Abstract
Calcium can ameliorate Na+ toxicity in plants by decreasing Na+ influx through nonselective cation channels. Here, we show that elevated external [Ca2+] also inhibits Na+-induced K+ efflux through outwardly directed, K+-permeable channels. Noninvasive ion flux measuring and patch-clamp techniques were used to characterize K+ fluxes from Arabidopsis (Arabidopsis thaliana) root mature epidermis and leaf mesophyll under various Ca2+ to Na+ ratios. NaCl-induced K+ efflux was not related to the osmotic component of the salt stress, was inhibited by the K+ channel blocker TEA+, was not mediated by inwardly directed K+ channels (tested in the akt1 mutant), and resulted in a significant decrease in cytosolic K+ content. NaCl-induced K+ efflux was partially inhibited by 1 mm Ca2+ and fully prevented by 10 mm Ca2+. This ameliorative effect was at least partially attributed to a less dramatic NaCl-induced membrane depolarization under high Ca2+ conditions. Patch-clamp experiments (whole-cell mode) have demonstrated that two populations of Ca2+-sensitive K+ efflux channels exist in protoplasts isolated from the mature epidermis of Arabidopsis root and leaf mesophyll cells. The instantaneously activating K+ efflux channels showed weak voltage dependence and insensitivity to external and internal Na+. Another population of K+ efflux channels was slowly activating, steeply rectifying, and highly sensitive to Na+. K+ efflux channels in roots and leaves showed different Ca2+ and Na+ sensitivities, suggesting that these organs may employ different strategies to withstand salinity. Our results suggest an additional mechanism of Ca2+ action on salt toxicity in plants: the amelioration of K+ loss from the cell by regulating (both directly and indirectly) K+ efflux channels.
Sodium toxicity is one of the main reasons for poor plant performance in saline environments. Several approaches are routinely used in agriculture to decrease Na+ toxicity, with application of Ca2+-containing compounds (such as lime or gypsum) to soils being probably the most effective among them (Bergmann, 1992; Rengel, 1992). It is widely reported that supplemental Ca2+ ameliorates Na+ toxicity symptoms in different plant species (for review, see Laüchli, 1990; Rengel, 1992; Hasegawa et al., 2000). Nevertheless, the mechanisms of Ca2+ amelioration at the cellular level are not fully understood.
It is becoming clear that to decrease Na+ toxicity, Ca2+ interacts with numerous intracellular and extracellular targets. Recently, the SOS stress-signaling pathway was identified to be a pivotal regulator of plant ion homeostasis under saline conditions. According to the suggested model, salinity causes a rise in cytosolic [Ca2+] ([Ca2+]cyt; White and Broadley, 2003), which activates the SOS3-SOS2 protein kinase pathway. This leads to increased expression of the plasma membrane Na+ efflux transporter SOS1 and possibly restricts Na+ influx through the plasma membrane high-affinity K+ transporter HKT1, so helping to maintain cellular K+/Na+ homeostasis (Liu and Zhu, 1998; Halfter et al., 2000; Ishitani et al., 2000; Zhu, 2003). The extent of the rise in [Ca2+]cyt (and, therefore, encoded information) is thought to depend on the concentration of extracellular Ca2+ (Yokoi et al., 2002). Direct experimental evidence supporting this model is still lacking.
An increase in external [Ca2+] can stimulate plasma membrane H+-ATPase activity via Ca2+-calmodulin-dependent protein kinases (Klobus and Janicka-Russak, 2004). Under salt stress conditions, increased plasma membrane H+-ATPase activity is necessary for repolarization of membrane voltage (after depolarization by Na+ influx), maintaining membrane integrity and ionic homeostasis. Na+ efflux by the SOS1 Na+-H+ antiporter ultimately relies on H+-ATPase activity. Indeed, loss of root endodermal plasma membrane H+-ATPase activity results in salt sensitivity in Arabidopsis (Arabidopsis thaliana; Vitart et al., 2001). Ca2+ stimulation of the SOS3-SOS2 pathway also appears to enhance vacuolar Na+ sequestration by Na+-H+ antiporters (Zhu, 2003). Thus, Ca2+, as an intracellular regulator, plays an important role in salinity tolerance.
Apart from effects on intracellular targets, supplemental Ca2+ may provide rapid, yet long-term protection against salinity stress via its stabilizing effect on cell wall components, plasma membrane lipids, and proteins (Rengel, 1992; Kinraide, 1998; Cramer, 2002). Many reports have recently demonstrated that elevated extracellular [Ca2+] inhibits the plasma membrane inwardly directed, Na+-permeable nonselective cation channels (NSCCs) that mediate toxic Na+ influx (Amtmann and Sanders, 1999; White, 1999; Demidchik et al., 2002b; Tester and Davenport, 2003). For example, in Arabidopsis root epidermis, 0.1 mm extracellular Ca2+ blocked one-half of the Na+ influx current through NSCCs; this blocking effect saturated at [Ca2+] higher than 2 mm (2–20 mm Ca2+ caused about 75%–80% inhibition; Demidchik and Tester, 2002). The Km values for Ca2+-induced block of NSCCs and 22Na+ uptake by Arabidopsis roots are remarkably similar (Demidchik and Tester, 2002; Essah et al., 2003). Down-regulation of Na+ influx through NSCCs could be the crucial mechanism in Ca2+ amelioration of salt toxicity, especially as other Na+-transporting systems are not particularly sensitive to Ca2+. Sodium influx through the (heterologously expressed) wheat (Triticum aestivum) low-affinity cation transporter, LCT1, was also inhibited by elevated external [Ca2+] (Amtmann et al., 2001). However, as concentrations below 10 mm Ca2+ were not effective and soil [Ca2+] are usually well below this level (Tisdale et al., 1993), the physiological importance of the Ca2+ effect on LCT1 is unclear (Amtmann et al., 2001). Another possible route for Na+ toxic influx in Arabidopsis, HKT1, showed no sensitivity to external Ca2+ (Tyerman and Skerrett, 1999; Rus et al., 2001).
It has long been known that salt tolerance is determined not only by a plant's ability to restrict Na+ accumulation in the cytosol, but also by its capacity to maintain a high cytosolic K+ to Na+ ratio (Maathuis and Amtmann, 1999; Chen et al., 2005). There is strong evidence that regulation of plasma membrane K+ transport by elevated Ca2+ may be as important as the restriction of Na+ uptake through NSCCs. It is known that Ca2+ sustains K+ transport and K+/Na+ selectivity at the plasma membrane in Na+-challenged plants (Laüchli, 1990; Maathuis and Amtmann, 1999). Arabidopsis sos3 plants (defective in Ca2+-based signaling) accumulate more Na+ and retain less K+ than wild type, consistent with a failure to regulate SOS1 and HKT1 (Liu and Zhu, 1998; Zhu, 2003). Recent experiments in our laboratory also suggested that SOS1 mutation affects intracellular K+ homeostasis, with a plasma membrane depolarization-activated, outward-rectifying K+ channel being a likely target (Shabala et al., 2005a). Supplemental Ca2+ in a physiological concentration range (1–10 mm) reduced, or even completely prevented, Na+-induced K+ efflux from salinized root and leaf tissues in broad bean (Vicia faba) and barley (Hordeum vulgare) plants (Shabala, 2000; Shabala et al., 2003, 2005b). However, Ca2+-sensitive, K+-permeable transporters that are targeted by supplemental Ca2+ to prevent K+ loss have never been delineated.
In this work, the above issues were addressed by combined application of a range of electrophysiological techniques (noninvasive ion flux measuring [MIFE] technique, multibarreled ion-selective electrodes for intracellular measurements, patch-clamp technique, membrane potential measurements) to study intracellular K+ homeostasis and K+ fluxes and currents through plasma membrane K+-permeable channels in Arabidopsis root and leaf mesophyll cells. We show that in both root and leaf cells, elevated external [Ca2+] can decrease or fully prevent Na+-induced K+ loss by inhibition of plasma membrane outwardly directed, K+-permeable channels. This effect on K+ channels could be another critical process in Ca2+ amelioration of NaCl-induced toxicity.
