Abstract
The exogenous addition of salicylic acid (SA) was previously shown to inhibit indeterminate but not determinate-type nodulation. We sought to extend these results by modulating endogenous levels of SA through the transgenic expression of salicylate hydroxylase (NahG) in both stably transformed Lotus japonicus and composite Medicago truncatula plants. NahG expression in L. japonicus resulted in a marked reduction of SA levels. This reduction correlated with an increase in the number of infections and mean nodule number when compared to controls. However, a complicating factor was that NahG-expressing plants had greater root growth. Spot inoculations of NahG-expressing L. japonicus plants confirmed increased nodulation in these plants. Consistent with the reported inhibitory effects of exogenous SA on indeterminate-type nodulation, NahG expression in M. truncatula plants led to enhanced nodulation and infection. These data point to an important role for SA-mediated plant defense pathways in controlling nodule formation on both determinate and indeterminate nodule-forming hosts.
Salicylic acid (SA) is a phenolic compound made throughout the plant kingdom via the phenylpropanoid pathway. Research efforts over the past decade have studied this molecule to elucidate its many roles in plant physiology. Many reports have demonstrated that SA is a key molecule in plant disease resistance. Although the actual mechanism of SA's action is not understood, it is clear that SA is intimately involved in the induction of both the hypersensitive response (HR) and systemic acquired resistance (Durner et al., 1997; Feys and Parker, 2000). Studies adding exogenous SA to plants suggest that this compound can enhance defense gene induction, the production of hydrogen peroxide (H2O2), and cell death (Draper, 1997). Moreover, certain Arabidopsis (Arabidopsis thaliana) mutants produce elevated levels of SA and show constitutive expression of pathogenesis-related genes and in some cases HR lesion formation even in the absence of pathogen challenge (Shah et al., 2001). Conversely, plants that express the bacterial nahG gene, encoding salicylate hydroxylase, are unable to accumulate SA and are more susceptible to several pathogens (Gaffney et al., 1993). SA levels can also affect the interaction of plants with symbiotic microorganisms. For example, Medina et al. (2003) found that tobacco (Nicotiana tabacum) plants expressing NahG had enhanced mycorrhizal fungal infection, while plants constitutive for SA expression exhibited reduced infection.
Plant-interacting microbes differ with respect to the nature of the responses that they elicit in their respective hosts. In an incompatible plant-pathogen interaction, the host plant induces a defense response, either the HR, systemic acquired resistance, or both, that limits pathogen invasion and spread. However, in the case of symbiotic bacteria in the genera Rhizobium, Bradyrhizobium, Allorhizobium, Mesorhizobium, and Sinorhizobium, an obvious defense response is usually not elicited. Instead, a beneficial relationship is established that results in nodule formation and atmospheric nitrogen fixation. Both partners benefit from this relationship as the legume plant is provided with a ready source of fixed nitrogen and the bacteria is, in turn, provided with a protected environment and usable carbon sources (Stacey et al., 1995).
It was suggested that a defense response could be elicited during some rhizobial-plant interactions and this could play an important role in determining host range or regulating nodule formation (Mellor and Collinge, 1995). For example, Vasse et al. (1993) reported that a plant defense response could be involved in the formation of aborted infection threads during normal infection of alfalfa (Medicago sativa) by Sinorhizobium meliloti. This was supported by the microscopic observation of localized root cell necrosis and accumulation of phenolic compounds at the site of infection thread arrest. It is well established that only a small percentage of the infection sites that are initiated are successful and it is possible that induction of a defense response could be responsible for limiting successful infection (for review, see Mellor and Collinge, 1995). In addition, Santos et al. (2001) reported that alfalfa responds to wild-type S. meliloti by the transient production of reactive oxygen species, termed the oxidative burst. Alfalfa roots inoculated with rhizobial exopolysaccharide mutants that are unable to fix nitrogen appear to exhibit a defense response (Niehaus et al., 1993). In this case, microscopy revealed a thickening of the cell walls in contact with the exopolysaccharide mutant rhizobia. Moreover, pea (Pisum sativum) roots inoculated with a lipopolysaccharide-defective mutant showed a phenotype reminiscent of the HR, including reduced nodule colonization by the mutant, callose deposition leading to thickened host cell walls, and sporadic host cell death (Perotto et al., 1994).
Martínez-Abarca et al. (1998) showed that SA accumulated in alfalfa roots inoculated with a nodC mutant of S. meliloti unable to synthesize the lipochitin Nod signal required for infection. This same report also showed that exogenous addition of SA resulted in both reduced and delayed nodule formation on alfalfa roots inoculated with wild-type S. meliloti. Subsequently, Bueno et al. (2001) showed a decrease in antioxidant enzyme activities and an increase in H2O2 accumulation in alfalfa roots following inoculation with a S. meliloti nodC mutant, as well as an increase in lipoxygenase activity after inoculation with the wild-type strain.
Most recently, van Spronsen et al. (2003) reported that exogenous SA addition inhibited indeterminate nodulation (e.g. with a persistent meristem) of vetch (Vicia sativa) but not determinate nodulation (e.g. with no persistent meristem) of Lotus japonicus. They correlated this response with the fact that Rhizobium leguminosarum bv viciae, which nodulates vetch, produces a lipochitin nodulation signal with a 18:4 fatty acid. They postulated that this fatty acid may be active in oxylipin signaling, which is known to be inhibited by SA. Rhizobia that form determinate nodules produce Nod signals lacking polyunsaturated fatty acids and, thus, these signals may act in a different way. However, the theory of van Spronsen et al. (2003) is inconsistent with two reports showing that the addition of exogenous SA to soybean (Glycine max) seedlings inhibited early nodulation (Lian et al., 2000; Sato et al., 2002). Soybean forms determinate nodules.
