Abstract
Plant development requires regulation of both cell division and differentiation. The class 1 KNOTTED1-like homeobox (KNOX) genes such as knotted1 (kn1) in maize (Zea mays) and SHOOTMERISTEMLESS in Arabidopsis (Arabidopsis thaliana) play a role in maintaining shoot apical meristem indeterminacy, and their misexpression is sufficient to induce cell division and meristem formation. KNOX overexpression experiments have shown that these genes interact with the cytokinin, auxin, and gibberellin pathways. The L1 layer has been shown to be necessary for the maintenance of indeterminacy in the underlying meristem layers. This work explores the possibility that the L1 affects meristem function by disrupting hormone transport pathways. The semidominant Extra cell layers1 (Xcl1) mutation in maize leads to the production of multiple epidermal layers by overproduction of a normal gene product. Meristem size is reduced in mutant plants and more cells are incorporated into the incipient leaf primordium. Thus, Xcl1 may provide a link between L1 division patterns, hormonal pathways, and meristem maintenance. We used double mutants between Xcl1 and dominant KNOX mutants and showed that Xcl1 suppresses the Kn1 phenotype but has a synergistic interaction with gnarley1 and rough sheath1, possibly correlated with changes in gibberellin and auxin signaling. In addition, double mutants between Xcl1 and crinkly4 had defects in shoot meristem maintenance. Thus, proper L1 development is essential for meristem function, and XCL1 may act to coordinate hormonal effects with KNOX gene function at the shoot apex.
Cell division and differentiation are tightly linked processes in plant development. In recent years, several genes that are involved in controlling shoot apical meristem (SAM) cell division and differentiation have been identified. The class 1 KNOTTED1-like homeobox (KNOX) genes such as knotted1 (kn1) in maize (Zea mays) and SHOOTMERISTEMLESS (STM) in Arabidopsis (Arabidopsis thaliana) are expressed throughout the SAM and down-regulated in both incipient leaf primordia and developing leaves (Vollbrecht et al., 1990; Smith et al., 1992; Jackson et al., 1994), indicating that these genes play a role in maintaining SAM indeterminacy. Loss-of-function mutations in Arabidopsis STM lead to loss of shoot meristems (Barton and Poethig, 1993; Long et al., 1996). In addition, overexpression of KN1 in tobacco (Nicotiana tabacum) and another class 1 KNOX gene, KNAT1 (also known as BREVIPEDICELLUS [BP]), in Arabidopsis lead to the production of lobed leaves with ectopic meristems (Sinha et al., 1993; Chuck et al., 1996). In maize, ectopic expression of the KNOX genes kn1, rough sheath1 (rs1), and gnarley1 (gn1) in dominant mutants causes abnormal cell divisions in the leaf (Becraft and Freeling, 1994; Schneeberger et al., 1995; Foster et al., 1999). These results indicate that misexpression of KNOX genes leads to similar outcomes and is sufficient to induce cell division and meristem formation.
The control of cell division and differentiation by KNOX genes probably occurs through modulation of hormonal pathways. KNOX overexpressing tobacco leaves display a delay in senescence similar to that seen in plants with increased cytokinin levels (Kusaba et al., 1998). Expressing KN1 under the control of the senescence-associated gene promoter in tobacco leads to increased levels of cytokinin and a delay in leaf senescence, suggesting that KN1 acts to increase cytokinin levels (Ori et al., 1999). In tobacco, ectopic KNOX gene expression directly down-regulates the gibberellic acid biosynthesis gene, GA20 oxidase, through binding of target sites in the promoter and first intron (Sakamoto et al., 2001). Furthermore, inducible expression of maize KN1 in Arabidopsis leads to the down-regulation of GA20 oxidase (Hay et al., 2003). KNOX genes have also been shown to coordinately regulate GA and cytokinin activity in Arabidopsis (Jasinski et al., 2005; Yanai et al., 2005). The maize semaphore mutation leads to ectopic expression of KNOX genes and also disrupts polar auxin transport (Scanlon et al., 2002).