RESULTS
NaCl Induces Ca2+-Sensitive K+ Efflux in Both Arabidopsis Roots and Leaf
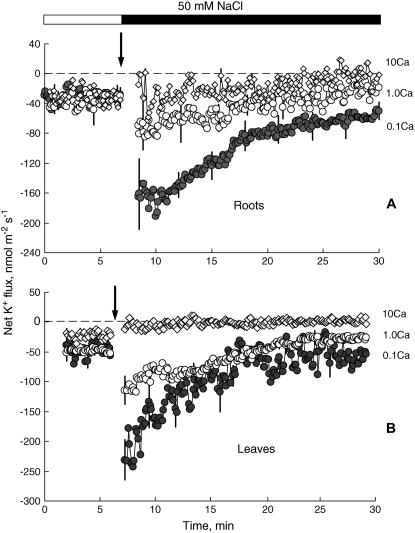
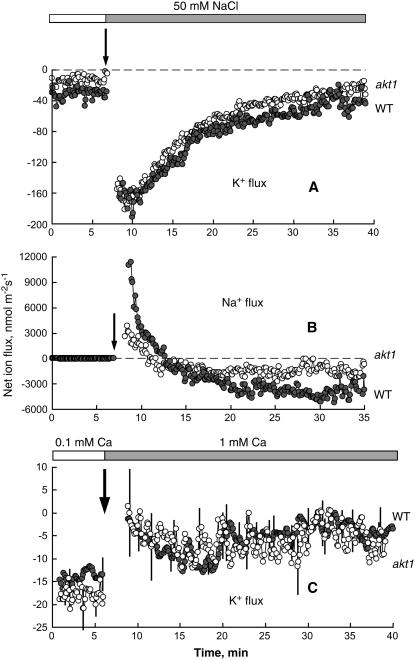
Mild salt stress in Arabidopsis seedlings is caused by 50 mm NaCl (Elphick et al., 2001). In the presence of 0.1 mm Ca2+, 50 mm NaCl added to the bath caused significant net K+ efflux from both Arabidopsis intact roots (mature epidermis) and leaf tissue (mesophyll segments; Fig. 1). The peak mean ± se K+ efflux (−182 ± 21.1 and −232 ± 8.5 nmol m−2 s−1 for roots and leaves, respectively; n = 7) was measured 1 to 1.5 min after salt application. During the next 15 to 20 min, the K+ flux gradually recovered but always remained negative (net efflux). No significant (P > 0.05) difference between leaf and root K+ flux responses to NaCl was found. Increasing the [Ca2+] in the bath from 0.1 to 1 mm caused a 3-fold reduction in salt-induced K+ efflux (Fig. 1). High bathing Ca2+ (10 mm) completely prevented salt-induced K+ efflux from both the root (Fig. 1A) and leaf (Fig. 1B) tissues. The ameliorative effect of increasing Ca2+ on K+ flux was also observed after long-term exposure to NaCl. Addition of 1 mm Ca2+ to tissue incubated in 0.1 mm Ca2+ and 50 mm NaCl for 3 h caused a significant (P < 0.05; Student's t test) shift in mean ± se steady-state K+ flux toward influx for roots (31.3 ± 3.3 nmol m−2 s−1; n = 6) and leaves (14.6 ± 1.9 nmol m−2 s−1; n = 5).
Figure 1.
Effect of salinity (50 mm NaCl) on net K+ fluxes measured from Arabidopsis (wild-type Columbia; A) root mature epidermis and leaf mesophyll tissue (B) at various bathing [Ca2+]. Data are mean ± se (n = 7). For all MIFE data, the sign convention is “influx positive.” Ionic concentration of the bath was as follows (in mm): 0.2 K+, 2 MES/4 Tris, pH 5.8 (adjusted with HCl), plus a required concentration of Ca2+.
NaCl-Induced K+ Efflux Is Unrelated to Cl− or Osmotic Shock and Sensitive to TEA+
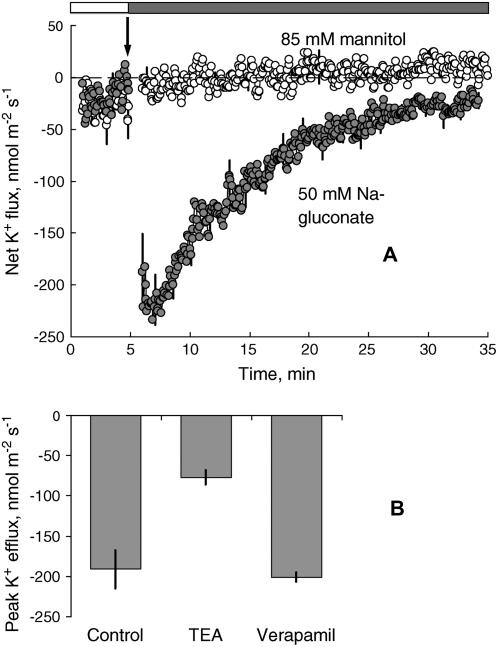
The NaCl-induced K+ efflux responses were not related to Cl−, as 50 mm sodium gluconate caused essentially identical responses to 50 mm NaCl from Arabidopsis roots (Fig. 2A; n = 6). When isotonic mannitol solution (85 mm) was added instead of 50 mm NaCl, no K+ efflux was observed (Fig. 2A). Similar results were also observed when fluxes were measured from leaf mesophyll tissue (data not shown). NaCl-induced K+ efflux was significantly inhibited by the K+ channel blocker TEA+ (70% inhibition; P < 0.05) but not by the Ca2+ channel blocker verapamil (Fig. 2B; n = 8). Sensitivity to TEA+ is an inherent property of K+ channels, making it possible to distinguish their conductance from all other cation-permeable channels (such as NSCCs and Ca2+ channels), which are TEA+ insensitive. Therefore, our data provide strong evidence that the greater part of the root and leaf NaCl-induced K+ effluxes was mediated by outwardly directed, K+-selective channels.
Figure 2.
Specificity and pharmacology of net K+ flux responses. A, Transient K+ fluxes in response to isotonic mannitol (white symbols) and sodium gluconate (gray symbols). Fluxes were measured from the mature epidermis of Arabidopsis wild-type roots after 1 to 1.5 h incubation in basic measuring solution. Data are mean ± se (n = 8). B, Effect of cation channel blockers (TEACl, 10 mm; verapamil, 20 μm) on the magnitude of the peak K+ efflux measured in response to 50 mm NaCl. Data are mean ± se (n = 6–8).
NaCl-Induced K+ Efflux Is Preceded by Na+ Influx
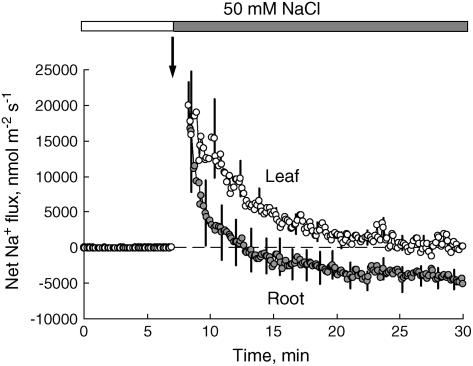
Immediately after the onset of salt stress, dramatic net Na+ influx was measured in both leaf and root tissue (Fig. 3; n = 6 and 8, respectively). Peak Na+ influx preceded peak K+ efflux by approximately 0.5 to 1 min (for comparison, see Fig. 1, A and B). In roots, Na+ influx was short lived, with net Na+ efflux measured 6 to 8 min after the beginning of treatment. Net Na+ flux returned back to zero level within 1 h (data not shown). No such efflux was measured from Arabidopsis leaf mesophyll samples, where net Na+ flux peaked immediately after NaCl treatment and gradually returned back to zero level within 15 min (Fig. 3). No significant effect (n = 5; P > 0.05) of TEA+ (20 mm) on Na+ influx was found when tested on root tissue (data not shown).
Figure 3.
Salinity-induced net Na+ flux kinetics measured from Arabidopsis wild-type root (mature epidermis; gray symbols) and leaf tissue (mesophyll; white symbols). Bath [Ca2+] was 0.1 mm. Data are mean ± se (n = 6–8).
Elevated Ca2+ Reduces NaCl-Induced Plasma Membrane Depolarization in Arabidopsis
As it is known that Na+ influx depolarizes the plasma membrane (Shabala et al., 2003), the observed K+ efflux may originate from depolarization-activated K+ channels (DAPCs). Accordingly, it was hypothesized that ameliorative effects of supplemental Ca2+ could be attributed, at least partially, to less dramatic membrane depolarization under these conditions. Indeed, high Ca2+ concentrations in the bath caused a statistically significant (P < 0.05) reduction in the magnitude of NaCl-induced membrane depolarization in epidermal root cells (Table I). Interestingly, elevated Ca2+ levels in the bath made plasma membrane potential less negative in the absence of salt stress (Table I). As a result, when confronted with salinity, the magnitude of membrane potential depolarization between various Ca2+ treatments was severalfold different (71.8 ± 4.1, 50.6 ± 2.7, and 29.4 ± 3.3 mV for 0.1, 1, and 10 mm Ca2+, respectively; n = 9).
