruère et al., 1999). To confirm and extend that result, we assayed protein phosphatase activities in extracts from hypocotyls of 6-d-old seedlings grown in the dark, low light, and high light (Supplemental Fig. 2). Under all light conditions, PP2A activity is lower in rcn1 hypocotyls than in wild-type hypocotyls (P ≤ 0.002), and the magnitude of the reduction in PP2A activity is similar in each case. These data demonstrate a consistent regulatory effect of RCN1 on PP2A activity in hypocotyls under all three light conditions, suggesting that the control of light-grown hypocotyl elongation is uncoupled from RCN1 regulation of PP2A.
Etiolated But Not Light-Grown rcn1 Seedlings Overproduce Ethylene
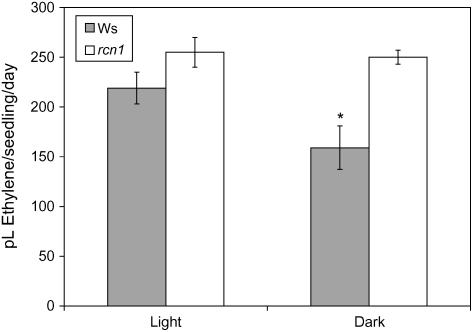
rcn1 seedlings were previously reported to overproduce ethylene in the dark, and blocking ethylene synthesis restored normal hypocotyl elongation (Larsen and Chang, 2001). However, light-dependent changes in ethylene biosynthesis have been reported for Arabidopsis (Guzman and Ecker, 1990; Vogel et al., 1998; Woeste et al., 1999; Vandenbussche et al., 2003). Therefore, we asked whether the near wild-type phenotypes of light-grown rcn1 hypocotyls might be correlated with normal ethylene production under these light conditions. We measured ethylene production by wild-type and rcn1-1 seedlings grown in the dark and in high light. As shown in Figure 2, dark-grown rcn1-1 seedlings produce elevated levels of ethylene as compared to wild-type seedlings, consistent with a prior report (Larsen and Chang, 2001). However, when grown in constant light, rcn1 and wild-type seedlings produce similar amounts of ethylene (Fig. 2). Strikingly, rcn1-1 seedlings produce ethylene at comparable levels in the dark and light, while wild-type seedlings show increased ethylene production when grown in constant light. These data indicate that ethylene overproduction in rcn1 is dark dependent. Furthermore, they implicate RCN1 in the negative regulation of ethylene biosynthesis in the dark.
Figure 2.
Ethylene synthesis is elevated in dark-grown rcn1 seedlings but not in light-grown seedlings. Ethylene levels were determined by gas chromatography for Ws and rcn1 seedlings grown in high light or darkness. There is significantly less ethylene synthesis (P < 0.05) in Ws in the dark than in either Ws in the light or rcn1 in the dark.
Auxin Transport Is Elevated in Dark-Grown rcn1 Hypocotyls
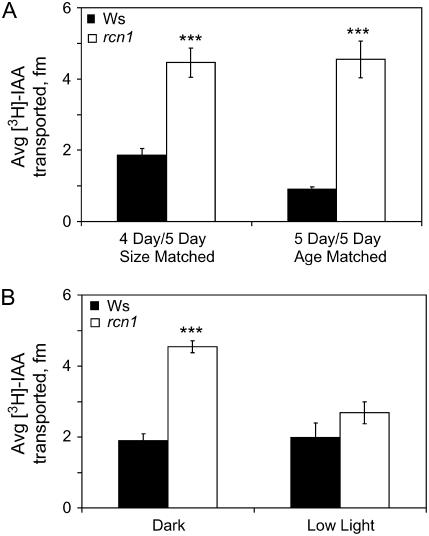
We assayed the effect of the rcn1 mutation on basipetal transport of 3H-IAA in the hypocotyl, as this has not been previously measured. When comparing IAA transport in the wild type and a mutant with altered hypocotyl elongation, two approaches can be used to control for differences in length. Seedlings can be used in transport assays at the same age, and transport can be compared at a constant distance from the site of IAA application, which may span a different range of tissues. Alternatively, the age of seedlings may be adjusted to yield hypocotyls that are matched in size, so that the amount of IAA transported into identical segments can be compared. These experiments were performed both ways comparing IAA transport in dark-grown hypocotyls of rcn1 and Wassilewskjia (Ws) matched for either age or size (Fig. 3A). Using both approaches, it is clear that the rcn1 hypocotyls exhibit a 2- and 4-fold increase in basipetal IAA transport compared to wild-type hypocotyls. The greater thickness of rcn1 hypocotyls may contribute to increased IAA transport; however, based on the diameters reported above, the cross-sectional area of an rcn1 hypocotyl is predicted to be only 1.4-fold larger than that of the wild type, while IAA transport increases 2.2- to 4-fold. Additionally, the elevated IAA transport in this tissue parallels the elevated IAA transport in intact rcn1 roots.
Figure 3.
IAA transport is elevated in dark-grown rcn1 seedlings. A, IAA transport was compared in dark-grown seedlings matched for age or for size. B, IAA transport was compared for dark- and low light-grown seedlings. Values shown represent averages (±se) for 30 to 40 seedlings from four separate experiments, and the differences between Ws and rcn1 under each condition were compared by Student's t test. ***, P < 0.0005.
Another important question is whether the elevation in IAA transport in rcn1 is light modulated, like the growth phenotypes and RCN1 expression patterns described above. For low light-grown hypocotyls, plants of similar age were used, because rcn1 hypocotyl length is near wild type under these conditions. We compared IAA transport in size-matched wild-type and mutant seedlings grown under low light or in the dark (Fig. 3B). Consistent with the other phenotypes, basipetal IAA transport differences between rcn1 and Ws are more profound in dark-grown seedlings. In this case, auxin transport in wild-type hypocotyls does not significantly change as a function of light level (P > 0.5) when the seedlings are matched for size (5-d dark-grown seedlings are compared to 7-d low light-grown seedlings). While transport is higher in etiolated rcn1 hypocotyls than Ws (P < 0.001), IAA transport is near wild-type levels in light-grown rcn1 seedlings. This is consistent with the RCN1 protein negatively regulating auxin transport in the dark.
An additional possibility is that the rcn1 mutation alters the response to auxin and thereby indirectly affects auxin transport. However, the dose-dependent growth inhibition by IAA is nearly identical in rcn1 and wild-type hypocotyls (Supplemental Fig. 3), suggesting that the rcn1 mutation does not alter IAA response, consistent with earlier experiments showing normal hypocotyl sensitivity to 2,4-dichlorophenoxyacetic acid in rcn1 seedlings (Garbers et al., 1996). We also examined expression of the auxin-responsive DR5-GUS reporter to test for altered auxin sensitivity and/or distribution in rcn1 hypocotyls. Under all conditions, we find that the DR5-GUS reporter is expressed at low levels in wild-type and rcn1 mutant shoots (Supplemental Fig. 4). These data provide no support for the hypothesis that rcn1 hypocotyl phenotypes reflect altered responses to endogenous auxin levels.