KNOX genes have also been implicated in cell differentiation pathways. For example, bp mutants show an increase in lignin biosynthetic gene expression (Mele et al., 2003). In contrast, plants mutant for the rice (Oryza sativa) OSH15 gene (orthologous to BP) show no meristem defects but have reduced hypodermal sclerenchyma (Sato et al., 1999), suggesting a difference in response between monocots and dicots. A genetic approach may help to dissect out these diverse influences of KNOX genes and plant hormones on shoot morphogenesis.
Higher angiosperm SAMs are organized into layers, with the L1 (or outer layer, also known as the tunica) dividing predominantly in the anticlinal plane and giving rise to the epidermis, and the internal layer(s), the L2 in maize and L2 and L3 in Arabidopsis, making up the body (or corpus) of the meristem. Mosaic analysis experiments showed that in Kn1 mutants, ectopic expression of KN1 in the inner layers of the leaf was sufficient to induce periclinal divisions in the L1, leading to knot formation (Hake and Freeling, 1986; Sinha and Hake, 1990). The KN1 protein has been shown to move between the L2 and L1 in meristems (Lucas et al., 1995; Kim et al., 2002, 2003); however, the functional significance of this intercellular transport in meristem maintenance has yet to be determined. Since knots form over major lateral veins, it has been suggested that auxin signaling may play a role in conditioning the kn phenotype (Gelinas et al., 1969). In a recent study on the relationship between polar auxin transport and leaf initiation, auxin was proposed to move from leaf primordia to the apical dome through the L1 layer (Benkova et al., 2003; Reinhardt et al., 2003b). The role of the L1 in meristem activity is just beginning to be understood. Microsurgical removal of the L1 in tomato (Lycopersicon esculentum) apices leads to a cessation of leaf initiation and an eventual differentiation of cells in the underlying central zone of the meristem (Reinhardt et al., 2003a). Once the L1 of the meristem is removed, it never grows back. Thus, underlying cell layers cannot be respecified as L1 cells. This suggests a strict lineage-based maintenance of the L1 and also indicates that the L1 is necessary for the maintenance of indeterminacy in the underlying meristem layers. Additional evidence for an L1 role in meristem function comes from a study in which a double mutant combination of two Arabidopsis L1-specific glabra 2-like homeobox genes, protodermal factor 2 and Arabidopsis thaliana meristem layer 1, leads to the formation of a disorganized shoot apex during embryogenesis and problems in leaf initiation and development. Leaves that are initiated by the double mutants do not have an epidermis, indicating that these two genes are necessary for epidermal identity (Abe et al., 2003). These instances of L1 involvement in meristem function have relied on ablation of the L1 by physical or genetic mechanisms. It is possible that other, more subtle changes involving division or differentiation patterns in the L1 could also affect meristem function, perhaps by disrupting hormone transport pathways.
Maize mutants defective in epidermal division and differentiation are useful for elucidating the mechanisms responsible for coordinating cell division and differentiation. During epidermal development, protoderm cells divide predominantly in the anticlinal plane. The Extra cell layers1 (Xcl1) mutation causes protoderm cells to divide in oblique periclinal orientations, which leads to the production of multiple epidermal layers (Kessler et al., 2002). Dosage analysis of the Xcl1 mutation showed that it is a hypermorphic mutation leading to overproduction of a normal gene product. Increased levels of XCL1 are thought to prolong cell division during leaf development, such that cells that have already perceived epidermal differentiation signals divide in altered planes and differentiate according to lineage instead of position. While the most apparent Xcl1 phenotype is the multiple epidermal layers that lead to thicker leaves, Xcl1 also affects the SAM. Xcl1 meristems are 35% shorter than normal sibling meristems and also recruit more cells into the incipient leaf primordia (P0; Kessler et al., 2002). This indicates that XCL1 may not only be involved in controlling protoderm division and differentiation patterns, but also plays an earlier role in regulating meristem size and leaf initiation and may provide a link between L1 division patterns, hormonal pathways, and meristem maintenance.