Table I.
Steady-state values of membrane potential of Arabidopsis root epidermal cells in control and after 1 h of 50 mm NaCl treatment at various calcium concentrations
Means ± se (n). Treatments with different superscript letters are significantly different at P < 0.05.
| [Ca2+] in the Bath | Membrane Potential
|
|
|---|---|---|
| Control | 50 NaCl | |
| mm | mV | |
| 0.1 | −127.5 ± 4.8 (9)a | −55.7 ± 3.4 (9)c |
| 1.0 | −118.5 ± 3.7 (9)a,b | −67.9 ± 1.7 (9)d |
| 10 | −103.7 ± 4.8 (9)b | −74.3 ± 1.7 (9)e |
K+ Efflux from the Cell Results in a Reduction in Cytosolic K+ Content
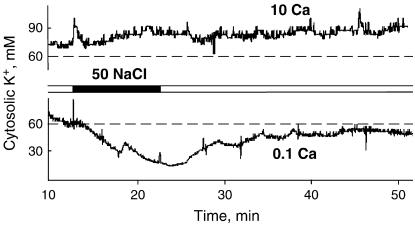
Noninvasive measurements of K+ fluxes from NaCl-stressed roots were complemented by measuring changes in the cytosolic free K+ concentrations ([K+]cyt) using multibarreled ion-selective microelectrodes. Under low (0.1 mm) Ca2+ conditions, imposition of salt stress caused a rapid and substantial decline in [K+]cyt (Fig. 4). The effect was reversible, and removal of NaCl stress resulted in a rapid recovery of [K+]cyt to almost the original level (in short-term experiments), most likely as a result of the buffering role of the vacuole. High Ca2+ levels in the bath efficiently prevented the NaCl-induced decrease in [K+]cyt (Fig. 4). The difference between Ca2+ treatments is statistically significant at P < 0.05 (53 ± 16 mm and 5 ± 6 mm changes for low and high Ca2+, respectively; n = 5). Taken together, our results suggest that noninvasive K+ flux measurements (using the MIFE technique) and cytosolic K+ measurements (using impaled multibarreled microelectrodes) are complementary, at least in short-term salinity experiments.
Figure 4.
Effect of salinity (50 mm NaCl) on changes in cytosolic free K+ concentration in mature epidermal Arabidopsis (wild-type Columbia) root cells at low (0.1 mm) and high (10 mm) bathing [Ca2+]. One (of five) representative example for each treatment is shown.
K+ Inward Rectifier Is Not Involved in NaCl-Induced K+ Flux
To discount the involvement of plasma membrane hyperpolarization-activated K+ channels, tests were conducted on the akt1 mutant that lacks such inwardly directed K+ channels (Hirsch et al., 1998). No significant difference (P > 0.05) was found between NaCl-induced K+ (Fig. 5A) and Na+ (Fig. 5B) flux responses between root mature epidermis of wild type (Arabidopsis Columbia ecotype) and akt1. K+ fluxes from salinized wild-type and akt1 roots also responded similarly to supplemental Ca2+ (Fig. 5C), suggesting that the AKT1 K+ inward-rectifying channel does not mediate the observed ameliorative effects of supplemental Ca2+ on K+ transport in salinized Arabidopsis roots.
Figure 5.
A and B, Salinity-induced net K+ (A) and Na+ (B) flux kinetics measured from mature root epidermis of Arabidopsis wild-type (gray symbols) and akt1 (white symbols) plants. Roots were incubated in basic measuring solution for about 1 h before 50 mm NaCl was added. Data are mean ± se (n = 6–9). C, Effect of supplemental Ca2+ on net K+ fluxes measured from the mature epidermis of Arabidopsis wild-type (gray symbols) and akt1 (white symbols) roots in the presence of 50 mm NaCl. Data are mean ± se (n = 6–8). Roots were exposed to salinity in low Ca2+ (0.1 mm) solution for 3 h prior to Ca2+ treatment.
Overall, our results show that elevated [Na+] induces Ca2+-sensitive net K+ efflux and that this efflux is likely to be mediated by activation of plasma membrane TEA+-sensitive, outwardly directed K+ channels. To understand the basis of Ca2+ amelioration, modes of Ca2+ action on Arabidopsis root and leaf K+ efflux channels were investigated using the patch-clamp technique. For roots, vectorial effects of Na+ on either side of the membrane were also examined.
K+ Efflux Channels in Root Epidermal Protoplasts Are Blocked by External Ca2+ and Na+
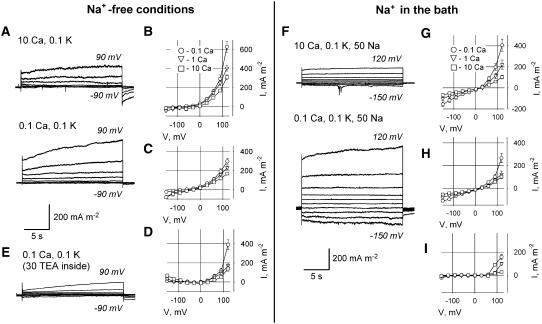
Depolarizing voltage pulses activated large outward currents (Fig. 6, A–E; 0.1 and 80 mm extracellular and intracellular K+, respectively). When 30 mm TEA+ replaced K+ in the pipette solution (PS), outward currents decreased severalfold (Fig. 6E; higher [TEA+] caused seal instability). This demonstrates that outward currents were mainly mediated by K+ efflux.
Figure 6.
Whole-cell plasma membrane currents in protoplasts derived from Arabidopsis mature root epidermis in control (Na+-free) conditions (A–E) and in 50 mm extracellular Na+ solution (F–I). A, Typical currents (recorded from the same protoplast) in 10 mm (top) and 0.1 mm (bottom) extracellular Ca2+ in control solution. B to D, Mean ± se I-V relationships (n = 4–9) for total (B), instantaneous (C), and time-dependent (D) currents measured at 0.1, 1, and 10 mm extracellular Ca2+ in Na+-free solution. F, Typical currents (recorded from the same protoplast) in 10 mm (top) and 0.1 mm (bottom) extracellular Ca2+ with 50 mm Na+ present in the bath. G to I, Mean I-V relationships (n = 5–6) for total (G), instantaneous (H), and time-dependent (I) currents measured at 0.1, 1, and 10 mm extracellular Ca2+ in the presence of 50 mm Na+ in the bath. Time-dependent currents were calculated as described in “Materials and Methods.” In all sections except E, PS contained 50 mm potassium gluconate and 30 mm KCl. E, Typical currents in 0.1 mm extracellular Ca2+ when K+ salts in PS were replaced by 30 mm TEACl. Extracellular K+ was 0.1 mm throughout. All concentrations are given in mm.
Measured mean Erev (−77 mV at 0.1 mm external Ca2+ for total current) was much closer to EK (calculated as −163 mV) than to ECl (calculated as 116 mV), showing K+ selectivity of the dominating conductance. Elevated extracellular [Ca2+] partially blocked K+ efflux currents (Fig. 6, A–D). Mean ± se K+ outward current (activated by 19.4-s-long, single-step depolarization from −70–120 mV) was 628 ± 59 mA m−2 (n = 4) with 0.1 mm Ca2+ in the bath. This current was 30% to 40% smaller in 1 mm Ca2+ (403 ± 32 mA m−2; n = 4) and approximately 50% smaller in 10 mm Ca2+ (308 ± 26 mA m−2; n = 4). Mean Erev was shifted toward EK (calculated as −163 mV) from Erev = −5 mV to Erev = −77 mV when [Ca2+] decreased from 10 mm to 0.1 mm, suggesting that elevated [Ca2+] decreased membrane selectivity to K+.
The outward K+ current comprised rapidly activated (instantaneous) and slowly activated (time-dependent) components. At 120 mV and 0.1 mm extracellular Ca2+, the mean time-dependent current was the greatest (Fig. 6, C and D) and had more pronounced rectification. Elevated extracellular [Ca2+] inhibited amplitude and weakened the rectification of both current components (Fig. 6, C and D, respectively). The time-dependent component of the outward K+ currents was more sensitive to Ca2+ than the instantaneous component (Fig. 6, C and D). These results show that at least two groups of cation channels mediate K+ efflux from Arabidopsis root epidermis protoplasts. These two groups have different kinetics, rectification, and sensitivity to Ca2+.