Increased Gravitropic Curvature in Etiolated rcn1 Hypocotyls
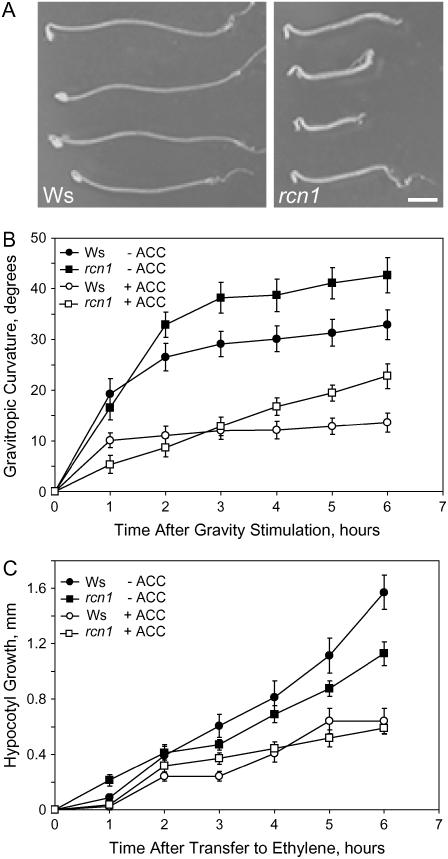
The effect of the rcn1 mutation on hypocotyl gravitropism has not been previously reported. We assayed the curvature of size-matched etiolated seedlings (Fig. 4). In the first hour after seedlings are reoriented by 90°, gravitropic curvature is similar in wild type and rcn1. The rate of curvature slows after 1 to 2 h in wild-type seedlings, but remains high in rcn1 seedlings until between 2 and 3 h after gravitropic stimulation. The net result is a faster gravitropic response in rcn1 hypocotyls (Fig. 4B). Consistent with this kinetic analysis, etiolated rcn1 hypocotyls achieve a greater angle of gravitropic curvature 6 h after reorientation than do wild-type hypocotyls (Table I). Because rcn1 hypocotyls elongate at a slower rate during this period (Fig. 4B), this increased bending is not the trivial result of faster growth.
Figure 4.
Hypocotyls of the rcn1 mutant exhibit enhanced gravity response in the absence or presence of ACC. Etiolated seedlings were transferred under a dim green safelight to agar plates containing 0 or 10 μm ACC at 5 d after germination. The plates were immediately reoriented by 90° relative to gravity and digital photographs were taken each hour for 6 h. Representative assay results 6 h after reorientation on medium without ACC are shown (A). Curvature (B) and elongation (C) were measured on sequential digital photographs. Each data point represents the average (±se) for 10 plants. Scale bar = 2 mm.
Table I.
Gravitropic responses of rcn1 and wild-type hypocotyls
| Angle of Curvaturea
|
P Valuesb | ||
|---|---|---|---|
| Ws | rcn1 | ||
| degrees | |||
| Etiolatedc | 34.6 ± 1.2 | 43.3 ± 2.4 | 0.0017 |
| Low lightd | 40.6 ± 2.8 | 40.0 ± 2.9 | 0.89 |
Gravitropic curvature of hypocotyls was measured 6 h after reorientation for etiolated seedlings and 24 h after reorientation for seedlings grown in low light.
P values were obtained by two-tailed Student's t test for equal variance and are the comparison of curvature in Ws versus rcn1.
Each value is the average ±se for at least 40 plants from four experiments.
Each value is the average ±se for at least 30 plants from three experiments.
In contrast, when seedlings are grown in low light, hypocotyls of both genotypes respond much more slowly to gravitropic reorientation, such that the curvature at 24 h is similar to that of dark-grown seedlings after 6 h (Table I). Under these conditions, we detect no difference in gravitropic responses of wild-type and rcn1 seedlings at 24 h (Table I), nor are differences observed at earlier time points after gravity stimulation (data not shown). Like the elongation and IAA transport phenotypes of rcn1 hypocotyls, the faster gravity response phenotype is reduced in the presence of light.
ACC Treatment Inhibits Hypocotyl Elongation and Gravity Response, But Not Auxin Transport
Initial characterizations of rcn1-1 (Garbers et al., 1996) and rcn1-2 (the eer1 allele; Larsen and Chang, 2001; Larsen and Cancel, 2003) indicated altered response of rcn1 seedlings to germination and growth in the presence of exogenous ethylene or the ethylene precursor, ACC. As ethylene inhibits hypocotyl elongation in dark-grown seedlings, enhanced response to ethylene has been suggested to account for the shorter hypocotyl in etiolated rcn1 seedlings (Larsen and Chang, 2001). The effect of ACC on hypocotyl gravitropism in rcn1 has not been examined previously.
We examined the short-term effect of ACC on growth and gravity response in rcn1 measured at 2 and 6 h after reorientation (Table II). Although there may be a short lag before ethylene synthesis increases in ACC-containing media, growth inhibition is already evident 1 h after transfer (Fig. 4). In the absence of ACC, wild-type seedlings have greater growth, but less curvature after gravity stimulation, than rcn1. ACC doses of 1 μm or more reduce both hypocotyl elongation and hypocotyl gravity response in wild-type hypocotyls with a dose-dependent effect, while a lower dose (0.1 μm) has no significant effect on either response. Short-term exposure to ACC inhibits hypocotyl elongation and gravity response similarly in rcn1 and wild-type seedlings, although both responses show slightly greater inhibition at 1 μm ACC in rcn1 hypocotyls. We also examined the effect of growth and gravity response of Ws and rcn1 seedlings in response to short-term treatment with 0.5 μLL−1 ethylene gas. We found similar results with these ethylene treatments, in that reductions in growth and gravity response were equivalent in rcn1 and Ws (data not shown). ACC and ethylene may inhibit gravity response directly or may block curvature indirectly by reducing elongation. Most importantly, in both the presence of ACC or ethylene, rcn1 plants grow more slowly than the wild type but still show a greater gravitropic response.
Table II.