In this study, we utilized double mutants between Xcl1 and dominant KNOX mutants to gain an understanding of the mechanisms coordinating cell division and differentiation and to try to tease apart the effects of these diverse KNOX genes on downstream parts of the pathway. Xcl1 suppresses the Kn1 phenotype, possibly through modulation of gibberellin (GA) levels. In combination with Xcl1, both Gn1 and Rs1 displayed synergistic phenotypes in which leaf development and meristem maintenance were affected in a manner similar to that seen when seedlings are treated with polar auxin transport inhibitors. In addition, double mutants between Xcl1 and another epidermal division and differentiation mutant, crinkly4 (cr4), failed to maintain a shoot meristem. Thus, proper L1 development is essential for meristem function, and XCL1 may play a role in coordinating hormonal effects at the shoot apex.
RESULTS
Xcl1
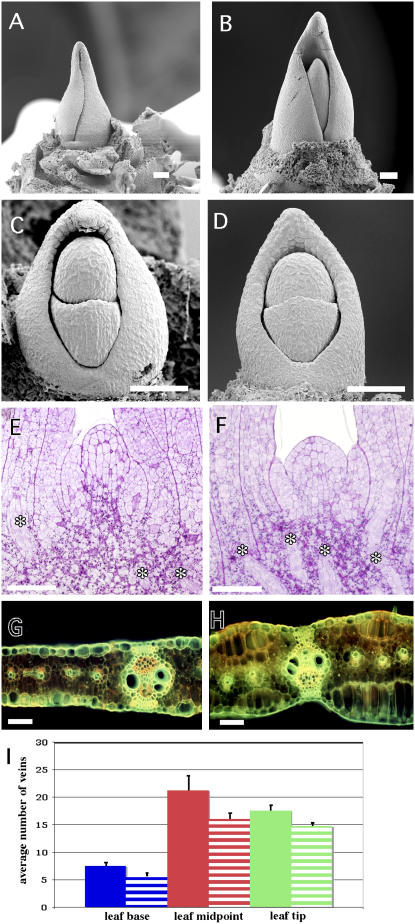
Xcl1 is a semidominant mutation that affects both meristems and leaves (Kessler et al., 2002). In wild-type SAMs, Plastochron 3 (P3) and higher leaf primordia completely encircle the SAM (Fig. 1A), while in Xcl1, leaves up to P4 do not completely encircle the SAM (Fig. 1B), indicating that Xcl1 leaves are narrow from early in development. By P2, wild-type (Fig. 1, C and E) and Xcl1 (Fig. 1, D and F) leaf primordia look very similar, although the apical dome is shorter in Xcl1 than in wild type. The most conspicuous Xcl1 phenotype is the presence of multiple epidermal layers (Fig. 1, G compared to H) that have been shown to arise from oblique divisions in the L1 early in leaf development (Kessler et al., 2002). In addition, Xcl1 mutant leaves have fewer intermediate veins (Fig. 1I).
Figure 1.
Wild-type (A, C, E, and G) and Xcl1 (B, D, F, and H) meristem and leaf phenotypes. A, Wild-type meristem with P3 completely encircling the meristem and margins overlapping. B, Xcl1 meristem with P3 margins that do not overlap. C, Dissected wild-type meristem at the P1/P2 stage. D, Dissected Xcl1 meristem at P1/P2 showing shorter apical dome. E, Median longitudinal section of a wild-type meristem showing vascular traces (*) into P2 and P3. F, Median longitudinal section of an Xcl1 meristem showing vascular traces (*) extending higher into the meristem. G, Transverse section of a wild-type leaf. H, Transverse section of an Xcl1 leaf showing multiple epidermal layers. I, Average number of veins at three different positions in wild-type (solid bars) and Xcl1 (striped bars) leaves. Scale bars = 100 μm.