NaCl (50 mm) was applied to the bathing medium to mimic the early stages of salt stress. Addition of NaCl to the bathing solution dramatically destabilized patch seals. In six protoplasts from 22 patches, responses to extracellular 50 mm NaCl were successfully recorded at two Ca2+ concentrations (1 and 10 mm); in five of those six protoplasts, the NaCl responses at all three [Ca2+] levels (0.1, 1, and 10 mm) were recorded (Fig. 6, F–I). Addition of elevated [Ca2+] to a background of 50 mm external NaCl inhibited mean K+ efflux currents (from 406 ± 56 mA m−2 [n = 5] at 0.1 mm Ca2+ to 220 ± 44 mA m−2 [n = 6] and 102 ± 18 mA m−2 [n = 6] at 1 and 10 mm Ca2+, respectively, as measured at 120 mV). Extracellular Na+ blocked the time-dependent K+ efflux current, with the current measured in 0.1 mm CaCl2 and 50 mm NaCl being one-half that measured in 0.1 mm CaCl2 alone. The Na+-insensitive, time-dependent current (measured in the presence of 50 mm NaCl) was very sensitive to elevated [Ca2+] (Fig. 6I, squares). These data suggest that Na+ and Ca2+ might target different types of outwardly rectifying, K+-permeable channels. The blocking effect of Na+ on the instantaneous K+ efflux current was much weaker than on the time-dependent current (Fig. 6H).
Addition of 50 mm NaCl to the bath also caused a large Ca2+-sensitive inward Na+ current (Fig. 6, F–H). This current shared characteristics with the Na+ influx current through NSCCs in mature epidermis protoplasts (ATP-free PS) previously reported by Demidchik and Tester (2002) and Demidchik et al. (2002a).
Effect of Internal Na+ on Ca2+-Sensitive K+ Efflux Channels in Root Epidermal Protoplasts
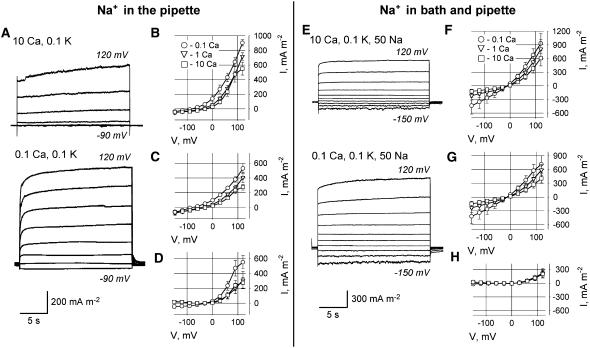
Addition of 50 mm sodium gluconate solely to the PS (with 30 mm KCl and 50 mm potassium gluconate still present in PS) caused an approximately 50% increase in the mean ± se total efflux current (up to 903 ± 43, 717 ± 49, and 552 ± 89 mA m−2 at external 0.1, 1, and 10 mm Ca2+, respectively, as measured in four to five protoplasts at 120 mV), suggesting that K+ efflux channels allowed Na+ efflux (Fig. 7, A–D). Both instantaneous and time-dependent current components increased, showing that they both are permeable to Na+. In these conditions, the blocking effect of elevated extracellular [Ca2+] was qualitatively similar to the effect in NaCl-free conditions. In the presence of 50 mm NaCl in both pipette and bath solutions, an extracellular [Ca2+] increase from 0.1 to 10 mm caused about a 1.5-fold reduction of total outward cation current (from 1,133 ± 208–607 ± 167 mA m−2, respectively, as measured for four protoplasts at 120 mV; Fig. 7). Application of salt to both sides of the plasma membrane induced an approximate 2-fold decrease in mean time-dependent outward cation current at 0.1 mm Ca2+ (V = 120 mV) as compared to salt-free conditions and fully inhibited its Ca2+ sensitivity (Fig. 7H; n = 4). As a result, Ca2+ blockage of the total outward cation current (Fig. 7F) was solely the result of suppression of the instantaneous current.
Figure 7.
Whole-cell plasma membrane currents (protoplasts derived from Arabidopsis mature root epidermis) with 50 mm Na+ present in the patch pipette (A–D), and when 50 mm Na+ was present both in the bath and PS (E–H). A, Typical currents (recorded from the same protoplast) in 10 mm (top) and 0.1 mm (bottom) extracellular Ca2+ for Na+ present in PS only. B to D, Mean ± se I-V relationships (n = 4–5) for total (B), instantaneous (C), and time-dependent (D) currents measured at 0.1, 1, and 10 mm extracellular Ca2+. E, Typical currents (recorded from the same protoplast) in 10 mm (top) and 0.1 mm (bottom) extracellular Ca2+ for symmetrical 50 mm Na+ conditions. F to H, Mean ± se I-V relationships (n = 4–5) for total (F), instantaneous (G), and time-dependent (H) currents measured at 0.1, 1, and 10 mm extracellular Ca2+. Time-dependent currents were calculated as described in “Materials and Methods.” PS contained 50 mm potassium gluconate, 30 mm KCl, and 50 mm sodium gluconate. Extracellular K+ was 0.1 mm. All concentrations are given in mm.
Surprisingly, Ca2+-sensitive Na+ influx currents increased dramatically when 50 mm NaCl was applied to both sides of the plasma membrane (Fig. 7E) as compared to conditions when this salt concentration was only added to the bath (Fig. 6E). This increase was not due to Cl− efflux because when 50 mm NaCl was added to the pipette, the inward current was much smaller (Fig. 7A). This might suggest the existence of positive feedback regulation of toxic Na+ uptake by accumulated Na+ and, if so, be important for understanding mechanisms of plant salt tolerance.
K+ Efflux Channels in Mesophyll Protoplasts Are Sensitive to External Ca2+ and Na+
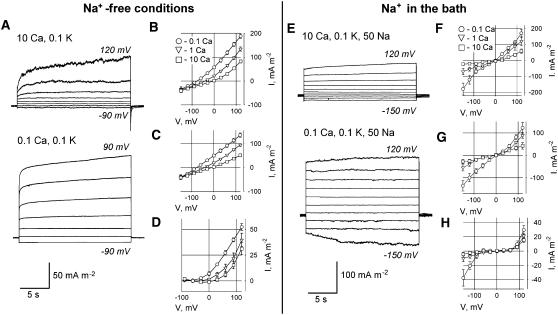
In leaf mesophyll protoplasts, the success rate for forming stable, high-resistance seals in 10 mm CaCl2 bathing solution was approximately 5%. In NaCl-free conditions, the density of K+ efflux current in mesophyll protoplasts (Fig. 8B) was about 3 times smaller than in root protoplasts (Fig. 6B). The contribution of mean ± se time-dependent current (52.2 ± 3.3 mA m−2; V = 120 mV; n = 4) to the total K+ outward current (187.9 ± 9.3 mA m−2; V = 120 mV; n = 4) at 0.1 mm extracellular Ca2+ was much lower than in root protoplasts (391 ± 52 and 628 ± 59 mm m−2, respectively; n = 3). Elevated Ca2+ caused up to 2-fold reduction of the total K+ outward current, mainly affecting the instantaneous current (Fig. 8C). Similar to root protoplasts, a decrease in extracellular [Ca2+] shifted Erev toward EK, suggesting that elevated Ca2+ reduces membrane selectivity to K+. If at 0.1 mm extracellular Ca2+, 80 mm K+ in the PS was replaced by 30 mm TEA+ (30 mm TEACl), 5 times smaller outward currents were recorded (37.9 ± 7.3 mA m−2; V = 120 mV; n = 4), indicating an involvement of K+ channels in catalyzing the outwardly directed conductance in mesophyll protoplasts.
Figure 8.
Whole-cell plasma membrane currents in protoplasts derived from Arabidopsis leaf mesophyll tissue in control (Na+-free) conditions (A–D) and in 50 mm extracellular Na+ solution (E–H). A, Typical currents (recorded from the same protoplast) in 10 mm (top) and 0.1 mm (bottom) extracellular Ca2+ in control solution. B to D, Mean ± se I-V relationships (n = 4–11) for total (B), instantaneous (C), and time-dependent (D) currents measured at 0.1, 1, and 10 mm extracellular Ca2+ in Na+-free solution. E, Typical currents (recorded from the same protoplast) in 10 mm (top) and 0.1 mm (bottom) extracellular Ca2+ with 50 mm Na+ present in the bath. F to H, Mean I-V relationships (n = 5–6) for total (F), instantaneous (G), and time-dependent (H) currents measured at 0.1, 1, and 10 mm extracellular Ca2+ in the presence of 50 mm Na+ in the bath. Time-dependent currents were calculated as described in “Materials and Methods.” PS contained 50 mm potassium gluconate and 30 mm KCl. Extracellular K+ was 0.1 mm throughout. All concentrations are given in mm.