In this work, we initially set out to repeat the exogenous SA studies of Martínez-Abarca et al. (1998) and van Spronsen et al. (2003) using the symbiosis between the model legume L. japonicus and Mesorhizobium loti, which results in determinate nodules. However, we found that exogenous levels of SA that inhibited nodulation also strongly reduced the growth of the bacterial symbiont. Since endogenous SA levels would mediate any effect of SA on nodulation, we opted to modulate these levels by construction of transgenic plants expressing salicylate hydroxylase, encoded by the bacterial nahG gene. Decreased SA levels in the nahG plants correlated with a significant increase in the number of infections and mean nodule number when compared to wild-type controls. However, root growth of these plants was also enhanced. When this factor was considered, no significant differences were apparent between the transgenic and control L. japonicus plants. However, when we eliminated the effect of root growth by spot inoculation, there was a significant increase in nodule formation on the NahG-expressing roots. To examine the role of endogenous SA in indeterminate nodulation, transformed, composite Medicago truncatula plants were constructed expressing NahG in their roots. These NahG-expressing plants showed enhanced nodulation and infection. Our data would suggest that SA levels impact nodulation on both determinate and indeterminate nodule-forming species.
RESULTS
Exogenous SA Inhibits Growth of M. loti
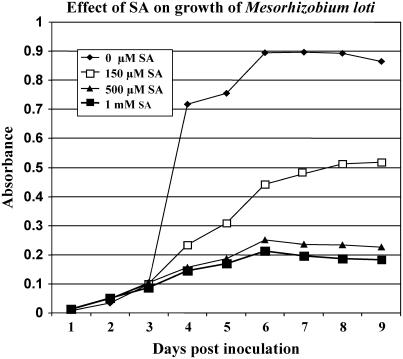
Previously, Martínez-Abarca et al. (1998) reported that exogenously applied SA at a level of 25 μm would inhibit nodulation of alfalfa. Addition of exogenous SA to vetch roots showed a similar response with significant inhibition of nodulation occurring at levels >5 μm (van Spronsen et al., 2003). In contrast to these results, our own results and those of van Spronsen et al. (2003) showed that SA levels up to 100 μm had no apparent effect on nodulation of L. japonicus roots. However, nodulation was significantly reduced when >100 μm SA was added (data not shown). These nodulation experiments were carried out in similar growth, nutrient, and pH conditions as those used in the studies of Martínez-Abarca et al. (1998). Since these SA levels were in excess of those used by Martínez-Abarca et al. (1998) and van Spronsen et al. (2003), experiments were performed to test whether the response seen was due to detrimental effects on symbiont growth or due to effects on the plant host. Initial experiments showed that the addition of SA levels up to 1 mm had no detrimental effect on M. loti growth (data not shown). However, these experiments were performed in rich medium (see “Materials and Methods”) and may not reflect the nutritional environment of the root rhizosphere. Therefore, we repeated these experiments by adding increasing levels of SA to M. loti growing in minimal medium. Figure 1 shows that M. loti growth (as measured by O.D.600) was adversely affected at all of the SA concentrations tested. This strong effect of SA addition on bacterial growth made it impossible to use this approach to accurately gauge the possible role of SA in modulating the nodulation response.
Figure 1.
Effect of salicylic acid on growth of M. loti in minimal media. The growth of a M. loti culture in minimal medium was measured (O.D.600) in the absence or presence of various levels of SA. Black diamonds represent the culture without added SA. Black squares indicate the culture with 150 μm SA. Black triangles represent the culture with 500 μm SA. Finally, the white squares represent the culture containing 1 mm SA.
Construction and Genetic Analysis of NahG Transgenic L. japonicus
If SA is playing a role in nodulation, then the important parameter to be measured is not exogenous SA levels but endogenous SA levels. Indeed, both Martínez-Abarca et al. (1998) and Blilou et al. (1999) reported that endogenous SA levels significantly increased in alfalfa and pea roots, respectively, when inoculated with noncompatible rhizobia. However, this response was not found in vetch roots (van Spronsen et al., 2003). To modulate endogenous SA levels, transgenic L. japonicus plants were constructed by Agrobacterium-mediated hypocotyl transformation in which the bacterial nahG gene, encoding salicylate hydroxylase, was constitutively expressed from the strong Cauliflower mosaic virus (CaMV) 35S promoter.
Following transformation, primary (T1) NahG transgenic L. japonicus plants were selfed and segregation of the transgene was analyzed in T2 and T3 generation seeds. Lines GB8.10 T3 and GD9.6 T2 segregated 3:1 for the transgene and each had one T-DNA insertion by Southern blotting. Segregation in lines GI3.8 T3, GK2.8 T3, and GK6.7 T3 indicated that they were homozygous for the transgene. The first two of these lines had one copy of the transgene, while line GK6.7 T3 had two copies. With the exception of the nodulation assay, further analysis was conducted only with these lines showing either one or two copies of the transgene.
NahG-Expressing Transgenic L. japonicus Plants Have Increased Nodulation
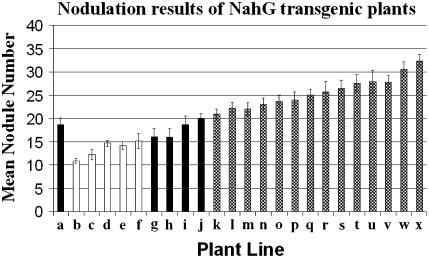
Transgenic plants expressing NahG (as determined by northern analysis; data not shown) were analyzed for their nodulation phenotype 4 weeks after inoculation with M. loti. Figure 2 presents the results from three independent experiments. The bars are color coded to denote different statistical groups. Bars that are black, white, or black and white checkered have mean nodule numbers that are statistically even, lower, or higher, respectively, than the mean nodule numbers on wild-type controls. Fourteen of the 26 lines tested exhibited a significantly higher mean nodule number, ranging from 22% to 78% higher than the wild-type control. It is notable that all of the lines that contained either a single or double copy of the nahG transgene were found in the statistical group with greater nodulation than wild type. Many of the lines that statistically fall in the even or lower groups were found to have two or more copies of the transgene. Therefore, we interpret these results to suggest that increasing transgene copy number was detrimental to nodulation. This effect may be the result of general detrimental effects on plant metabolism, perhaps due to the toxic effects of overproduction of catechol, the product of the salicylate hydroylase (van Wees and Glazebrook, 2003). Moreover, pleiotropic effects due to NahG expression have been noted before. For example, Heck et al. (2003) showed that not all of the effects on plant disease resistance in NahG expression plants could be attributed solely to a reduction in SA levels. In addition to the use of wild-type controls, the nodulation assays also included plant lines that went through the tissue culture process but ended up having no insertion of the transgene. These plants were essentially wild-type plants but had experienced the same treatments as the generated transgenic NahG lines. These plants showed similar nodulation results to the wild-type control plants, indicating that the tissue culture process itself could not explain the enhanced nodulation phenotype.