Kn1;Xcl1
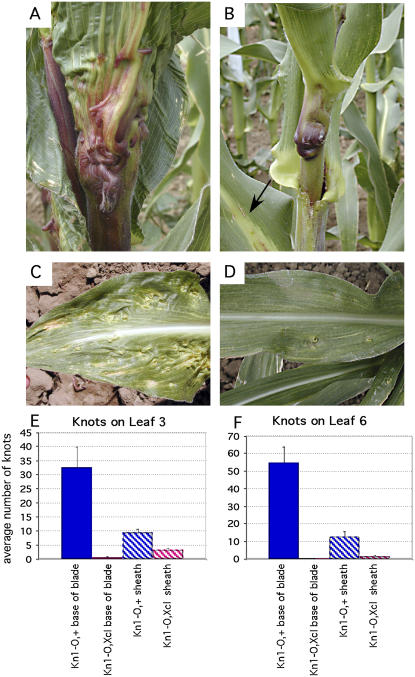
The reduction in meristem height seen in the Xcl1 single mutants (Kessler et al., 2002) indicates that Xcl1 may play a role in regulating the function of Kn1, a class 1 KNOX gene expressed in the SAM. To examine this possibility, double mutants were made between Xcl1 and two different dominant alleles of Kn1, Kn1-N2, and Kn1-O. Both of these mutations cause ectopic expression of KN1 in leaves that leads to the formation of large protrusions, or knots, over the major veins. Kn1-O is a spontaneous mutation that was caused by a duplication at the KN1 locus leading to a dominant, neomorphic phenotype (Veit et al., 1990). Large knots protrude from the abaxial side of the leaf at the blade-sheath junction and sheath identity is pushed up into the blade (Fig. 2A). Ligule displacement into the blade and ectopic ligules on the adaxial side of the blade midrib are also prominent features of Kn1-O leaves (Gelinas et al., 1969; Hake and Freeling, 1986; Sinha and Hake, 1994). These phenotypes are most evident in adult leaves, although ligule displacement can also be seen in juvenile leaves.
Figure 2.
Xcl1 suppresses Knotted mutations. A, Kn1-O adult leaf with knots at the blade-sheath junction. B, Kn1-O;Xcl1 double mutant with suppressed knots at the blade-sheath junction. Ectopic ligule on the adaxial side of leaf blade is indicated by an arrow. C, Kn1-N2 adult leaf has knots primarily on the blade and not the sheath. D, Kn1-N2;Xcl1 adult leaf has a reduced number of knots on the blade. E, Comparison of the number of knots in Kn1-O (blue) and Kn1-O;Xcl1(pink) leaf 3 (from the tassel), blade (solid), and sheath (striped). F, Comparison of the number of knots on leaf 6 (from tassel).
Xcl1;Kn1-O double mutants have a suppressed Kn1-O phenotype. Instead of a huge mass of knotted tissue at the blade-sheath boundary (Fig. 2A), one large protrusion was seen in every leaf near the top of the sheath in double mutants (Fig. 2B). However, ectopic ligules were still seen on the adaxial blade midrib, indicating that this aspect of the Kn1-O phenotype was not affected by the presence of the Xcl1 mutation (Fig. 2B, arrow). The number of knots in the sheath and at the base of the blade were determined in Kn1-O single mutants and Kn1-O;Xcl1 double mutants at two different positions on the plant (Fig. 2, E and F). Both the third and sixth leaf from the tassel had significantly fewer knots when Xcl1 was present, indicating that the effect of Xcl1 on knot formation was similar in all adult leaves.
While the Kn1-O allele primarily affects the blade/sheath junction of adult leaves, the Kn1-N2 allele causes knots to form primarily on leaf blades (Fig. 2C). In Kn1-N2, both juvenile and adult leaf blades have a severe knotted phenotype. Since the timing of Kn1-N2 knot formation is different than in Kn1-O, crosses were made with Xcl1 to see if there was a difference in knot suppression. In Kn1-N2;Xcl1 double mutants, knot development in adult leaf blades was also significantly inhibited (Fig. 2D). Seedling leaves showed a similar effect in that double mutants had only one to three knots on leaves 1 and 2, while Kn1-N2 single mutants had more than 10 knots on these leaves. It is interesting to note that while both Kn1-N2 and Kn1-O mutant leaves are wider than wild-type or Xcl1 leaves, double mutant Kn1;Xcl1 leaves are narrow like Xcl1 leaves (Fig. 2, A–D). Segregation analysis on an F2 Kn1-N2;Xcl1 family showed that while most of the phenotypic classes were represented in the expected proportions, two classes deviated significantly from the numbers expected for an additive interaction (Table I). Fewer Kn1-N2/?;Xcl1/Xcl1 (strong Xcl1 phenotype, very mild knots) than expected were found, and more Kn1-N2/Kn1-N2;Xcl1/Xcl1 (strong Xcl1 phenotype, no knots) individuals than expected were found. This result indicates that in some homozygous Xcl1 seedlings, the Kn1-N2 mutation was present, but knot formation was completely suppressed.