Addition of 50 mm NaCl to the bathing solution strongly destabilized the seals, particularly at 0.1 mm [Ca2+]. From 42 patches tested in these conditions, only five protoplasts showed stable seal characteristics at all three bath [Ca2+] examined (Fig. 8, E–H). In the presence of 50 mm NaCl, the Ca2+ sensitivity of instantaneous and time-dependent outward currents resembled that in NaCl-free conditions (compare Fig. 8, C and G). Addition of 10 mm Ca2+ significantly shifted Erev of time-dependent, outwardly directed conductance toward ECa (compare Fig. 8, D and H).
DISCUSSION
Cytosolic K+ is homeostatically maintained at a steady-state level (Leigh, 2001), but under saline conditions it dramatically decreases (Carden et al., 2003). This is further supported by our data (see Fig. 4) showing rapid and substantial decline in [K+]cyt upon NaCl treatment at low external Ca2+. This effect appears to be prolonged, as in barley, where 8-d-long treatment with 200 mm NaCl resulted in the cytosolic [K+] dropping from 70 to 39 mm (Carden et al., 2003). K+ loss per se is harmful for cell physiology and biochemistry. The problem is exacerbated when K+ loss is accompanied by accumulation of Na+ that replaces K+ in metabolic reactions, thus further impairing enzymatic activity and disturbing metabolism. Under high external [NaCl], Na+ accumulates in the cytosol in the 50 to 200 mm range (Binzel et al., 1988; Maathuis and Amtmann, 1999; Flowers and Hajibagheri, 2001), resulting in many-fold decline in cytosolic K+ to Na+ ratio and eventual cell death. Elevation of external Ca2+ can ameliorate toxicity symptoms induced by salinity (LaHaye and Epstein, 1969; Läuchli, 1990; Martinez and Läuchli, 1993; Reid and Smith, 2000; Cramer, 2002), including excessive loss of K+ (Shabala et al., 2003, 2005b). Here, we report a mechanism of salinity-induced K+ loss from plant cells and a mechanism for its prevention by high [Ca2+], namely, K+ efflux through outwardly directed, K+-permeable channels and their regulation (block) by elevated extracellular Ca2+.
The initial small but significant (P < 0.05) net K+ efflux from both root and leaf tissues is likely to be attributed to the difference in K+ level in the growth and measuring media (Fig. 1). Following the standard procedure, plants were grown in a K+-rich (20 mm) Murashige and Skoog medium. Such “luxury” conditions could make high-affinity K+ transport systems redundant. Earlier, a substantial down-regulation of AtHAK5 transporter by elevated K+ levels was shown for both Arabidopsis root and leaf tissues (Ahn et al., 2004; Armengaud et al., 2004). To enable the optimal signal to noise ratio during measurements, plants were measured in much poorer K+ conditions (0.2 mm K+ in the bath). The time passed since roots were transferred from the petri dish to the measuring chamber (approximately 1 h) is probably not long enough to up-regulate the expression of high-affinity K+ transporters to compensate for K+ movement down the electrochemical gradient. Thus, net initial K+ efflux was measured in steady-state conditions (Fig. 1).
There are several lines of evidence suggesting that the observed NaCl-induced K+ originates from the plasma membrane rather than the apoplast (e.g. resulting from the Donnan exchange in the cell wall; Ryan et al., 1992; Arif and Newman, 1993). First, K+ flux was sensitive to TEA+, a known K+ channel blocker (White, 1997). Second, qualitatively similar kinetics of K+ efflux was measured from leaf mesophyll tissue and protoplasts (where the cell wall is absent) isolated from this tissue (data not shown). Third, we have earlier shown that, regardless of the amount of Ca2+ in the bath solution (0.1, 1, and 10 mm concentrations tested), transient NaCl-induced kinetics of Ca2+ flux from the cell wall was not significantly affected (figure 4 in Shabala and Newman, 2000). Fourth, qualitatively similar results are observed between the effects of Ca2+ on net K+ flux measured with MIFE and those on outward K+ currents measured from protoplasts in patch-clamp experiments (Figs. 1 and 6, respectively). Taken together, this suggests that the overall contribution (if any) of the Donnan exchange in the cell walls toward the NaCl-induced K+ fluxes measured in our experiments was negligible.
Using the MIFE technique, we have demonstrated that treatment of intact Arabidopsis leaf and root with 50 mm NaCl induced TEA+-sensitive K+ efflux and this was fully prevented by elevated external Ca2+. Thus, it is shown that NaCl-induced loss of K+ is catalyzed by K+ channels (by TEA+ sensitivity) and that regulating these channels by Ca2+ underlies a Ca2+ amelioration of this toxic effect. This effect is unrelated to the osmotic component of salt stress and is not caused by Cl−, indicating the possibility of Na+-specific effects. To fully address this issue, a range of monovalent cation salts has to be tested. Unfortunately, due to methodological issues (e.g. a poor K+ LIX selectivity between K+ and Cs+), such experiments are currently not feasible. Recent experiments on barley mesophyll tissues also showed TEA+ sensitivity of NaCl-induced K+ efflux (Shabala et al., 2005b). Also, previous studies on root and leaf of barley, bean, and wheat suspension cells (Babourina et al., 2000; Shabala, 2000; Shabala et al., 2003, 2005a, 2005b) demonstrated magnitudes of K+ efflux that were similar to Arabidopsis. Taken together, these results suggest that data for Arabidopsis can be extrapolated to agricultural plants (at least to barley and beans) and can therefore be used in applied plant biology. Another important finding of this work is that such physiologically different plant tissues as root epidermis and leaf mesophyll share similar ion channel-mediated mechanisms of Na+-induced K+ loss and its amelioration by Ca2+.
There are two known TEA+-sensitive, K+-transporting systems in the Arabidopsis plasma membrane: hyperpolarization- and depolarization-activated K+ channels or, in other terminology, K+ inward and outward rectifiers, respectively (Maathuis and Sanders, 1995; Véry and Sentenac, 2003). A contribution from the third system—voltage-insensitive, outwardly directed NSCCs—cannot be completely ruled out, although White and Tester (1992) showed that TEA+ permeates but does not block root NSCCs. Since Na+-induced, TEA+-sensitive K+ efflux was not affected in akt1 plants, we can rule out the idea that this efflux was caused by Na+ block of K+ influx channels. So, the activation of DAPCs and NSCCs most likely underlies the Na+-induced K+ efflux. This is consistent with the strong NaCl-induced depolarization found in our experiments (Table I) and reported elsewhere for both root and leaf tissues (Cakirlar and Bowling, 1981; Babourina et al., 2000; Horie et al., 2001; Laurie et al., 2002; Shabala et al., 2003). This depolarization is caused by the massive Na+ influx and preceding K+ efflux, at least in intact Arabidopsis roots (Figs. 1A and 3). Thus, in addition to the direct blockage of K+ efflux channels (as discussed below) by elevated Ca2+, their indirect control by Ca2+-sensitive, Na+-dependent membrane depolarization also is likely (Table I; Fig. 6).
Arabidopsis DAPCs and NSCCs can be distinguished by their activation kinetics, voltage dependence, and pharmacology (Maathuis and Sanders, 1995; White, 1997; Romano et al., 1998; Demidchik and Tester, 2002; Demidchik et al., 2002b). Arabidopsis root cortex DAPC was shown to be weakly selective between K+ and Na+ (Maathuis and Sanders, 1995). Blom-Zandstra and Vogelzang (2003) also reported that outward-rectifying cation currents in the pith cells of sweet pepper (Capsicum annuum) were permeable to Na+. Additionally, it is known that DAPCs and NSCCs exist in Arabidopsis plasma membrane of root and leaf at the single-channel level (Spalding et al., 1992; Romano et al., 1998; Demidchik and Tester, 2002). Indeed, the K+ efflux currents that we found in whole-cell recordings contained two current components with contrasting kinetics (time-dependent and instantaneous, respectively) and rectification (steep and weak, respectively). Both these components were poorly permeable for TEA+, conducted K+ and Na+, and were sensitive to elevated Ca2+. Based on kinetics and voltage dependence, we suggest that these two components were mediated by DAPCs and NSCCs.