Figure 2.
Nodulation results of the NahG transgenic L. japonicus plants. Four-week-old plants were inoculated with 1 mL of a M. loti culture at an O.D.600 of 0.1. The plants were harvested 4 weeks postinoculation and nodule numbers enumerated. a, Wild type; b, GK1.9 T3; c, GB8.9P T3; d, GB3.14P T3; e, GB3.13 T3; f, GK6.1 T3; g, GH3 T2; h, GD14 T2; i, GH6 T2; j, GB4.9 T3; k, GI1.1 T3; l, GI3.11 T3; m, GB3B T3; n, GB3.8 T3; o, GI3.15 T3; p, GK6.6 T3; q, GK2.8 T3; r, GD9.6 T2; s, GB8.10 T3; t, GB8.19 T3; u, GI3.8 T3; v, GK3.6 T3; w, GK6.7 T3; and x, GK1.13 T3. It should be noted that plant lines generated from the same callus, and thus having the same letter designation, can vary genetically due to somoclonal variation and gene copy segregation. Plant lines marked with black, white, and checkered bars are statistically different groups. The lines with white bars have a mean nodule number significantly lower than the lines with black bars, α = 0.05 (using a Student's t test). The lines with black bars have a mean nodule number significantly higher than the lines with white bars, but are still significantly lower than the lines with checkered bars, α = 0.05. And finally, the lines with checkered bars have a mean nodule number statistically higher than the lines with black or white bars, α = 0.05. This figure represents data collected from three independent experiments where n = 30. Error bars represent se.
L. japonicus NahG Transgenics Showing Increased Nodulation Have Reduced SA Content
NahG expression should correlate with a significant decrease in endogenous SA content. HPLC analysis confirmed that total SA levels (free SA and SA conjugated with Glc) in NahG lines showing increased nodulation were greatly reduced in both leaf and root tissues compared to wild-type plants (Table I). Since the levels of free SA in wild-type L. japonicus were found to be very low (48 ± 17 ng g−1 fresh weight) in roots and undetectable in leaves, analysis of free SA was not performed for the transgenic lines. The finding that the NahG lines had low or undetectable SA levels is consistent with the notion that reduced endogenous SA correlates with higher nodulation. Those lines (i.e. GK1.9 T3, GB3.13 T3, GI1.1 T3, and GH3 T2) shown in Figure 2 showing normal or reduced nodulation did not show a significant change in SA levels (data not shown).
Table I.
Total SA content in wild-type and NahG L. japonicus
Leaves and roots from 4-week-old plants were harvested and analyzed by HPLC for total SA (free SA and SA conjugated with Glc). The values presented are the average of at least three replicates ± se. ND, Not detectable; NA, not analyzed.
| Line | nahG Copy Number | Leaves | Roots |
|---|---|---|---|
| ng g−1 fresh weight | ng g−1 fresh weight | ||
| Wild type | 0 | 824 ± 124 | 603 ± 132 |
| GI3.8 T3 | 1 | ND | ND |
| GK2.8 T3 | 1 | ND | ND |
| GB8.10 T3 | 1 | ND | NA |
| GD9.6 T2 | 1 | 168 ± 16 | NA |
| GK6.7 T3 | 2 | ND | ND |
| GK1.13 T3 | 2 | ND | ND |
L. japonicus NahG Lines Have Enhanced Infection Thread Formation
As discussed, previous published reports suggested that a plant defense response could lead to an arrest of infection thread development or growth. If SA is involved in this phenomenon, then one would expect to see an increase in infection thread number or growth in L. japonicus plants with reduced levels of SA. Alternatively, the NahG lines might show the same number of total infections, but more of these infections could lead to nodule formation due to the lack of a defense response. To examine these possibilities, wild-type and NahG plants were inoculated with a M. loti strain carrying a hemA-lacZ fusion (Schauser et al., 1998). Subsequent staining of roots infected with this strain using 5-bromo-4-chloro-3-indolyl-β-galactoside allowed visualization of the bacterial infection thread. Infection threads were counted on wild-type and NahG-expressing plants using a stereomicroscope and the results are shown in Table II. When counting total infection threads/plant, the results showed that the NahG-expressing plants had a significantly higher number of infection threads than the wild type. In addition to infection thread numbers, the infection zone (i.e. area above the root tip at the time of inoculation, which is the primary area of infection on the root, was measured; compare with Calvert et al., 1985) of NahG transgenic plants was significantly larger. In addition to the size of the infection zone, the number of infection zones found on each plant was significantly increased in the NahG lines.
Table II.