Table I.
Segregation of Kn1-N2;Xcl1 family
In an F2 Kn1-N2;Xcl1 family, two classes (bold) deviated significantly from the numbers expected for an additive interaction. Fewer Kn1-N2/?;Xcl1/Xcl1 (strong Xcl1 phenotype, very mild knots) and more Kn1-N2/kn1-N2;Xcl1/Xcl1 (strong Xcl1 phenotype, no knots) individuals than expected were found. The χ2 value rejects the segregation ratio/null hypothesis. Σχ2 = 18.1; critical value = 16.75 (P = 0.005, 5 degrees of freedom).
| Genotype | Expected Ratio | Expected No. | Observed No. | χ2 |
|---|---|---|---|---|
| kn1/kn1;xcl1/xcl1 | 1/16 | 3.75 | 5 | 0.42 |
| kn1/kn1;Xcl1/xcl1 | 2/16 | 7.5 | 12 | 2.7 |
| kn1/kn1;Xcl1/Xcl1 | 1/16 | 3.75 | 10 | 10.4 |
| Kn1/?;xcl1/xcl1 | 3/16 | 11.25 | 10 | 0.14 |
| Kn1/?;Xcl1/xcl1 | 6/16 | 22.5 | 18 | 0.9 |
| Kn1/?;Xcl1/Xcl1 | 3/16 | 11.25 | 5 | 3.5 |
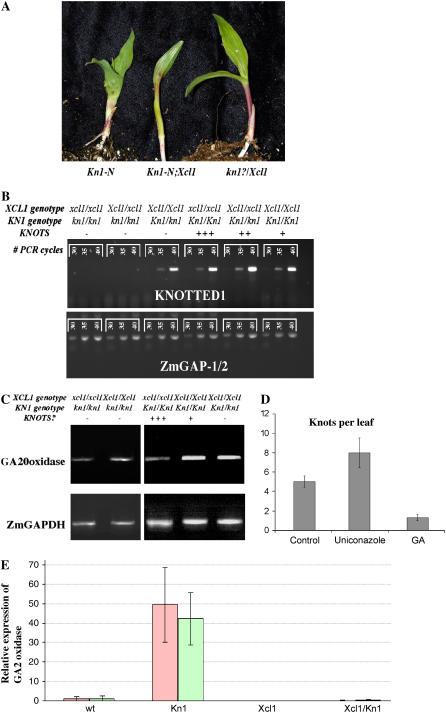
The suppression of Kn1 leaf phenotypes when Xcl1 is present suggests that Xcl1 may affect the ectopic expression of KN1 in double mutants. This hypothesis was tested in a Kn1-N2;Xcl1 segregating family. Individuals were genotyped based on the presence of an rDotted transposable element inserted in the fourth intron of Kn1-N2 (Sinha, 1990; N. Sinha, unpublished data). Primers that spanned exon/exon junctions were used to specifically amplify cDNAs to examine the expression of KN1 in expanded leaf 1 of each of the mutant classes (Fig. 3A) by semiquantitative reverse transcription (RT)-PCR with GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE (ZmGAPDH) as the constitutively expressed control (Richert et al., 1996). As expected, in wild-type and Xcl1 individuals, we did not detect KN1 transcript in leaves, but double mutant individuals with knots had KN1 transcript at similar levels to that seen in Kn1-N2 leaves. In seedlings that were homozygous for Xcl1 and heterozygous for Kn1-N2 but did not have any visible knots on leaf 1, KN1 transcript was detected at similar levels to that seen in individuals with knots (Fig. 3B), indicating that Xcl1 suppression of the Kn1-N2 phenotype in leaves is not at the transcriptional level.