In NaCl-free conditions, the time-dependent and instantaneous K+ efflux currents of root protoplasts were approximately equal (Fig. 6, C and D). In contrast, the leaf protoplast's instantaneous current was 2 to 3 times larger than its time-dependent current (Fig. 8, C and D). In general, this shows that root cells better select for K+ over other cations than leaf cells (probably because their function is in selective uptake of ion from the soil solution) and that NSCCs play dominating roles in catalyzing K+ efflux in the leaf. Root protoplast time-dependent currents were more sensitive to elevated external [Ca2+] than instantaneous currents. In contrast, instantaneous currents in mesophyll protoplasts revealed higher sensitivity to Ca2+ than time-dependent currents (Fig. 8, C and D). This suggests that both DAPCs and NSCCs differ in root and leaf (at least they have different Ca2+-binding sites). They could also have different molecular origins. Unfortunately, only a few candidate genes have been characterized so far in Arabidopsis (for review, see Shabala, 2003; Véry and Sentenac, 2003). AKT2/3 is an instantaneously activated K+-permeable channel, showing only a weak rectification that can be efficiently blocked by physiological concentrations of Ca2+ (Marten et al., 1999; Lacombe et al., 2000; Cherel et al., 2002). Recent studies have shown AKT2/3 to be in mesophyll (Dennison et al., 2001). To our knowledge, there is no clear candidate channel for the mesophyll time-dependent conductance. Arabidopsis guard cell GORK is a steeply rectifying, slowly activating K+ efflux channel but it is not expressed in the mesophyll (Hosy et al., 2003). Arabidopsis root outwardly directed NSCCs and DAPCs have not been characterized at the molecular level, although AtGORK is expressed in both root hairs and root epidermal cells (Ivashikina et al., 2001).
One of key results of this study is that presence of Na+ at different sides of the plasma membrane dramatically changes K+ efflux conductances and, particularly, their Ca2+ sensitivity (that is critical for Ca2+ amelioration). Extracellular Na+ (50 mm) had no effect on either root or leaf instantaneous K+ efflux currents, but dramatically decreased time-dependent currents in both cell types (Figs. 6 and 8). These results are consistent with the generally accepted Na+ insensitivity of NSCCs (for review, see Demidchik et al., 2002b) and high Na+ sensitivity of DAPCs (Tyerman et al., 1997; Volkov et al., 2004). In leaf protoplasts, extracellular Na+ completely blocked Ca2+-sensitive, time-dependent K+ efflux currents, but NSCCs remained responsive to Ca2+ as in Na+-free conditions (Fig. 8). So, under saline conditions, Ca2+ prevents K+ loss from the leaf tissue almost exclusively through the inhibition of instantaneously activated voltage-independent conductance (most likely mediated by NSCCs). When external 10 mm Ca2+ and 50 mm Na+ were applied together to root protoplasts, the time-dependent K+ efflux currents almost disappeared (Fig. 6I). Thus, in saline conditions, Ca2+ can probably switch off DAPC-mediated root K+ loss.
As mentioned above, according to different estimates, under salt stress cytosolic [Na+] can reach up to 200 mm level, with most authors favoring values in the 50 to 100 mm range (Maathuis and Amtmann, 1999). We used 50 mm Na+ because it did not disturb the gigaohmic seal and corresponded to the conditions of a mild salt stress, or the initial step of more strong stress, when the plant activates defense mechanisms. We have tested how Na+ accumulation can affect K+ efflux currents and their Ca2+ sensitivity in Arabidopsis root epidermis protoplasts. Addition of 50 mm Na+ solely to the PS resulted in an increase of both instantaneous and time-dependent currents, demonstrating both are permeable to Na+ (Fig. 7, C and D). However, this did not affect Ca2+ sensitivity of efflux currents. When 50 mm Na+ was added to both pipette and bath (mimicking chronic salinity stress), time-dependent efflux currents lacked Ca2+ sensitivity (Fig. 7H), but instantaneous currents were still inhibited by elevated [Ca2+] (Fig. 7G). This resembled the behavior of K+ efflux currents of leaf protoplasts measured in Na+-free PS (Fig. 8, C and D). Intriguingly, in contrast to K+ efflux currents, application of Ca2+ only weakly inhibited time-dependent and instantaneous Na+ efflux currents, suggesting additional beneficial effects of this divalent cation for achieving a healthy K+ to Na+ ratio.
In conclusion, the data reported here show that K+ loss caused by NaCl is due to Na+-induced, TEA+-sensitive K+ efflux, most likely mediated by two groups of outwardly directed, K+-permeable channels: DAPCs and NSCCs. Elevated Ca2+ regulates both these channels and prevents K+ loss in roots and leaves. Under saline conditions, Na+ significantly inhibits DAPCs but not NSCCs. As a result, under these conditions, the Ca2+-sensitive component of K+ efflux is largely determined by NSCCs. These results significantly challenge the conventional view of the mechanisms of ameliorative Ca2+ action in plants. It appears that in addition to the widely accepted inhibiting effect of Ca2+ on Na+ influx through NSCCs, apoplastic Ca2+ also prevents K+ loss from the cell by regulating (both directly and indirectly) K+ efflux channels. Further experiments using K+ transport mutants (and, specifically, those for K+ efflux channels) will provide more conclusive and specific evidence for the ameliorative effects of Ca2+ on K+ efflux channels.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana; wild-type Columbia and akt1 mutant) seeds were obtained from Nottingham Arabidopsis Stock Centre (Nottingham, UK). Plants were grown vertically at 22°C (100 μmol m−2 s−1 irradiance; 16-h daylength) in sterile conditions on full-strength Murashige and Skoog medium (Duchefa) with 1% (w/v) Suc and 0.35% (w/v) Phytagel (Sigma). Seven- to 15-d-old plants were used for root measurements. For measurements on leaf mesophyll, 7-d-old seedlings were transplanted into soil (fertilized potting mix; see Shabala [2000] for details) and grown in pots until approximately 3 weeks old. Fully grown rosette leaves were excised and used in both MIFE and patch-clamp experiments.
Measurements of Net K+ and Na+ Fluxes
Net fluxes of K+ and Na+ were measured noninvasively using ion-selective vibrating microelectrodes (the MIFE technique; University of Tasmania, Hobart, Australia), generally as described in our previous publications (Shabala et al., 1997; Shabala and Lew, 2002). Microelectrodes were constructed from silanized borosilicate glass capillaries using ionophore cocktails 60031 for K+ and 71176 for Na+ (Sigma-Aldrich). The electrodes were calibrated in sets of standard solutions (0.1–1 mm for K+; 0.2–50 mm for Na+) before and after use. Electrodes with a response of less than 50 mV per decade and correlation less than 0.999 were discarded.
The ion-selective electrodes were mounted on a manipulator providing three-dimensional positioning and positioned 20 μm above the tissue surface. During measurements, a computer-controlled stepper motor moved the electrodes between 20 to 50 μm from the tissue surface at a frequency of 0.1 Hz. The recorded potential differences were converted into electrochemical potential differences using the calibrated Nernst slopes of the electrodes. The initial 1- to 2-s interval after electrode movement was ignored to allow for settling of the system. Ion fluxes were calculated by MIFEFLUX software as described by Newman (2001).
The procedure of isolation of the leaf mesophyll segments for MIFE measurements was adopted from Shabala and Newman (1999) and Shabala (2000). Briefly, epidermal tissue of leaf segments was removed from the abaxial leaf surface with fine forceps. Small (3- × 4-mm) segments were cut, avoiding major venation, and left floating peeled-side down on the surface of aerated experimental solution. To avoid possible confounding effects of tissue damage during epidermis removal, ion flux measurements were started several hours after preparation (Shabala and Newman, 1999). For MIFE measurements of Arabidopsis roots, an apical root segment (approximately 8 mm long) was excised and used. Root or leaf segments were mounted horizontally in a Plexiglas holder and placed in a 4-mL measuring chamber. The chamber containing a sample (a leaf segment or an excised root apex) was filled with basic measuring solution containing 0.2 mm KCl, 2 mm MES/4 mm Tris (pH 5.8 adjusted with HCl) and a required amount of CaCl2, and placed on a three-dimensional hydraulic manipulator as described by Shabala (2000). Steady-state ion fluxes were measured for 5 to 10 min. Then the test treatment was applied and measurements of transient ion flux kinetics were taken for another 40 to 50 min.