NahG infection events relative to L. japonicus root growth
Data taken from 10 plants per line. Plants were germinated 7 d and then planted for 14 d before inoculation. Plants were harvested 2 weeks postinoculation and scored for infection events. Groups marked a, b, or c were statistically significant at α = 0.05 by a Student's t test. Asterisk (*) indicates total root length = tap root + lateral roots.
| Wild Type | GI3.8 T3 (One Copy) | GK2.8 T3 (One Copy) | GK6.7 T3 (Two Copies) | |
|---|---|---|---|---|
| Average nodule number/plant | 8a | 12b | 11b | 11b |
| Average number of infections/plant | 168a | 219b | 289c | 275c |
| Average number of infections/average total root length (cm)* | 7a | 6a | 7a | 7a |
| Average infection zone length (mm) | 6.1a | 7.7b | 7.7b | 8.5c |
| Average number of infection zones/plant | 3a | 4b | 5c | 4b |
| Average number of infections/infection zone length (mm) | 27.5a | 28.4a | 37.5b | 32.4b |
| Average tap root length (cm) | 6.2a | 8.4a | 10.7c | 12.7c |
| Average lateral root length (cm) | 19.8a | 26.7b | 27.9b | 24.3b |
| Average number of lateral roots/plant | 6a | 9b | 9b | 12c |
L. japonicus NahG Lines Have Enhanced Root Growth
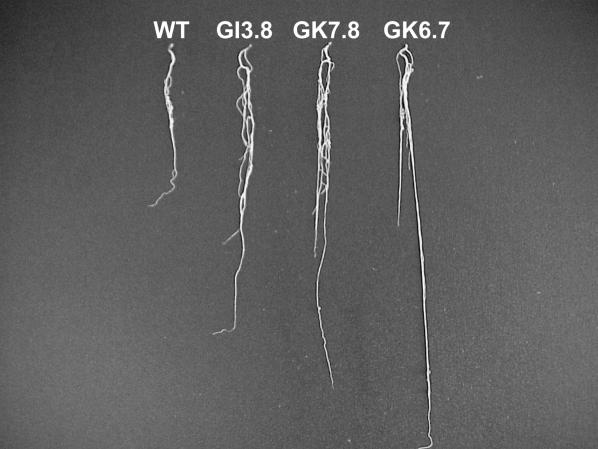
Keeping in mind the diverse roles of SA in plant physiology, including its role as a growth regulator (Gutiérrez-Coronado et al., 1998; Lian et al., 2000), we examined general root growth parameters of the NahG-expressing plants. Figure 3 shows that representative plants from the NahG lines had significantly increased root size compared to wild-type controls. This phenotype was apparent with plants grown in nitrogen-free medium (allowing nodulation) and with 10 mm nitrate (where nodulation is inhibited). Furthermore, Table II shows root size data from three independent experiments. Compared to controls, the NahG lines had significantly increased tap root and lateral root length, as well as increased lateral root numbers/plant. Relative to root growth, scored by the number of infections/centimeter of total root (i.e. tap root and lateral roots), the NahG lines did not have increased infection numbers. Similarly, the increase in root length could also contribute to larger and more abundant infection zones on the NahG-expressing plants. However, it is important to remember that only a certain portion of the root can be infected by rhizobia. Therefore, the infection zone length, and not total root length, is perhaps a better parameter to measure when investigating the role of SA in nodulation. By this measure, there was a slight, but significant, increase in infections in NahG-expressing roots (Table II).
Figure 3.
NahG lines have increased root growth. Five-week-old wild-type and NahG roots were harvested and measured for tap root size, lateral root number, and lateral root size. A, A representative wild-type root. B to D, Representative NahG roots (B, GI3.8; C, GK2.8; and D, GK6.7). Results are representative of three independent experiments.
Spot Inoculation of NahG-Expressing L. japonicus
To remove the complication of enhanced root growth, the roots of the various NahG-expressing plants were spot inoculated. The results clearly showed that the NahG-expressing plants exhibited enhanced nodulation (Table III). The rate of nodule formation (as measured by the appearance of nodules) was not affected. Therefore, it would appear that reduction of SA resulted in greater susceptibility to infection by M. loti.
Table III.
Spot inoculation of NahG-expressing L. japonicus
Inoculations were performed as described in “Materials and Methods.” Data (average ± se) were taken from 10 or more plants per line at the times shown. dpi, Days postinoculation.
| Line | Transgene Copy Number | Average Nodule Number/Plant
|
|||
|---|---|---|---|---|---|
| 5 dpi | 6 dpi | 7 dpi | 10 dpi | ||
| Wild type | 0 | 0.65 ± 0.13 | 1.70 ± 0.28 | 2.85 ± 0.36 | 3.1 ± 0.25 |
| GA6T3 | 0 | 0.95 ± 0.31 | 2.1 ± 0.35 | 2.18 ± 0.39 | 2.6 ± 0.29 |
| GB8.10 | 1 | 1.46 ± 0.45a | 2.82 ± 0.44a | 3.97 ± 0.47a | 4.2 ± 0.45a |
| GD9.6 | 1 | 1.37 ± 0.30a | 3.64 ± 0.48a | 3.98 ± 0.47a | 3.99 ± 0.41a |
| GI3.8 | 1 | 0.77 ± 0.60 | 2.63 ± 0.45a | 3.65 ± 0.65a | 3.78 ± 0.42 |
| GK2.8 | 1 | 1.52 ± 0.58a | 2.37 ± 0.29a | 2.77 ± 0.54 | 3.62 ± 0.36 |
| GK6.7 | 2 | 1.22 ± 0.27a | 2.96 ± 0.15a | 4.28 ± 0.31a | 4.35 ± 0.38a |
| GB3B | 3 | 1.25 ± 0.57a | 2.35 ± 0.32a | 3.37 ± 0.65a | 4.38 ± 0.66a |
Values significantly different (α = 0.05) by Student's t test when compared to wild type at the same dpi.
Nodulation of NahG-Expressing M. truncatula Roots
The data above indicates that NahG expression results in a small, but significant increase in nodulation, suggesting that determinate nodulating plants, like L. japonicus, may respond similarly to SA as indeterminate nodule-forming plants, like M. truncatula. To examine this more directly, we constructed composite M. truncatula transgenic plants using transformation with Agrobacterium rhizogenes. Such plants have been used commonly to examine nodulation, including analyzing phenotypes resulting from RNAi expression (e.g. Boisson-Dernier et al., 2001; Kumagai and Kouchi, 2003).