ACC sensitivity of gravitropism and elongation in etiolated wild-type and rcn1 hypocotyls
| Growtha
|
Gravitropic Responsea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ecotype | ACC | 2 h
|
6 h
|
2 h
|
6 h
|
||||
| Elongation | Elongation | Curvature | Curvature | ||||||
| μm | mm | %b | mm | %b | degrees | %b | degrees | %b | |
| Ws | 0 | 0.40 ± 0.06 | 100 | 1.29 ± 0.11 | 100 | 23.3 ± 2.0 | 100 | 29.4 ± 2.0 | 100 |
| Ws | 0.1 | 0.35 ± 0.04 | 89 | 1.10 ± 0.07 | 85 | 23.6 ± 1.6 | 101 | 34.8 ± 2.1 | 118 |
| Ws | 1 | 0.29 ± 0.04 | 73 | 0.70 ± 0.09c | 54 | 16.3 ± 1.8c | 70 | 25.3 ± 1.7 | 86 |
| Ws | 10 | 0.23 ± 0.04c | 57 | 0.50 ± 0.05c | 39 | 11.1 ± 1.2c | 48 | 16.4 ± 1.2c | 56 |
| rcn1 | 0 | 0.37 ± 0.04 | 100 | 1.0 ± 0.05 | 100 | 29.5 ± 1.6 | 100 | 44.6 ± 1.8 | 100 |
| rcn1 | 0.1 | 0.30 ± 0.03 | 81 | 0.74 ± 0.05 | 73 | 25.4 ± 2.3 | 86 | 39.5 ± 2.2 | 89d |
| rcn1 | 1 | 0.19 ± 0.02c | 52d | 0.37 ± 0.03c | 37d | 15.0 ± 1.5c | 51d | 27.7 ± 1.9c | 62d |
| rcn1 | 10 | 0.25 ± 0.03c | 67 | 0.45 ± 0.03c | 45 | 12.5 ± 1.2c | 42 | 22.7 ± 1.2c | 51 |
The average and se of 30 seedlings from three separate experiments are reported. Seedlings were grown for 5 d before transfer to control or ACC containing media and were immediately reoriented 90° relative to gravity.
The elongation and curvature values were normalized to untreated controls in the same genotype with no added ACC.
P < 0.05 for comparison of the growth or gravity response in ACC treatments to the untreated control for each genotype using a Student's t test.
P < 0.05 for comparison of the growth or gravity response in rcn1 to Ws normalized to the untreated control of each genotype at each ACC dose using a Student's t test.
To provide greater insight into the responses to ACC, we performed a kinetic analysis of growth and gravitropic bending in seedlings grown on agar containing 0 and 10 μm ACC (Fig. 4). Treatment with 10 μm ACC inhibits the initial rate of curvature of both wild-type and rcn1 seedlings. As in the preceding experiment, the gravity response of rcn1 hypocotyls remains greater than that of the wild type in the absence of ACC, but also in the presence of ACC. Both genotypes exhibit about 50% inhibition of curvature at 10 μm ACC, relative to the untreated controls. ACC at 0.1 μm has no inhibitory effect on gravity response in wild-type or mutant seedlings, and in many, but not all, experiments this dose increases gravitropic bending of wild type. Treatment with 1.0 μm ACC produces a weaker inhibitory effect on the rate of curvature than 10 μm in both genotypes (data not shown). These results are consistent with similar effects of ACC on gravity response in rcn1 and wild-type seedlings.
To further explore the relationship between ethylene response and auxin transport in rcn1, we examined the effect of ACC on hypocotyl IAA transport in wild-type and rcn1 seedlings. We asked whether ACC modulates IAA transport in wild-type hypocotyls and whether there are differences in ACC's effect on IAA transport in rcn1. At all ACC doses, transport in rcn1 is greater than in wild-type seedlings, and both genotypes show only subtle changes in IAA transport in response to added ACC (Supplemental Fig. 5). This result suggests that under these growth and assay conditions, IAA transport is not regulated by ACC or the resulting ethylene synthesis. Furthermore, ACC does not affect root basipetal IAA transport, which is measured in intact and unwounded root tissues (Buer et al., 2006).
EIN2, But Not ETR1, Is Required for Maximal Hypocotyl Gravitropism
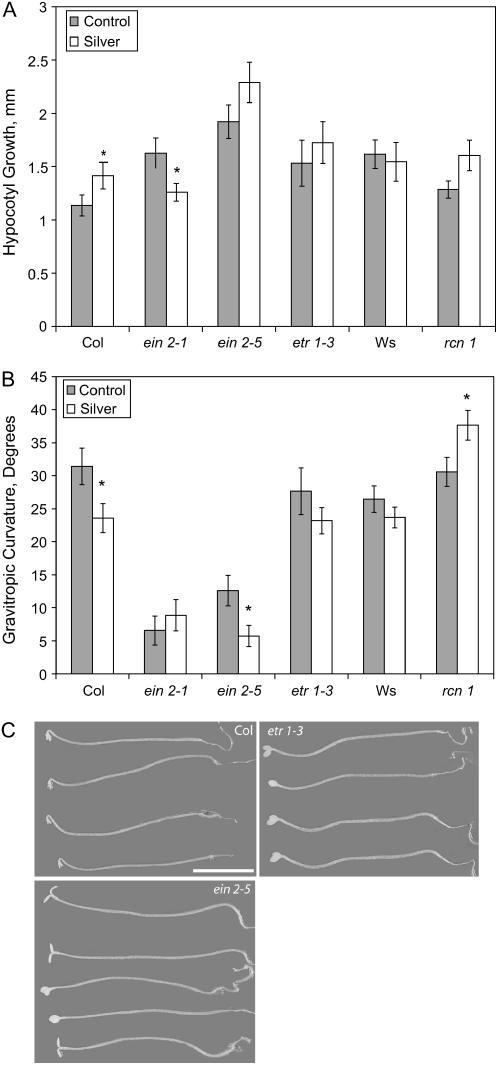
We examined the gravitropic response of seedlings that carry mutations in ethylene-signaling components and seedlings that were treated with the ethylene-signaling inhibitor, silver nitrate. We used the ethylene insensitive mutants, ethylene resistant1-3 (etr1-3), ethylene insensitive2-1 (ein2-1), and ein2-5, and compared their hypocotyl growth and gravitropic responses to those of wild type and rcn1 in the presence and absence of silver nitrate (Fig. 5). As reported previously, both silver nitrate and mutations that block ethylene signaling significantly increase the elongation of hypocotyls (Guzman and Ecker, 1990; Kieber et al., 1993). Unlike elongation, gravity response is not statistically altered in etr1-3 (P > 0.05). In contrast, silver treatment reduced gravity response in Columbia (Col) seedlings and significantly increased the gravitropic response in rcn1 seedlings (P < 0.05), indicating that ethylene signaling is not required for the enhanced gravity phenotype in rcn1.
Figure 5.