Protoplast Isolation
Protoplasts were isolated from Arabidopsis root mature epidermis using the enzymatic digestion procedure described by Demidchik et al. (2002a). Isolated protoplasts were kept on ice up to 10 h in holding solution containing (in mm) 1 CaCl2, 1 MgCl2, 3 KCl, 10 Glc, pH 5.7 (1 mm MES/0.5 mm Tris base), osmolality 300 mOsM adjusted with d-sorbitol (SigmaUltra; used in all patch-clamp experiments). Protoplasts from leaf mesophyll cells were isolated from plasmolyzed leaves using a procedure adapted from Elzenga et al. (1991). Briefly, adaxial leaf epidermis was removed with forceps (Dumont No. 7). Peeled leaves were floated (peeled-side down) on enzyme solution (2% [w/v] Cellulase Onozuka RS [Yakult Honsha], 1.2% [w/v] cellulysin [CalBiochem], 0.1% [w/v] pectolyase Y-23 [Yakult Honsha], 0.1% [w/v] bovine serum albumin [Sigma], 10 mm KCl, 10 mm CaCl2, 2 mm MgCl2, 2 mm MES, pH 5.7 adjusted with Tris base, 700 mOsM adjusted with d-sorbitol) at 30°C with rotation (60 rpm). After 7 to 10 min of incubation, leaves were transferred into wash solution (as above minus enzymes) and washed twice, for 3 and 5 min, respectively, at 30°C with rotation (60 rpm). Protoplasts were released by gently shaking the plasmolyzed leaf tissue in 1 mL of an ice-cold release solution (10 mm CaCl2, 5 mm KCl, pH 5.7, 380 mOsM adjusted with d-sorbitol). The preparation was twice centrifuged for 4 min at 70g to 80g and resuspended in a fresh release solution. The supernatant was discarded and the remaining 0.2-mL aliquot containing mesophyll protoplasts was kept on ice for patch-clamp experiments.
Patch-Clamp Electrophysiology
Protoplasts of 15 to 20 μm diameter were patch clamped in the whole-cell mode. GΩ resistance seals were obtained in sealing solution containing 10 to 20 mm CaCl2, 0.1 mm KCl, 2 mm MES, pH 5.7 adjusted with Tris, 300 mOsM (adjusted by d-sorbitol). After seal formation, Ca2+ concentration in the bathing solution was reduced. Measurements were carried out sequentially at three bath [Ca2+] levels: 10, 1, and 0.1 mm. This order minimized seal breakdowns. [Ca2+] lower than 0.1 mm were not used because they caused seal instability. If extracellular 0.1 mm Ca2+ did not cause breakdown of the high-resistance seal, then Ca2+ was elevated to 1 then 10 mm to ensure that there was no seal resistance change. KCl (0.1 mm) was added to all bathing solutions to facilitate EK calculations. Bathing solution was adjusted to 300 (root protoplasts) or 380 mOsM (leaf protoplasts) with d-sorbitol. Basic PS contained the following (in mm): 2 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, 0.8 CaCl2 (100 nm free Ca2+), pH 7.2 adjusted by Tris base. KCl (30 mm), potassium gluconate (50 mm), sodium gluconate (50 mm), and TEACl (30 mm) were added to PS as required and CaCl2 adjusted to maintain 100 nm free Ca2+. Addition of 0.5 mm Mg-ATP to PS prevented rundown of K+ efflux currents for at least the first 30 to 40 min after seal formation (in a few patches, K+ current rundown was quicker; such patches were discarded). Pipette Ca2+ activities were calculated using GEOCHEM (Parker et al., 1995).
Recording equipment was as described by Demidchik et al. (2002a). Liquid junction potentials (which were not more than 10 mV) were calculated using JPCalc 2.20 software (P.H. Barry, University of New South Wales, Australia). Membrane potentials were clamped at −70 mV throughout experiments, as specified in the figure legends. Voltage pulses were applied in steps. Instantaneous currents were sampled 50 ms after the capacitance spike caused by the V pulse. Time-dependent currents were calculated as differences between maximal currents (usually about 50 ms before the end of the V pulse) and instantaneous currents. Curve fitting was mostly done using Statistica 6.0 (StatSoft) and SigmaPlot 4.01 (CPSS).
Cytosolic K+ Measurements
Double-barreled potassium-selective microelectrodes were made, calibrated, and used as described previously (Walker et al., 1998; Carden et al., 2003). The K/Na selectivity of the valinomycin sensor in the PVC microelectrodes was 3.5, indicating more than three orders of magnitude difference in selectivity between K+ and Na+. Previous studies (Coombs et al., 1994; Walker et al., 1995) also showed the absence of any effect of cytosolic solution on the K+ electrode slope or the detection limit within the concentration range expected in this study.
Excised pieces of Arabidopsis root like those used for vibrating ion-selective microelectrode measurements were placed in a Plexiglas chamber and perfused with basic measuring solution, to which 50 mm NaCl was added for the salt treatments. During the intracellular measurements, the root was held between small two Plexiglas blocks that were attached to the base of the chamber using silicone grease.
Membrane Potential Measurements
Conventional KCl-filled Ag/AgCl microelectrodes with tip diameter approximately 0.5 μm were used to measure membrane potential of epidermal cells in mature zone of Arabidopsis roots essentially as described by Shabala et al. (2005b). Root segments were excised and mounted in the holder as described above and left to equilibrate in either basic salt medium or the NaCl-containing solution for 50 to 60 min. Steady-state membrane potential values were measured from at least five individual plants for each treatment, with not more than three measurements taken from any one root. Measurements were always made within 80 min of the excision and mounting. Membrane potentials were recorded for 1.5 to 2 min after the potential stabilized following cell penetration.
Acknowledgments
We thank Dr. Romola Davenport for useful discussions.
This work was supported by Aus Industry (no. S00112661), Department of Education, Science and Training (no. CG040074), and University of Tasmania Institutional Research Grants Scheme (no. S0011864) grants to S.S.; an Australian Research Council grant (no. A00105708) to I.A.N.; and a Leverhulme Trust project grant (no. F/09 741/C) to J.M.D.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sergey Shabala (sergey.shabala@utas.edu.au).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.082388.