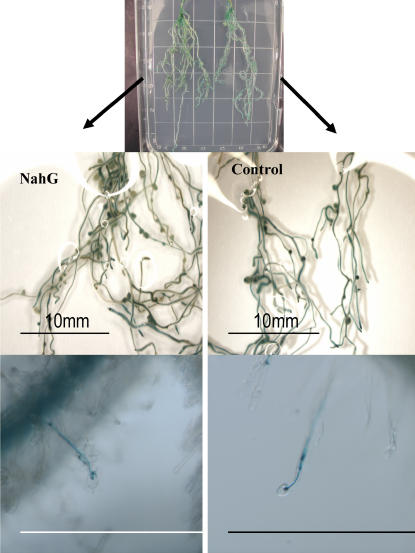
Infection of M. truncatula expressing NahG with a S. meliloti strain expressing β-galactosidase allowed measurement of both nodules and infections (Table IV). NahG expression resulted in a doubling of the nodules/root and infections/root (Fig. 4) with no apparent affect on root growth.
Table IV.
Nodulation and infection of M. truncatula composite roots expressing NahG
Data (average ± se) were taken from 10 plants.
| Genotype | Number of Nodules/Root | Number of Infections/Root | Root Length Inoculated (cm) | Root Length Uninoculated (cm) |
|---|---|---|---|---|
| NahG expressing | 11.3 ± 0.3 | 38 ± 10 | 5.3 ± 0.4 | 4 ± 0.3 |
| Empty vector control | 6 ± 0.4 | 14 ± 8 | 4.9 ± 0.3 | 4 ± 0.3 |
Figure 4.
Nodulation of composite M. truncatula roots by S. meliloti. Top, NahG-expressing and nontransgenic (control) plants growing side by side on an agar plate. Middle left, Composite roots expressing NahG and nodulated by S. meliloti; note increased number of nodules in relation to the middle, right section (control plants nodulated by S. meliloti). Bottom left, Transgenic roots. Right, Nontransgenic roots. Pictures show blue staining of β-galactosidase demonstrating images used to count the number of infections on roots.
In repeated experiments, the levels of SA in the M. truncatula transgenic roots were quite variable and we were unable to demonstrate a significant reduction in either free or total SA levels in these roots. We attribute this to the fact that A. rhizogenes transformation of M. truncatula, unlike L. japonicus, results in the production of chimeric roots (i.e. possessing both transformed and untransformed tissue). For example, Collier et al. (2005) in a screen of several plant species found up to 23% chimeric, transformed roots on M. truncatula, whereas other plants produced very few. The composite nature of the NahG-expressing plants and the likely presence of chimeric roots likely resulted in wild-type tissue in our root preparations leading to the variability found in the SA measurements.
DISCUSSION
In the L. japonicus-M. loti model system, the results of adding exogenous SA clearly showed a detrimental effect on the bacterial symbionts. All SA levels tested inhibited bacterial growth in minimal medium in a dose-dependent manner. Therefore, in this system, addition of exogenous SA could not be used to examine the role of this signal molecule in nodulation. To modulate endogenous SA levels, transgenic L. japonicus plants expressing NahG were constructed. Consistent with the postulated role of SA signaling in limiting nodulation, 14 out of 26 NahG transgenic lines showed a significant increase in nodulation compared to wild-type controls. Since the majority of the remaining lines had two or more transgene insertions, we interpret their lack of nodulation response to the detrimental effects of multiple T-DNA insertions. For example, multiple insertions could lead to silencing of the transgene consistent with the fact that these plants showed normal SA levels. All of the single copy lines showed significantly higher nodulation. As expected, these NahG transgenic plants showed significantly lower levels of endogenous SA. The increased nodulation in the NahG-expressing plants could be due to a larger number of infection events and/or to an increase in the number of successful infections. To examine these possibilities, we visualized infection by 5-bromo-4-chloro-3-indolyl-β-galactoside staining of roots infected with a M. loti strain expressing a hemA-lacZ fusion. These data showed that the NahG lines had roughly the same number of successful versus aborted infection threads. However, the total number of infection events on the NahG plants was significantly higher than on wild-type plants. These results support the hypothesis that a SA-mediated defense response may be involved in the plant's ability to autoregulate nodulation at the step of infection thread formation. Importantly, the root infection zone was also significantly increased in the NahG lines, suggesting that SA might play multiple roles in controlling nodulation. The increased infection zone in the NahG plants suggests that the presence of SA in Lotus can reduce the potential of the root for rhizobial infection.
It is widely accepted that plants have the ability to autoregulate nodulation and it is clear that the nodulation process can be autoregulated at different stages of the nodulation process (for review, see Caetano-Anolles and Gresshoff, 1991). One possibility is that a localized defense response may be involved in limiting nodulation. It has long been known that few root hairs are infected and only a small percentage of those infected result in nodule formation (Calvert et al., 1985). This indicates that the infection process, including infection thread and nodule formation, is a highly regulated step. Cytological studies in the alfalfa-S. meliloti symbioses showed that after initial nodule formation, subsequent infections were aborted. The aborted infections were accompanied by a HR-like defense response that included necrosis of infection thread cells and accumulation of phenolics and pathogenesis-related proteins (Vasse et al., 1993). Keeping in mind the importance of SA in the HR, the increased nodulation seen in the NahG-expressing transgenic plants is consistent with the hypothesis that a SA-mediated defense response is involved in limiting nodulation. Microscopy analysis would suggest that this SA-mediated response limits infection thread initiation and not infection thread growth.