Reduced ethylene perception enhances growth but does not impair gravitropic response. Seedlings were grown for 4 d in the dark and then transferred to fresh control media or media supplemented with 100 nm silver nitrate. After 18 h, seedlings were reoriented 90° relative to gravity and the amount of growth (A) and the gravitropic response (B) was quantified 6 h after reorientation. The average and se of 25 to 35 seedlings from three separate experiments are reported. C, Representative images of the seedlings after 6 h are shown. Student's t tests were used to determine statistical differences between growth and gravity response between untreated and silver-treated seedlings within genotypes (white bars). *, P < 0.05. Scale bar = 5 mm.
Surprisingly, both ein2-1 and ein2-5 exhibit striking gravitropic defects (P < 0.0005). The kinetics of the ein2-5 and ein2-1 hypocotyl gravitropic responses were examined and a delay is evident at all times between 1 and 24 h after reorientation (data not shown). A partial growth randomization phenotype has been noted previously for the ein2-1 mutant (Golan et al., 1996). This result is consistent with a second, alternative mode of EIN2 action that is independent of ethylene signaling, as previously proposed (Gazzarrini and McCourt, 2003).
Ethylene Signaling Is Not Required for Enhanced Gravity Response in rcn1
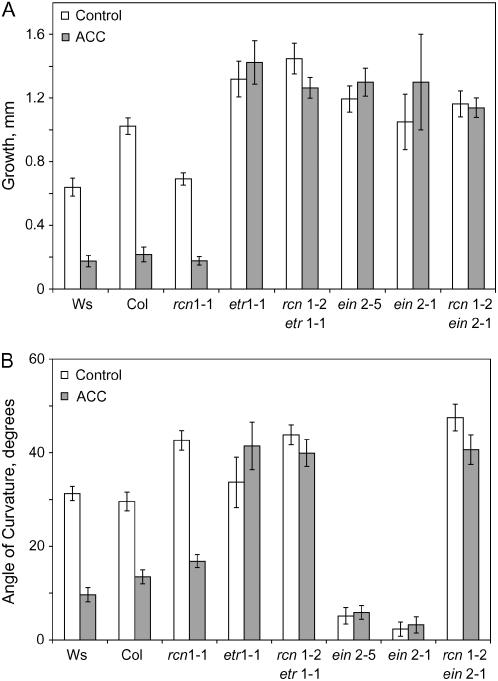
To determine whether rcn1 phenotypes depend on an intact ethylene response, we compared the growth and gravity responses of ethylene insensitive rcn1-2 ein2-1 and rcn1-2 etr1-1 double mutants (Larsen and Chang, 2001) 6 h after transfer to media containing ACC (Fig. 6). In the presence of ACC, wild-type seedlings (Col and Ws) and rcn1-2 are significantly inhibited in growth (P < 0.0005), while the ein2-5, etr1-1, ein2-1 rcn1-2, and etr1-1 rcn1-2 mutants show the expected insensitivity to ACC (P > 0.05). Thus, the reduced elongation of rcn1 hypocotyls appears to be ethylene dependent, as reported previously (Larsen and Chang, 2001). Similarly, ACC statistically reduces gravitropism in Col, Ws, and rcn1-1 (P < 0.0005).
Figure 6.
Ethylene resistance does not impede rcn1 hypocotyl gravity response. Seedlings of the indicated genotypes were grown in the dark for 5 d and transferred to media with or without ACC. Each value represents the average (±se) for growth and gravity response 6 h after transfer and reorientation with two or three separate experiments averaged (n = 17 to 30 seedlings).
Strikingly, however, gravity responses of the ein2-1 rcn1-2 and etr1-1 rcn1-2 double mutants resemble those of the rcn1 parent and are clearly different from the etr1 and ein2 parents (Fig. 6B; P < 0.0005). In the absence of added ACC, etr1-1 responds to reorientation with a gravitropic curvature that is not significantly different from wild type (P > 0.05). As described above, the ein2-1 and ein2-5 mutants have a drastically reduced gravity response relative to Col (P < 0.0005). The reduction in gravitropic response of the two ein2 alleles in this assay is even more dramatic than shown above due to small differences in germination conditions in this experiment.
The most important finding is that gravitropic curvature in the rcn1-2 etr1-1 and rcn1-2 ein2-1 double mutants is significantly enhanced in the absence of ACC relative to wild type (Fig. 6B; P < 0.0005), resembling the rcn1 single mutant parent, rather than the etr1 or ein2 parents. This result is consistent with the enhanced gravitropic response in silver-treated rcn1 plants observed above (Fig. 5). As expected, gravity responses of wild-type and rcn1 seedlings are inhibited by ACC (P < 0.0005), while ein2-5, ein2-1, etr1-1, and both double mutants show no significant reductions in response by ACC (P > 0.05). Thus, gravitropic responses in rcn1-2 etr1-1 and rcn1-2 ein2-1 double mutants resemble the response of the rcn1 single mutant but are ethylene insensitive. These results show that the enhanced gravitropic curvature of rcn1 does not require an intact ethylene response pathway.
DISCUSSION
The goal of this work was to characterize the role of RCN1-regulated PP2A activity in controlling hypocotyl growth and gravitropism. We also aimed to dissect the interplay between regulation of auxin transport and ethylene signaling and synthesis, as each of these processes involves potential targets for PP2A activity and is thought to influence growth and gravity response. Previous work has demonstrated roles for RCN1-regulated PP2A activity in control of auxin transport in roots (Garbers et al., 1996; Deruère et al., 1999; Rashotte et al., 2001; Shin et al., 2005). Like roots, etiolated hypocotyls of rcn1 also have altered growth characteristics and sensitivity to auxin transport inhibitors (Garbers et al., 1996; Deruère et al., 1999; Larsen and Chang, 2001), suggesting that the RCN1 protein regulates responses in multiple tissues. However, a detailed characterization of auxin transport and dependent physiological processes in rcn1 hypocotyls had not been performed.
RCN1 Regulates Auxin Transport and Gravity Response in Shoots and Roots
We found that IAA transport and gravitropism were both increased in dark-grown rcn1 hypocotyls. Because a previous report indicated that rcn1 exhibited defects in hypocotyl growth in dark-, but not light-grown seedlings (Deruère et al., 1999), we examined auxin transport in seedlings grown in high and low light, in addition to darkness. Like the hypocotyl elongation phenotype, the altered auxin transport and gravitropic response phenotypes are observed only in dark-grown rcn1 seedlings.