References
- Ahn SJ, Shin R, Schahtman DP (2004) Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol 134: 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann A, Fischer M, Marsh EL, Stefanovic A, Sanders D, Schachtman DP (2001) The wheat cDNA LCT1 generates hypersensitivity to sodium in a salt-sensitive yeast strain. Plant Physiol 126: 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann A, Sanders D (1999) Mechanisms of Na+ uptake by plant cells. Adv Bot Res 29: 75–112 [Google Scholar]
- Arif I, Newman IA (1993) Proton efflux from oat coleoptile cells and exchange with wall calcium after IAA or fusicoccin treatment. Planta 189: 377–383 [DOI] [PubMed] [Google Scholar]
- Armengaud P, Breitling R, Amtmann A (2004) The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol 136: 2556–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babourina O, Leonova T, Shabala S, Newman I (2000) Effect of sudden salt stress on ion fluxes in intact wheat suspension cells. Ann Bot (Lond) 85: 759–767 [Google Scholar]
- Bergmann W (1992) Nutritional Disorders of Plants: Development, Visual and Analytical Diagnosis. Gustav Fischer, Jena, Germany
- Binzel ML, Hess FD, Bressan RA, Hasegawa PM (1988) Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol 86: 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom-Zandstra M, Vogelzang SA (2003) A short-term NaCl exposure increases the Na+ conductance of outward-rectified cation currents in the pith cells of sweet pepper. Bioelectrochemistry 60: 47–55 [DOI] [PubMed] [Google Scholar]
- Cakirlar H, Bowling DJF (1981) The effect of salinity on the membrane potential of sunflower roots. J Exp Bot 32: 479–485 [Google Scholar]
- Carden DE, Walker DJ, Flowers TJ, Miller AJ (2003) Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol 131: 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28: 1230–1246 [Google Scholar]
- Cherel I, Michard E, Platet N, Mouline K, Alcon C, Sentenac H, Thibaud JB (2002) Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell 14: 1133–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs HV, Miller AJ, Sanders D (1994) Disruptive effects of protein on performance of liquid membrane-based ion-selective microelectrodes. Am J Physiol Cell Physiol 267: C1027–C1035 [DOI] [PubMed] [Google Scholar]
- Cramer G (2002) Sodium-calcium interactions under salinity stress. In A Laüchli, U Lüttge, eds, Salinity. Environment-Plants-Molecules. Kluwer, Dordrecht, The Netherlands, pp 205–227
- Demidchik V, Bowen HC, Maathuis FJM, Shabala SN, Tester MA, Davies JM (2002. a) Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J 32: 799–808 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M (2002. b) Nonselective cation channels in plants. Annu Rev Plant Biol 53: 67–107 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128: 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison KL, Robertson WR, Lewis BD, Hirsch RE, Sussman MR, Spalding EP (2001) Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol 127: 1012–1019 [PMC free article] [PubMed] [Google Scholar]
- Elphick CH, Sanders D, Maathuis FJM (2001) Critical role of divalent cations and Na+ efflux in Arabidopsis thaliana salt tolerance. Plant Cell Environ 24: 733–740 [Google Scholar]
- Elzenga JTM, Keller CP, van Volkenburgh E (1991) Patch clamping protoplasts from vascular plants: method for the quick isolation of protoplasts having a high success rate of gigaseal formation. Plant Physiol 97: 1573–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M (2003) Sodium influx and accumulation in Arabidopsis. Plant Physiol 133: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers TJ, Hajibagheri MA (2001) Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant Soil 231: 1–9 [Google Scholar]
- Halfter U, Ishitani M, Zhu JK (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA 97: 3735–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51: 463–499 [DOI] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280: 918–921 [DOI] [PubMed] [Google Scholar]
- Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A (2001) Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J 27: 129–138 [DOI] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Poree F, Boucherez J, Lebaudy A, Bouchez D, Véry A-A, et al (2003) The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M, Liu JP, Halfter U, Kim CS, Shi WM, Zhu JK (2000) SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12: 1667–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle HH, Hedrich R (2001) K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett 508: 463–469 [DOI] [PubMed] [Google Scholar]
- Kinraide TB (1998) Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol 118: 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobus G, Janicka-Russak M (2004) Modulation by cytosolic components of proton pump activities in plasma membrane and tonoplast from Cucumis sativus roots during salt stress. Physiol Plant 121: 84–92 [DOI] [PubMed] [Google Scholar]
- Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud JB (2000) A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 12: 837–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaHaye PA, Epstein E (1969) Salt tolerance in plants: enhancement with calcium. Science 166: 395–396 [DOI] [PubMed] [Google Scholar]
- Läuchli A (1990) Calcium, salinity and the plasma membrane. In RT Leonard, PK Hepler, eds, Calcium in Plant Growth. American Society of Plant Physiologists, Rockville, MD, pp 26–35
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA (2002) A role for HKT1 in sodium uptake by wheat roots. Plant J 32: 139–149 [DOI] [PubMed] [Google Scholar]
- Leigh RA (2001) Potassium homeostasis and membrane transport. J Plant Nutr Soil Sci 164: 193–198 [Google Scholar]
- Liu JP, Zhu JK (1998) A calcium sensor homolog required for plant salt tolerance. Science 280: 1943–1945 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot (Lond) 84: 123–133 [Google Scholar]
- Maathuis FJM, Sanders D (1995) Contrasting roles in ion-transport of two K+-channel types in root-cells of Arabidopsis thaliana. Planta 197: 456–464 [DOI] [PubMed] [Google Scholar]
- Marten I, Hoth S, Deeken R, Ache P, Ketchum KA, Hoshi T, Hedrich R (1999) AKT3, a phloem-localized K+ channel, is blocked by protons. Proc Natl Acad Sci USA 96: 7581–7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Läuchli A (1993) Effects of Ca2+ on the salt-stress response of barley roots as observed by in-vivo 31P-nuclear magnetic resonance and in-vitro analysis. Planta 4: 519–524 [Google Scholar]
- Newman IA (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24: 1–14 [DOI] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL (1995) GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In RH Loeppert, AP Schwab, S Goldberg, eds, Chemical Equilibrium and Reaction Models, Special Publication 42. Soil Science Society of America, Madison, WI, pp 253–269
- Reid RJ, Smith FA (2000) The limits of sodium/calcium interactions in plant growth. Aust J Plant Physiol 27: 709–715 [Google Scholar]
- Rengel Z (1992) The role of calcium in salt toxicity. Plant Cell Environ 15: 625–632 [Google Scholar]
- Romano LA, Miedema H, Assmann SM (1998) Ca2+-permeable, outwardly-rectifying K+ channels in mesophyll cells of Arabidopsis thaliana. Plant Cell Physiol 39: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee B, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM (2001) AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA 98: 14150–14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Newman IA, Arif I (1992) Rapid calcium exchange for protons and potassium in cell walls of Chara. Plant Cell Environ 15: 675–683 [Google Scholar]
- Shabala L, Cuin TA, Newman I, Shabala S (2005. a) Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222: 1041–1050 [DOI] [PubMed] [Google Scholar]
- Shabala S (2000) Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant Cell Environ 23: 825–837 [Google Scholar]
- Shabala S (2003) Regulation of potassium transport in leaves: from molecular to tissue level. Ann Bot (Lond) 92: 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Newman I (1999) Light-induced changes in hydrogen, calcium, potassium, and chloride ion fluxes and concentrations from the mesophyll and epidermal tissues of bean leaves. Understanding the ionic basis of light-induced bioelectrogenesis. Plant Physiol 119: 1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Newman I (2000) Salinity effects on the activity of plasma membrane H+ and Ca2+ transporters in bean leaf mesophyll: masking role of the cell wall. Ann Bot (Lond) 85: 681–686 [Google Scholar]
- Shabala S, Shabala L, van Volkenburgh E (2003) Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Funct Plant Biol 30: 507–514 [DOI] [PubMed] [Google Scholar]
- Shabala S, Shabala L, van Volkenburgh E, Newman I (2005. b) Effect of divalent cations on ion fluxes and leaf photochemistry in salinised barley leaves. J Exp Bot 56: 1369–1378 [DOI] [PubMed] [Google Scholar]
- Shabala SN, Lew RR (2002) Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Slayman CL, Goldsmith MHM, Gradmann D, Bertl A (1992) Ion channels in Arabidopsis plasma membrane. Transport characteristics and involvement in light-induced voltage changes. Plant Physiol 99: 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot (Lond) 91: 503–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale SL, Nelson VL, Beaton JD, Havlin JL (1993) Soil Fertility and Fertilizers. Prentice Hall, Upper Saddle River, NJ
- Tyerman SD, Skerrett IM (1999) Root ion channels and salinity. Sci Hortic (Amsterdam) 78: 175–235 [Google Scholar]
- Tyerman SD, Skerrett M, Garrill A, Findlay GP, Leigh RA (1997) Pathways for the permeation of Na+ and Cl− into protoplasts derived from the cortex of wheat roots. J Exp Bot 48: 459–480 [DOI] [PubMed] [Google Scholar]
- Véry A-A, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Vitart V, Baxter I, Doerner P, Harper JF (2001) Evidence for a role in growth and salt resistance of a plasma membrane H+-ATPase in the root endodermis. Plant J 27: 191–201 [DOI] [PubMed] [Google Scholar]
- Volkov V, Wang B, Dominy PJ, Fricke W, Amtmann A (2004) Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant Cell Environ 27: 1–14 [Google Scholar]
- Walker DJ, Black CR, Miller AJ (1998) The role of cytosolic K+ and pH in the growth of barley roots. Plant Physiol 118: 957–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Walker DJ, Smith SJ, Miller AJ (1995) Simultaneous measurement of intracellular pH and K+ or
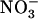 in barley root cells using triple-barreled, ion-selective microelectrodes. Plant Physiol 108: 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
in barley root cells using triple-barreled, ion-selective microelectrodes. Plant Physiol 108: 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar] - White PJ (1997) Cation channels in the plasma membrane of rye roots. J Exp Bot 48: 499–514 [DOI] [PubMed] [Google Scholar]
- White PJ (1999) The molecular mechanisms of sodium influx to root cells. Trends Plant Sci 4: 245–246 [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR (2003) Calcium in plants. Ann Bot (Lond) 92: 487–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Tester MA (1992) Potassium channels from the plasma membrane of rye roots characterized following incorporation into planar lipid bilayers. Planta 186: 188–202 [DOI] [PubMed] [Google Scholar]
- Yokoi S, Bressan RA, Hasegawa PM (2002) Salt stress tolerance of plants. In M Iwanaga, ed, Genetic Engineering of Crop Plants for Abiotic Stress. JIRCAS Working Report Number 23. Japan International Research Center for Agricultural Sciences, Ibaraki, Japan, pp 25–33
- Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6: 441–445 [DOI] [PubMed] [Google Scholar]