SA is a molecule that has a variety of effects in plant metabolism. For example, SA has been shown to affect root and shoot growth in soybean (Gutiérrez-Coronado et al., 1998; Lian et al., 2000) and abscission in peach (Prunus persica) and pepper (Capsicum annuum) leaves (Ferrarese et al., 1996). Therefore, we also examined the NahG-expressing, transgenic L. japonicus plants for general effects on plant growth. The NahG transgenic L. japonicus plants clearly showed increased root growth compared to wild-type plants. If root size is taken into account, then the number of infection events per centimeter of total root length was statistically equivalent in the NahG transgenic plants when compared to the wild type, as can be seen in Table II. Similar root growth effects were not observed in experiments with alfalfa (M.J. Soto, personal communication) and no mention was made of SA effects on root growth in vetch by van Spronsen et al. (2003). Since these studies dealt exclusively with exogenous addition of SA, the work is not directly comparable with the NahG transgenics where endogenous SA levels were affected.
To circumvent the issue of greater root growth in NahG-expressing plants, roots were spot inoculated and nodule formation measured over time. The results of these experiments are consistent with the notion that reduction of SA levels is responsible for increased infection.
The results of these experiments led us to examine the effects of endogenous SA levels on indeterminate nodulation of M. truncatula using roots transformed by A. rhizogenes. The results obtained were qualitatively the same as those found using L. japonicus. That is, both nodulation and infections were increased in the NahG-expressing plants.
In summary, consistent with the variety of physiological effects reported for SA, transgenic expression of NahG resulted in pleiotropic growth responses in L. japonicus. However, even when these effects are considered, nodulation and infection was significantly increased in plants expressing NahG. These results are consistent with the notion that SA-mediated plant defense pathways are involved in modulating legume infection both during indeterminate and determinate nodulation.
MATERIALS AND METHODS
Bacterial Strains
Bacterial strains used in this study are Mesorhizobium loti strain NZP2235 carrying a hemA-lacZ fusion (Schauser et al., 1998), Sinorhizobium meliloti ABS7M expressing an aminolevulinic acid synthetase-lacZ fusion (Leong et al., 1985), Agrobacterium rhizogenes AR10 (Stiller et al., 1997; Boisson-Dernier et al., 2001), and Agrobacterium tumefaciens strain LBA4404 (Stiller et al., 1997). M. loti NZP2235 was cultivated on yeast (Saccharomyces cerevisiae) mannitol broth medium (Handberg and Stougaard, 1992) or B-minimal medium (Niwa et al., 2001) at 30°C. S. meliloti was grown on tryptone-yeast extract broth (0.5% tryptone, 0.3% yeast extract, 0.13% CaCl2 6H2O [pH 7.0]) at 30°C (Platzer et al., 1997). The Agrobacterium strains were grown at 30°C on yeast extract peptone medium (Vervliet et al., 1975). Antibiotic concentrations were 2 μg/mL tetracycline and 100 μg/mL carbenicillin for M. loti NZP2235 and A. tumefaciens/A. rhizogenes, respectively.
Plant Material and Growth Conditions
Lotus japonicus ecotype Gifu seeds were originally provided by Dr. Jiri Stiller and were propagated under greenhouse conditions. Lotus seeds were scarified by rubbing briefly between two sheets of fine 150 grain sandpaper until the seed coat was visibly roughened. Alternatively, the scarification process was performed by treating with sulfuric acid for 3 min followed by washes with distilled water. The scarified seeds were then soaked in 3% H2O2, 95% ethanol for 15 min with shaking at room temperature. The seeds were then germinated in square petri dishes on a 0.5 cm stack of sterile Whatman number 1 filter paper soaked in sterile, distilled, deionized water. The petri dishes were then sealed with parafilm and placed in a Percival model CU-32L (Percival Scientific) incubator for 1 week with a light cycle of 8 h, 22°C day and 16 h, 20°C night. For segregation analysis, the seedlings were transferred to .5× B5 (Gamborg's B5 medium; Gamborg and Shyluk, 1970) agar plates containing 5 μg/mL G418 (Sigma) and allowed to grow in the light for 4 weeks before scoring for antibiotic resistance.
For infection and nodulation analysis, the germinated seedlings were transferred to Leonard jars and 4-inch diameter plastic pots containing sterile vermiculite, respectively. Two-week-old plants were inoculated with 1 mL each of a 3 d M. loti (hemA-lacZ) culture washed with sterile water and diluted to an O.D.600 of 0.1. The plants were allowed to grow as described above for an additional 2 weeks postinoculation before the roots were harvested for infection assays or for 4 weeks postinoculation for scoring nodulation. The plants were watered with B and D nutrient solution as described by Broughton and Dilworth (1971). The plants were removed from the Leonard jars by flooding gently with water. The roots were washed gently with water and subsequently detached from the plant using a razor blade. The detached roots were immediately fixed in glutaraldehyde and stained for LacZ (β-galactosidase) expression as described by Boivin et al. (1990). Roots were stored in the dark in sterile, distilled, water at 4°C until use.
The roots were measured and photographed using a stereoscope (Olympus SZX12) equipped with a Nikon DXM1200 digital camera. The infection zone was defined as an area on the root showing the most abundant infections (compare with Calvert et al., 1985). The edges of the infection zone were determined where no additional infections were apparent within 3 mm.
Spot inoculations of L. japonicus roots were done as originally described in Carlson et al. (1993) for soybean (Glycine max) roots. Five-day-old seedlings were placed in plastic pouches containing 5 mL of B & D nutrient solution and allowed to acclimate for 2 d. At the time of inoculation, the position of the smallest emergent root hairs, visible in a dissecting microscope at magnification ×50, and the root tip were marked on the top face of the plastic pouch. The top face of the plastic pouch was slit with a razor blade and rolled back to expose the root. Prior to inoculation a single Amberlite bead was transferred with forceps to a position above the root tip approximately 80% of the distance between the root tip and the smallest emergent root hairs. Droplets containing M. loti in a volume of 30 to 50 nL were delivered by micropipette to the same position as the Amberlite bead. The roots were shielded from the light using black paper and the plants were grown for 4 weeks in a growth chamber (see above). Roots were then examined for nodulation and infection as described above.
For SA analysis germinated Lotus seedlings were grown in hydroponic culture as described by Olivares et al. (1980).