As in the root tip, RCN1-controlled PP2A activity appears to act as a negative regulator of basipetal auxin transport (Rashotte et al., 2001). Paradoxically, loss of RCN1 function impedes gravitropic response in roots but enhances curvature in hypocotyls. Consideration of the differences in gravity response mechanisms in these two tissues suggests a hypothesis to explain the apparent contradiction. Roots sense gravity very locally in the columella cells in the root cap (Blancaflor et al., 1998), and auxin is redistributed from the root tip to one side of the root after gravity stimulation rather than being laterally transported across the root tip (Blancaflor and Masson, 2003). In roots, uniformly increased basipetal transport may impede the redistribution of auxin at the root tip, which is required to form a lateral auxin gradient and to achieve maximal gravitropic bending (Rashotte et al., 2001). In contrast, gravity perception in stems occurs in the starch sheath parenchyma tissues that run the length of the hypocotyl (Fukaki et al., 1998). Lateral auxin transport then is believed to occur in multiple tissues along the length of the hypocotyl (Blancaflor and Masson, 2003). Increased basipetal auxin transport would provide more auxin to the lateral transport stream and would thereby increase gravitropic bending. In contrast, the mdr1 mutant has reduced hypocotyl IAA transport (Noh et al., 2001) but has enhanced gravi- and phototropic responses. These differences may be due to specific effects of the mdr1 mutation on transporter localization or function (Noh et al., 2003) rather than the rcn1 mutation, which affects bulk polar auxin flow.
Although auxin transport is also regulated by IAA (Paponov et al., 2005), our data suggest that increased transport in rcn1 does not involve altered IAA response. Hypocotyls of rcn1 exhibit normal inhibition by exogenous IAA and show a wild-type DR5-GUS expression pattern (Supplemental Figs. 3 and 4).
Light Modulates the rcn1 Phenotype and Ethylene Synthesis
Etiolated rcn1 hypocotyls exhibit four striking phenotypes that are largely suppressed in light-grown seedlings. Overall hypocotyl elongation is strongly reduced, ethylene synthesis is increased, basipetal IAA transport is increased, and gravity response is increased in dark-grown, but not light-grown rcn1 seedlings. Suppression of these phenotypes by light does not involve production of an RCN1-independent PP2A enzyme, because activity assays show similar reductions in PP2A activity in seedlings from high light, low light, and dark-growth regimens (Supplemental Fig. 3) and light does not greatly reduce the overall accumulation of RCN1 protein (Zhou et al., 2004). The RCN1-GUS expression pattern suggests that local abundance of RCN1, i.e. strong accumulation in rapidly elongating cells (Supplemental Fig. 1), may be critical for promoting elongation in etiolated seedlings. Alternatively, light modulation of the rcn1 phenotype may be dependent upon the target(s) of PP2A dephosphorylation.
The etiolated growth phenotype of rcn1 is likely due to the elevated ethylene synthesis that is only found in dark-grown seedlings. Interestingly, while ethylene synthesis in the wild type is low in etiolated seedlings and increases in light-grown plants, ethylene production is high in both dark and light in rcn1, suggesting that RCN1 may negatively regulate ethylene synthesis in dark-grown seedlings. Consistent with this hypothesis, inhibition of protein phosphatase action increases the activity of at least one ACS isozyme, apparently by increasing the enzyme's stability (Spanu et al., 1994; for review, see Chae and Kieber, 2005). This result suggests a mechanism by which ACS activity and resulting ethylene synthesis may be increased in dark-grown rcn1 seedlings.
PP2A Activity Modulates Hypocotyl Elongation in Etiolated Seedlings through Ethylene Synthesis
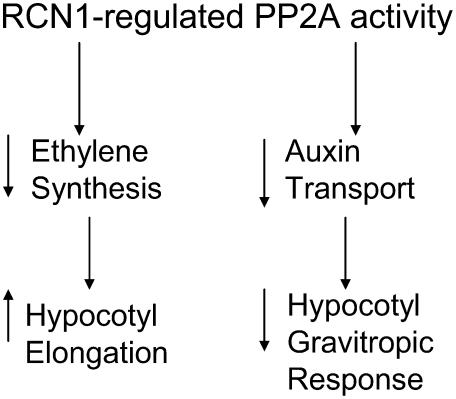
Our results are consistent with the hypothesis that the reduced elongation of etiolated rcn1 hypocotyls results from elevated ethylene synthesis and consequent inhibition of growth. Phosphatase inhibitor treatment enhances hypocotyl ethylene response in wild-type seedlings, producing a phenocopy of rcn1 (Larsen and Cancel, 2003). This result demonstrates that reduced PP2A activity in rcn1 impairs hypocotyl growth. The rcn1 hypocotyl elongation defect is reduced by the ethylene biosynthesis inhibitor aminoethoxyvinylglycine (Larsen and Chang, 2001), by the ethylene signaling inhibitor silver (Fig. 5A), and by mutations conferring ethylene insensitivity (Fig. 6A; Larsen and Chang, 2001). This growth effect of ethylene appears to be independent of auxin transport, as ACC treatment had little effect on IAA transport in either mutant or wild-type seedlings (Supplemental Fig. 5). Our results suggest a model in which RCN1-regulated PP2A activity acts through two parallel pathways (Fig. 7), modulating hypocotyl elongation via regulation of ethylene synthesis and gravitropism via regulation of auxin transport.
Figure 7.
Roles of RCN1-regulated PP2A activity in etiolated hypocotyls. RCN1-regulated PP2A activity controls hypocotyl elongation and gravity response through genetically separable pathways in dark-grown seedlings. PP2A activity controls elongation through a pathway requiring regulated ethylene biosynthesis and response, while PP2A control of gravity response involves regulation of auxin transport but is independent of ethylene response.
Although rcn1 hypocotyls exhibit slightly enhanced response to one ACC dose (1 μm), overall sensitivity to short-term ACC treatment is very similar in wild-type and rcn1 mutant hypocotyls (Table II), and rcn1 hypocotyls also show a normal response to ethylene treatment (data not shown). Our conclusion that rcn1 hypocotyls exhibit near-normal ethylene sensitivity contrasts with a previous report indicating that rcn1 hypocotyl elongation shows enhanced ethylene sensitivity (Larsen and Chang, 2001). In the previous report, hypocotyl elongation was measured after continuous growth in the presence of ACC (Larsen and Chang, 2001) rather than after a short-term ACC exposure, as used in this work. Recent work has clearly separated rapid and long-term growth effects of ethylene and has shown that these are mediated by different ethylene-signaling pathways (Binder et al., 2004a, 2004b). It is possible that the results reported here and the results of Larsen and Chang (2001) represent effects on the rapid and slower phases of growth, respectively. However, both reports are consistent with increased ethylene synthesis in the rcn1 mutant contributing to the inhibition of hypocotyl elongation. This hypothesis is further supported by the coordinate changes in elongation and ethylene synthesis we observe in etiolated and light-grown hypocotyls.