Figure 3.
Kn1-N2;Xcl1 seedling phenotypes. A, The number of knots on seedling leaves is reduced when Xcl1 is present. B, Semiquantitative RT-PCR of KN1 expression reveals that KN1 RNA is present in double mutant leaves with suppressed knots. Numbers in brackets indicate number of PCR cycles. Control RT-PCR for the constitutively expressed ZmGAPDH. C, GA20 oxidase levels are increased when Xcl1 is present and decreased in Kn1-N2 single mutants. D, Knots per leaf on the third leaf of Kn1-N2 heterozygote seedlings treated with Tween 20 (control), Uniconazole, or GA. Differences between Uniconazole and GA treatments were found to be statistically significant at P = 0.01 and differences between control and GA at P = 0.05 using ANOVA. Error bars are ses, n = 3. E, Detection of GA2 oxidase RNA levels with QRT-PCR in wild-type (wt), Xcl1, Kn1-N2 (Kn1), and Xcl1/Kn1-N2 seedlings. The data represents two replicate runs. RNA levels were normalized to ZmGAPDH mRNA and are shown relative to wild-type levels. Bars represent ses over four technical replicates.
GA levels have been shown to be reduced in tobacco leaves that overexpress Nicotiana tabacum homeobox 15 (NTH15; Tamaoki et al., 1997), and NTH15 was subsequently shown to directly suppress the GA biosynthesis gene GA20 oxidase (Sakamoto et al., 2001). In addition, inducible expression of maize KN1 in Arabidopsis led to down-regulation of GA20 oxidase (Hay et al., 2003). GA levels have been shown to be low in the SAM as a result of the activity of GA2 oxidase, and this enzyme was proposed to be KNOX regulated (Jasinski et al., 2005; Yanai et al., 2005). This data led us to hypothesize that changing GA levels would also affect the severity of the Kn1 phenotype. Applying GA to seedlings suppressed the Kn1-N2 phenotype, while the presence of the GA biosynthesis inhibitor, uniconazole, enhanced the Kn1-N2 phenotype (Fig. 3D). The results indicate that the suppression of Kn1-N2 by Xcl1 could be at the level of GA biosynthesis or signaling (Jasinski et al., 2005; Yanai et al., 2005). We identified maize expressed sequence tags that encode a GA20 oxidase gene and a GA2 oxidase gene. Levels of the ZmGA20 oxidase and ZmGA2 oxidase genes were examined by RT-PCR in Kn1-N2;Xcl1 segregants to determine if Xcl1 is regulating KNOX activity by altering GA levels (Fig. 3C). While no apparent alteration was seen in the GA20 oxidase levels, quantitative RT-PCR (QRT-PCR) revealed high induction of GA2 oxidase in the Kn1-N2 genotype, and the presence of the Xcl1 mutation drastically reduced the GA2 oxidase transcript levels (Fig. 3E) and decreased the number of knots, indicating that Xcl1 may change GA levels during leaf development, allowing for suppression of the Kn1-N2 phenotype in leaves.