Medicago truncatula (ecotype A17) seeds were surface sterilized by soaking in concentrated sulfuric acid for 10 min, 20% bleach for 3 min, and then washing thoroughly in sterile water. Sterilized seeds were grown under continuous light at 25°C on B & D medium agar (Broughton and Dilworth, 1971). B & D medium was dispensed into a 25 mL Nunc plate (243 × 243 × 18 mm) with 6% [w/v] Kalys agar (HP696-7470, Kalys).
Hairy root transformation of M. truncatula was performed as described by Boisson-Dernier et al. (2001). Transgenic roots were inoculated with 1 mL of S. meliloti strain ABS7M at 1 × 108 cells per plant and grown in sterilized perlite (Hummert) with B & D nutrient solution for 3 weeks. The culture inoculant was manually applied to each plant with a pipette. During harvesting, plants were washed with water and were immediately fixed in glutaraldehyde and stained for LacZ (β-galactosidase) expression as described by Boivin et al. (1990). Constitutive expression of green fluorescence protein (GFP), encoded by the pAKK1467B vector (C. Taylor, unpublished data), was used to distinguish transformed roots from adventitious roots. Phenotypic data was only scored from roots showing GFP expression.
Construction of NahG Transgenic L. japonicus
The nahG gene, originally from Pseudomonas putida, encodes salicylate hydroxylase, which catabolizes SA to catechol. The nahG construct was kindly provided by Dr. Kay Lawton (Syngenta Biotechnology). Construction of the plant binary vector pCIB200 expressing nahG behind the CaMV 35S promoter was previously described (Gaffney et al., 1993). This construct was used to transform L. japonicus ecotype Gifu via Agrobacterium-mediated hypocotyl transformation as described in Stiller et al. (1997).
Construction of Composite M. truncatula Plants Expressing NahG
In the case of A. rhizogenes transformation, the nahG gene was PCR amplified from pCIB200 by ExTaq polymerase (Takara) using forward primer (5′ GCC TCG AGA TGA AAA ACA ATA AAC TTG GCT TG 3′) and reverse primer (5′ GAC TTA AGC TAC CAT TTT CCC AAC CCA G 3′). The PCR product was inserted into the pGemTeasy vector (Promega). The resulting plasmid was then restricted using Sse8387I and the nahG gene inserted into binary vector pAKK1448B (Collier et al., 2005). This vector contains the GFP expressed from a CaMV 35s promoter; thus, allowing the detection of transformed hairy roots by GFP expression.
Analysis of SA Content
Leaf and root tissues from 4-week-old wild-type and transgenic nahG L. japonicus were collected, weighed, and frozen in liquid nitrogen. Between five to 15 plants were used for each SA measurement. Hairy-root transgenic M. truncatula plants were grown in perlite with B & D nutrient solution for 3 weeks before harvest. Approximately 12 plants were used for each SA measurement. For each sample, 0.1 to 0.3 g of the frozen tissue was extracted and quantitated for free and total SA (free and SA β-glucoside), essentially as described previously (Enyedi et al., 1992; Bowling et al., 1994). Ten microliters from a total volume of the final 150/80 μL of leaf/root methanolic extracts were injected into a 5 μm C18 column (4.6 × 150 mm; Varian). SA was separated isocratically with 30% (v/v) methanol containing 1% (v/v) acetic acid at a flow rate of 1 mL min−1. The temperature of the oven was 40°C. SA was detected with a Varian Prostar fluorescence detector using an excitation wavelength of 313 nm and an emission wavelength of 405 nm. Identification was determined by spiking a sample with an authentic standard.
Supplementary Material
This work was supported by the National Science Foundation, Plant Genome Program (grant no. DBI–0421620 to G.S.). M.J.S. was supported by a Ministerio de Educación y Ciencia (Spain) contract.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Gary Stacey (staceyg@missouri.edu).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.080986.
References
- Blilou I, Ocampo JA, García-Carrido JM (1999) Resistance of pea roots to endomycorrhizal fungus or Rhizobium correlates with enhanced levels of endogenous salicylic acid. J Exp Bot 50: 1663–1668 [Google Scholar]
- Boisson-Dernier A, Chabaud M, Garcia F, Becard G, Rosenberg C, Barker DG (2001) Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant Microbe Interact 14: 695–700 [DOI] [PubMed] [Google Scholar]
- Boivin C, Camut S, Malpica C, Truchet G, Rosenberg C (1990) Rhizobium meliloti genes encoding trignoellline are induced under symbiotic conditions. Plant Cell 2: 1157–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno P, Soto MJ, Rodríguez-Rosales MP, Sanjuan J, Olivares J, Donaire JP (2001) Time-course lipoxygenase, antioxidant enzyme activities and H2O2 accumulation during the early stages of Rhizobium-legume symbiosis. New Phytol 152: 91–96 [DOI] [PubMed] [Google Scholar]
- Caetano-Anolles G, Gresshoff PM (1991) Plant genetic-control of nodulation. Annu Rev Microbiol 45: 345–382 [DOI] [PubMed] [Google Scholar]
- Calvert HE, Pence MK, Pierce M, Malik NSA, Bauer WD (1985) Anatomical analysis of the development and distribution of rhizobium infections in soybean roots. Can J Bot 62: 2375–2384 [Google Scholar]
- Carlson RW, Sanjuan J, Bhat UR, Glushka J, Spaink H, Wijfjes AHM, van Brussel AAN, Stokkerman TJW, Peters K, Stacey G (1993) The structures and biological activities of the lipo-oligosaccharide nodulation signals produced by type I and type II strains of Bradyrhizobium japonicum. J Biol Chem 268: 18372–18381 [PubMed] [Google Scholar]
- Collier R, Fuchs B, Walter N, Lutke WK, Taylor CG (2005) Ex vitro composite plants: an inexpensive, rapid method for root biology. Plant J 43: 449–457 [DOI] [PubMed] [Google Scholar]