Elevated Ethylene Reduces Hypocotyl Gravity Response in Wild-Type Arabidopsis
The effect of ACC on gravitropic bending in etiolated Arabidopsis hypocotyls has not been well characterized previously. One earlier report noted a slight growth-randomizing effect of 1 mm ACC (Golan et al., 1996), but ACC doses ranging from 0.1 to 100 μm do not cause growth randomization in wild-type seedlings (Harper et al., 2000). Ethylene treatment has yielded inconsistent results in other plant species and tissues, clearly reducing gravitropic responses in some experiments (Wheeler and Salisbury, 1981; Wheeler et al., 1986; Lee et al., 1990; Kiss et al., 1999; Madlung et al., 1999), while in others it produced no effect (Kaufman et al., 1985; Harrison and Pickard, 1986; Woltering, 1991). A recent study indicated that ethylene inhibits elongation but increases gravitropic curvature in etiolated maize (Zea mays) roots (Chang et al., 2004). Several studies reported that low concentrations of ethylene affect the rate of gravitropic curvature, but not the final angle of orientation after gravitropic stimulation (Wheeler and Salisbury, 1981; Wheeler et al., 1986; Lee et al., 1990). The lack of kinetic data at early time points after gravitropic stimulation in several of these experiments may explain negative results (Kaufman et al., 1985; Harrison and Pickard, 1986; Woltering, 1991).
ACC treatment reduced the rate of hypocotyl gravitropic curvature in the first hours after gravity stimulation, with smaller effects on the later rate of curvature (Fig. 4). Inhibition is partial and shows a weak dose dependence in both wild-type and mutant seedlings. If wild-type seedlings are grown for multiple days on ACC before reorientation, the effect of ACC on gravitropic curvature is much weaker, consistent with an adaptation to the high levels of ethylene (data not shown). This may account for the lack of an ACC effect in earlier studies (Harper et al., 2000). In contrast, ethylene inhibition of elongation is maintained during long-term ACC exposure. Together, our data suggest that excess ethylene has a weak inhibitory effect on hypocotyl gravitropic response, with a much stronger inhibitory effect on hypocotyl elongation. Despite overproduction of ethylene, rcn1 seedlings exhibit enhanced gravitropic response. Our working model (Fig. 7) posits that the hypocotyl gravitropic phenotype of rcn1 seedlings involves different PP2A substrates than those responsible for the elongation defect.
Enhanced Gravitropic Response in rcn1 Is Not Mediated by Ethylene-Signaling Pathways
We examined the ethylene dependence of the rcn1 gravitropic phenotype. The rcn1-2 etr1-1 and rcn1-2 ein2-1 mutants have gravity responses that are identical to the rcn1 single mutant. Additionally, silver treatment of rcn1 seedlings further enhanced the gravity response, consistent with the enhanced gravitropic phenotype of rcn1 being independent of ethylene signaling. These results show that an intact ethylene signaling pathway is not required for the enhancement of gravity response in rcn1 hypocotyls and suggest that RCN1 may act downstream of EIN2 in gravitropic response.
We also assayed the effect of ACC treatments on the kinetics of gravity response in wild-type and rcn1 hypocotyls in parallel to the effects of ACC on hypocotyl elongation. Hypocotyl gravity response has a similar ACC dose response curve in rcn1 and wild-type seedlings. Although we have not ruled out an enhanced ethylene response in rcn1, as reported previously (Larsen and Chang, 2001; Larsen and Cancel, 2003), we did not find a consistent enhancement of ACC effects on either elongation or gravitropic response in rcn1. Only one ACC dose (1 μm) caused a more profound inhibition of elongation and gravity response in rcn1 hypocotyls. Together these results suggest that enhanced hypocotyl auxin transport and gravity response in rcn1 are independent of the enhanced ethylene synthesis and growth inhibition of this mutant.
EIN2 Is Required for Gravitropic Curvature But May Act in an Ethylene-Independent Fashion
Few reports in the literature have used mutants altered in ethylene signaling and/or synthesis to test for a role of ethylene response in gravitropic curvature. Although the eir1 mutant was isolated based on an ethylene-insensitive root elongation phenotype and later shown to have delayed root gravitropism, the primary defect in this mutant is linked to auxin transport (Luschnig et al., 1998). The delayed shoot gravitropism of the tomato (Lycopersicon esculentum) mutants never ripe (NR) and epi, are consistent with a role for ethylene in the early events of gravitropic response (Madlung et al., 1999). NR encodes an ETR1 homolog that is essential in fruit ripening, while epi overproduces ethylene (Klee and Tieman, 2002). The NR mutant is insensitive to the effect of exogenous ethylene on hypocotyl gravitropism (Madlung et al., 1999).
We examined the gravitropic response of plants with either genetic or chemical reductions in ethylene signaling to understand the role of ethylene signaling in this response. Surprisingly, this analysis revealed that EIN2 is required for hypocotyl gravitropic response. Both ein2-1 and ein2-5 single mutants are almost completely agravitropic, while etr1 mutant seedlings show no significant changes in hypocotyl gravity response. Furthermore, the inhibitory effect of ACC on hypocotyl gravitropism is lost in both ein2 and etr1, indicating that the effect of ACC is mediated by ETR1- and EIN2-dependent signaling. The agravitropic phenotype of ein2 is hypocotyl specific, as roots of both ein2 and etr1 have wild-type gravitropic responses (Roman et al., 1995; Buer et al., 2006).
The observation that etr1-1, unlike ein2, exhibits nearly normal gravitropism can be explained by two alternative models. This result may be consistent with a low level of residual ethylene signaling in etr1, possibly because etr1 affects the function of a subset of ethylene receptors. Alternatively, EIN2 may act through an ethylene independent pathway. To explore the first possibility further, we treated seedlings with doses of silver nitrate that reduces ethylene signaling. The silver treatment was effective in increasing hypocotyl elongation, but wild-type, etr1, and rcn1 seedlings did not exhibit the profound gravity defects associated with ein2. Our data therefore are more consistent with EIN2 acting in two pathways, including a separate pathway independent of ethylene signaling, as suggested previously (Gazzarrini and McCourt, 2003).
CONCLUSION
The complex interactions between auxin, ethylene, and light signaling require careful consideration in proposing mechanisms by which the RCN1 protein regulates hypocotyl growth and gravitropism. Several results point to ethylene overproduction as a key factor in the reduced elongation of etiolated rcn1 hypocotyls, while the increased auxin transport and gravity response phenotypes do not require ETR1- or EIN2-dependent ethylene signaling pathways. Furthermore, we have uncovered a role for EIN2 in controlling hypocotyl gravity response that is unique to this tissue and appears to be independent of the EIN2 role in mediating ethylene signaling. Future experiments will examine the EIN2-dependent gravity pathway and the apparent epistasis between ein2 and rcn1 in controlling gravitropic responses.
MATERIALS AND METHODS
Chemicals
NPA was purchased from Chemical Services. 5-Bromo-4-chloro-3-indolyl-β-d-GlcUA cyclohexylamine salt was purchased from Research Products International. Absolute ethanol was purchased from McCormick Distilling. Three to 5(n)-3H-IAA was purchased from Amersham (26 Ci/mmol) and from American Radiolabeled Chemicals (20 Ci/mmol). All other chemicals were purchased from Sigma.
Seed Germination and Plant Growth
Wild-type Arabidopsis (Arabidopsis thaliana) seed, ecotype Ws, and rcn1-1 were as used previously (Rashotte et al., 2001). The rcn1-2 allele, etr1-1, ein2-5, and the rcn1-2 etr1-1 and rcn1-2 ein2-1 double mutants (Larsen and Chang, 2001) were generously provided by Paul Larsen, and the ein2-1 mutant was obtained from the Arabidopsis stock center. Seeds were soaked in distilled water for 30 min and surface sterilized with 95% (v/v) ethanol for 5 min and 20% (v/v) bleach with 0.01% (v/v) Triton X-100 for 5 min. After five washes in sterile distilled water, seeds were germinated and grown on 9-cm petri plates containing sterile control medium (0.8% [w/v] agar [Sigma Type M, plant tissue culture], 1× Murashige and Skoog salts, pH 6.0, 1.5% [w/v] Suc, 1 μg mL−1 thiamine, 1 μg mL−1 pyridoxine HCl, and 0.5 μg mL−1 nicotinic acid). Seeds were pretreated in vertically oriented petri dishes with 18 h of continuous fluorescent light (90 μmol m−2 s−1) at room temperature (22°C) to ensure germination prior to moving into the dark for an additional 4 d in experiments involving etiolated hypocotyls. For PP2A assays, ethylene measurements, and double mutant analyses, seedlings were treated with 2 to 4 d of 4°C exposure to synchronize germination before returning to room temperature.
All experimental manipulation of etiolated hypocotyls were performed under green light at 2 μmol m−2 s−1 by filtering fluorescent light through green acrylic filter (ACR no. 2092). Hypocotyls used in experiments involving low-light conditions were grown on vertically oriented petri plates in a box with a neutral density filter on the top to reduce the fluorescent light intensity to 8 μmol m−2 s−1 at room temperature (22°C) for 5 d. Fluence rates were measured with a quantum meter (model BQM, Spectrum Technologies). Images of plants used for the phenotypic analysis were captured by Sony DSC-F505v digital camera. These electronic images were then used to quantify length and curvature using Adobe Photoshop.
Hypocotyl IAA Transport Assays
The hypocotyl basipetal IAA transport assay is modified from Rashotte et al. (2003). Transport was measured in etiolated rcn1 seedlings at 5 d after planting and in etiolated wild-type (Ws) seedlings at both 4 and 5 d after planting to allow seedlings to be matched for age and size. Low light-grown rcn1 and Ws seedlings were used 7 d after planting. Seedlings were transferred to either control plates, cotyledons were removed, and the tops aligned vertically on the plate, 1 h prior to the assay. Agar cylinders containing 100 nm 3H-IAA were applied to the tops of the hypocotyls and 3H-IAA transport was measured after 5 h by cutting a 5-mm segment from the bottom of the hypocotyl as described previously (Rashotte et al., 2003).
Analysis of Hypocotyl Gravity Response and Growth
Gravity response was measured in etiolated seedlings matched for size using 4-d-old Ws and 5-d-old rcn1. For seedlings grown in low light, 7-d-old seedlings were used for both genotypes. Seedlings were transferred to control plates with the apical hooks facing the same direction, such that the hook opening opposed the gravitropic curvature direction. Initial digital photographs were taken using a Sony Cybershot DSC-505v and the plates were then oriented 90° relative to the gravity vector with the apical hooks facing down so that hook opening and gravity response occurred in the same direction, and then placed in the dark. After 6 h, digital photographs were taken again and the images were analyzed for growth and angle of curvature in Adobe Photoshop. For the kinetic analysis of gravitropism in etiolated seedlings, photos of the plates were taken every hour under green light, maintaining the orientation of the plate, and then returning the plates immediately to the dark.
Gravitropism and growth assays were also carried out in the presence of ACC. Plants were germinated as described above and transferred to 1× Murashige and Skoog plates containing the indicated concentrations of ACC. For ethylene insensitive single and double mutants, plants were grown for 5 d and reoriented immediately after transfer to fresh media or media supplemented with 10 μm ACC. Assays were performed as described above and the new growth or angle of curvature was measured after 6 h.
For treatment with silver nitrate to inhibit ethylene signaling, the plants were transferred 4 d after plating to control media or media supplemented with 100 nm silver nitrate (17 μg mL−1). After 18 h, seedlings were reoriented relative to gravity by 90°, and the gravitropic angle was measured 6 h after reorientation.
Measurement of Ethylene Production
Ws-0 and rcn1 seed sterilization and ethylene measurements were conducted as described previously (Vogel et al., 1998). Arabidopsis seedlings were grown on 0.5× Murashige and Skoog medium containing 1% (w/v) Suc in 22-mL gas chromatography vials. Vials were incubated at 4°C for 6 d, light induced for 6 h, and then capped and incubated for 3 d at 23°C in the dark or under constant light. The accumulated ethylene was measured by gas chromatography as described by Vogel et al. (1998).
Supplementary Material
This work was supported by the National Aeronautics and Space Agency (grant no. NAG2–1507), by the Wake Forest University Science Research Fund and Research and Publication Funds (to G.K.M.), by the National Institute of Health (grant no. GM064425 to J.J.K.), and by the National Science Foundation (grant nos. IOB 0135458 and IOB 0446039 to A.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alison DeLong (alison_delong@brown.edu).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.083212.