- Draper J (1997) Salicylate, superoxide synthesis and cell suicide in plant defense. Trends Plant Sci 2: 162–165 [Google Scholar]
- Durner J, Shah J, Klessig DF (1997) Salicylic acid and disease resistance in plants. Trends Plant Sci 2: 266–277 [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin I (1992) Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA 89: 2480–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarese L, Moretto P, Trainotti L, Rascio N, Casadoro G (1996) Cellulase involvement in the abscission of peach and pepper leaves is affected by salicylic acid. J Exp Bot 47: 251–257 [Google Scholar]
- Feys B, Parker JE (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet 16: 449–455 [DOI] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756 [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Shyluk JP (1970) Culture of plant cells with ammonium salts as sole nitrogen source. Plant Physiol 45: 583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Coronado MA, Trejo-López C, Larqué-Saavedra A (1998) Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol Biochem 36: 563–565 [Google Scholar]
- Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2: 487–496 [Google Scholar]
- Heck S, Grau T, Buchala A, Metraux JP, Nawrath C (2003) Genetic evidence that expression of NahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J 36: 342–352 [DOI] [PubMed] [Google Scholar]
- Kumagai H, Kouchi H (2003) Gene silencing by expression of hairpin RNA in Lotus japonicus roots and root nodules. Mol Plant-Microbe Interact 16: 663–668 [DOI] [PubMed] [Google Scholar]
- Leong SA, Williams PH, Ditta GS (1985) Analysis of the 5′ regulatory region of the gene for ɛ–aminolevulinic acid synthetase of Rhizobium meliloti. Nucleic Acids Res 13: 5965–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian B, Zhou X, Miransari M, Smith DL (2000) Effects of salicylic acid on the development and root nodulation of soybean seedlings. J Agron Crop Sci 185: 187–192 [Google Scholar]
- Martínez-Abarca F, Herrera-Cervera JA, Bueno P, Sanjuan J, Bisseling T, Olivares J (1998) Involvement of salicylic acid in the establishment of the Rhizobium meliloti-alfalfa symbiosis. Mol Plant Microbe Interact 11: 153–155 [Google Scholar]
- Medina MJH, Gagnon H, Piche Y, Ocampo JA, Garrido JMG, Vierheilig H (2003) Root colonization by arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Sci 164: 993–998 [Google Scholar]
- Mellor RB, Collinge DB (1995) A simple model based on known plant defense reactions is sufficient to explain most aspects of nodulation. J Exp Bot 46: 1–18 [Google Scholar]
- Niehaus K, Kapp D, Puhler A (1993) Plant defense and delayed infection of alfalfa psuedonodules induced by an exopolysaccharide (EPS-I)-deficient Rhizobium meliloti mutant. Planta 190: 415–425 [Google Scholar]
- Niwa S, Kawaguchi M, Imaizumi-Anraku H, Chechetka SA, Ishizawa H, Ikuta A, Kouchi H (2001) Responses of a model legume Lotus japonicus to lipochitin oligosaccharide nodulation factors purified from Mesorhizobium loti JRL501. Mol Plant Microbe Interact 14: 848–856 [DOI] [PubMed] [Google Scholar]
- Olivares J, Casadesus J, Bedmar EJ (1980) Method for testing degree of infectivity of Rhizobium meliloti strains. Appl Environ Microbiol 39: 967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perotto S, Brewin NJ, Kannenberg EL (1994) Cytological evidence for a host defense response that reduces cell and tissue invasion in pea nodules by lipopolysaccharide-defective mutants of Rhizobium leguminosarum strain 3841. Mol Plant Microbe Interact 7: 99–112 [Google Scholar]
- Platzer J, Sterr W, Hausmann M, Schmitt R (1997) Three genes of a motility operon and their role in flagellar rotary speed variation in Rhizobium meliloti. J Bacteriol 179: 6391–6399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R, Hérouart D, Sigaud S, Touati D, Puppo A (2001) Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol Plant Microbe Interact 14: 86–89 [DOI] [PubMed] [Google Scholar]
- Sato T, Fujikake H, Ohtake N, Sueyoshi K, Takahashi T, Sato A, Ohyama T (2002) Effect of exogenous salicylic acid supply on nodulation formation of hypernodulating mutant and wild type of soybean. Soil Sci Plant Nutr 48: 413–420 [Google Scholar]
- Schauser L, Handberg K, Sandal N, Stiller J, Thykjaer T, Pajuelo E, Nielsen A, Stougaard J (1998) Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol Gen Genet 259: 414–423 [DOI] [PubMed] [Google Scholar]
- Shah J, Kachroo P, Nandi A, Klessig DF (2001) A recessive mutation in the Arabidopsis SS12 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J 25: 563–574 [DOI] [PubMed] [Google Scholar]
- Stacey G, Sanjuán J, Luka S, Dockendorff T, Carlson RW (1995) Signal exchange in the Bradyrhizobium-soybean symbiosis. Soil Biol Biochem 27: 473–483 [Google Scholar]
- Stiller J, Martirani L, Túppale S, Chian R, Chiurazzi M, Gresshoff PM (1997) High frequency transformation and regeneration of transgenic plants in the model legume Lotus japonicus. J Exp Bot 48: 1357–1365 [Google Scholar]
- van Spronsen PC, Tak T, Rood AMM, van Brussel AAN, Kijne JW, Boot KJM (2003) Salicylic acid inhibits indeterminate-type nodulation but not determinate-type nodulation. Mol Plant-Microb Interact 16: 83–91 [DOI] [PubMed] [Google Scholar]
- van Wees SCM, Glazebrook J (2003) Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. Plant J 33: 733–742 [DOI] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Truchet G (1993) Abortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. Plant J 4: 555–566 [Google Scholar]
- Vervliet G, Holsters H, Teuchy H, Van Montagu M, Schell J (1975) Characterization of different plaque-forming and defective temperate phages in Agrobacterium strains. J Gen Virol 26: 33–48 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.