References
- Abel S, Nguyen M, Chow W, Theologis A (1995) ASC4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. J Biol Chem 270: 19093–19099 [DOI] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057–4067 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8: 505–520 [Google Scholar]
- Bernasconi P (1996) Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiol Plant 96: 205–210 [Google Scholar]
- Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB (2004. a) Short-term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiol 136: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O'Malley RC, Wang W, Moore JM, Parks BM, Spalding EP, Bleecker AB (2004. b) Arabidopsis seedling growth response and recovery to ethylene: a kinetic analysis. Plant Physiol 136: 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee JJ, Peer WA, Murphy AS (2005) Auxin transport. Curr Opin Plant Biol 8: 494–500 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116: 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Masson PH (2003) Plant gravitropism: unraveling the ups and downs of a complex process. Plant Physiol 133: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer C, Sukumar P, Muday G (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis thaliana. Plant Physiol 140: 1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16: 1191–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HS, Kieber JJ (2005) Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci 10: 291–296 [DOI] [PubMed] [Google Scholar]
- Chang S, Kim Y-S, Lee J, Kaufman P, Kirakosyan A, Yun H, Kim T-W, Kim S, Cho M, Lee J, et al (2004) Brassinolide interacts with auxin and ethylene in the root gravitropic response of maize (Zea mays). Physiol Plant 121: 666–673 [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100: 469–478 [DOI] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Guern J (1998) Short-lived and phosphorylated proteins contribute to carrier-mediated efflux, but not to influx, of auxin in suspension-cultured tobacco cells. Plant Physiol 116: 833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong A, Mockaitis K, Christensen S (2002) Protein phosphorylation in the delivery of and response to auxin signals. Plant Mol Biol 49: 285–303 [PubMed] [Google Scholar]
- Deruère J, Jackson K, Garbers C, Söll D, DeLong A (1999) The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant J 20: 389–399 [DOI] [PubMed] [Google Scholar]
- Friml J (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Friml J, Wiœniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, et al (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M (1998) Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 14: 425–430 [DOI] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruère J, Bernasconi P, Soll D (1996) A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J 15: 2115–2124 [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S, McCourt P (2003) Cross-talk in plant hormone signalling: what Arabidopsis mutants are telling us. Ann Bot (Lond) 91: 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Blakeslee J, Bouchard R, Lee O, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer W, Bailly A, Richards EL, et al (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44: 179–194 [DOI] [PubMed] [Google Scholar]
- Golan A, Tepper M, Soudry E, Horwitz BA, Gepstein S (1996) Cytokinin, acting through ethylene, restores gravitropism to Arabidopsis seedlings grown under red light. Plant Physiol 112: 901–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MA, Pickard BG (1986) Evaluation of ethylene as a mediator of gravitropism by tomato hypocotyls. Plant Physiol 80: 592–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P, Pharis RP, Reid DM, Beall FD (1985) Investigations into the possible regulation of negative gravitropic curvature in intact Avena sativa plants and in isolated stem segments by ethylene and gibberellins. Physiol Plant 65: 237–244 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Edelmann RE, Wood PC (1999) Gravitropism of hypocotyls of wild-type and starch-deficient Arabidopsis seedlings in spaceflight studies. Planta 209: 96–103 [DOI] [PubMed] [Google Scholar]
- Klee H, Tieman D (2002) The tomato ethylene receptor gene family: form and function. Physiol Plant 115: 336–341 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Cancel JD (2003) Enhanced ethylene responsiveness in the Arabidopsis eer1 mutant results from a loss-of-function mutation in the protein phosphatase 2A A regulatory subunit, RCN1. Plant J 34: 709–718 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Chang C (2001) The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiol 125: 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Chang WK, Evans ML (1990) Effects of ethylene on the kinetics of curvature and auxin redistribution in gravistimulated toots of Zea mays. Plant Physiol 94: 1770–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A, Behringer FJ, Lomax TL (1999) Ethylene plays multiple nonprimary roles in modulating the gravitropic response in tomato. Plant Physiol 120: 897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P, Gausman H (1966) Effects of ethylene on auxin transport. Plant Physiol 41: 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6: 535–542 [DOI] [PubMed] [Google Scholar]
- Muday GK, Murphy AS (2002) An emerging model of auxin transport regulation. Plant Cell 14: 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS (2003) Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, et al (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Paponov I, Teal W, Trebar M, Blilou K, Palme K (2005) The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci 410: 170–177 [DOI] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) PIN gene expression and PIN localization are altered in flavonoid-deficient mutants. Plant Cell 16: 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, et al (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94: 1462–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Amakawa T, Goto N, Tsurumi S (2001) Auxin is a positive regulator for ethylene-mediated response in the growth of Arabidopsis roots. Plant Cell Physiol 42: 301–307 [DOI] [PubMed] [Google Scholar]
- Rashotte A, Chae H, Maxwell B, Kieber J (2005) The interaction of cytokinin with other signals. Physiol Plant 123: 184–194 [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13: 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Poupart J, Waddell CS, Muday GK (2003) Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol 133: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Shin HS, Guo Z, Blancaflor EB, Masson PH, Chen R (2005) Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases. Plant J 42: 188–200 [DOI] [PubMed] [Google Scholar]
- Spanu P, Grosskopf DG, Felix G, Boller T (1994) The apparent turnover of 1-aminocyclopropane-1-carboxylate synthase in tomato cells is regulated by protein phosphorylation and dephosphorylation. Plant Physiol 106: 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17: 2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle JC (1988) Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol 88: 795–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, May S, Williams L, Millner P, et al (2004) Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16: 3069–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Parry G, Graham N, Allen T, Bennett M (2002) Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol 49: 411–426 [DOI] [PubMed] [Google Scholar]
- Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, Lee OR, Richards EL, Murphy AS, Sato F, et al (2005) PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17: 2922–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Verbelen JP, Van Der Straeten D (2005) Of light and length: regulation of hypocotyl growth in Arabidopsis. Bioessays 27: 275–284 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJ, Harren FJ, Van Der Straeten D (2003) Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiol 133: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ (1998) Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA 95: 4766–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang NN, Shih MC, Li N (2005) The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J Exp Bot 56: 909–920 [DOI] [PubMed] [Google Scholar]
- Wheeler RM, Salisbury FB (1981) Gravitropism in higher-plant shoots. I. A role for ethylene. Plant Physiol 67: 686–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RM, White RG, Salisbury FB (1986) Gravitropism in higher-plant shoots. IV. Further studies on participation of ethylene. Plant Physiol 82: 534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste K, Vogel J, Kieber J (1999) Factors regulating ethylene biosynthesis in etiolated Arabidopsis thaliana seedlings. Physiol Plant 105: 478–484 [Google Scholar]
- Woltering EJ (1991) Regulation of ethylene biosynthesis in gravistimulated Kniphofia (hybrid) flower stalks. J Plant Physiol 138: 443–449 [Google Scholar]
- Woltering EJ, Balk PA, Nijenhuis-deVries MA, Faivre M, Ruys G, Somhorst D, Philosoph-Hadas S, Friedman H (2005) An auxin-responsive 1-aminocyclopropane-1-carboxylate synthase is responsible for differential ethylene production in gravistimulated Antirrhinum majus L. flower stems. Planta 220: 403–413 [DOI] [PubMed] [Google Scholar]
- Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A (2003) Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J Biol Chem 278: 49102–49112 [DOI] [PubMed] [Google Scholar]
- Yang S, Hoffman N (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35: 155–189 [Google Scholar]
- Zhou HW, Nussbaumer C, Chao Y, DeLong A (2004) Disparate roles for the regulatory A subunit isoforms in Arabidopsis protein phosphatase 2A. Plant Cell 16: 709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.