Gn1;Xcl1 and Rs1;Xcl1 Double Mutants
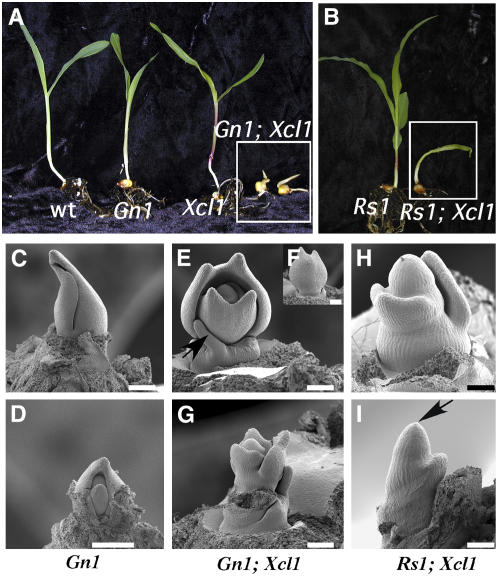
Gn1 and Rs1 are dominant mutations in duplicated KNOX genes (Becraft and Freeling, 1994; Schneeberger et al., 1995; Foster et al., 1999). Both of these mutations affect cell division at the blade-sheath junction of leaves. Since Gn1 and Rs1 have similar phenotypes to Kn1, we wanted to determine if Xcl1 would also suppress these phenotypes. When an F2 Gn1;Xcl1 population was examined, four phenotypic classes were observed in a 9:3:3:1 ratio (Fig. 4A; Table II). The largest phenotypic class was composed of seedlings with severely reduced and agravitropic shoot and root development. This group of seedling-lethal individuals did not resemble single Xcl1 or Gn1 mutants and represented genotypes that were either homozygous or heterozygous for both Gn1 and Xcl1. Rs1;Xcl1 double mutants had similar seedling phenotypes to Gn1;Xcl1 seedlings (Fig. 4, A and B). The seedling-lethal phenotypes of Gn1;Xcl1 and Rs1;Xcl1 double mutants suggest that meristem maintenance may be affected in the double mutants. Scanning electron microscopy (SEM) was used to examine SAMs in each of the phenotypic classes. Gn1 meristems are very similar to wild-type meristems at comparable stages of development (Fig. 4, C and D compared to Fig. 1, A and C). However, Gn1;Xcl1 meristems are strikingly different than either Gn1 or Xcl1 single mutant meristems. In double mutants, the shoot apices were 2 to 3 times larger than apices of either single mutant at similar stages of development (Fig. 4, E and F). When dissected down to P4, Gn1;Xcl1 meristems are very exposed, none of the leaves completely encircle the meristem, and the older leaves are often bifurcated (Fig. 4F). In addition, extremely reduced leaves with radial tips and aberrant phyllotaxy were sometimes observed in double mutants (Fig. 4, E and G). Rs1;Xcl1 meristems were very similar in appearance to the Gn1;Xcl1 meristems. SEMs showed that Rs1;Xcl1 apices were extremely large and leaf primordia did not encircle the meristem (Fig. 4H). In addition, some double mutant meristems failed to initiate leaves along one face of the meristem (Fig. 4I). The apical dome often ended in an acute tip, and there was considerable shoot expansion between successive leaf initiation events (Fig. 4, H and I). These results indicate that the presence of both Gn1 or Rs1 and Xcl1 leads to unique problems in normal meristem maintenance and leaf initiation.
Figure 4.
Seedling and meristem phenotypes of Gn1;Xcl1 and Rs1;Xcl1 double mutants. A, Gn1 seedlings have ligular displacement leading to erect leaves 1 and 2 compared to wild-type and Xcl1 seedlings. Gn1;Xcl1 double mutants (at right) have extremely reduced leaves, are agravitropic, and do not initiate lateral roots. B, Rs1 seedlings are similar to Gn1. A less severe double mutant with one leaf is shown at the right. C, Gn1 meristem at the P3 stage is very similar to wild type (Fig. 1A). D, Gn1 meristem dissected to P1/P2 stage. E, Gn1;Xcl1 apex appears much larger than Gn1 and leaves do not encircle the meristem. A delayed leaf with radial tip is also present (arrow). F, Rotated view of meristem in E reveals a bifurcated leaf primordium. G, Dissected Gn1;Xcl1 meristem (same as E) with aberrant phyllotaxy and leaf shape. H, Rs1;Xcl1 meristem with aberrant phyllotaxy and elongated internodes. I, Naked Rs1;Xcl1 meristem that has failed to initiate leaf primordia at regular intervals. Scale bars for all sections = 200 μm.
Table II.
Segregation of Gn1;Xcl1 F2 family
Four F2 phenotypic classes were observed in a Gn1;Xcl1 F2 population in a 9:3:3:1 ratio. The largest phenotypic class of seedling-lethal individuals did not resemble single Xcl1 or Gn1 mutants. The χ2 value fails to reject the segregation ratio/null hypothesis. Σχ2 = 4.2; critical value = 7.81 (P = 0.05).
| Genotype | Expected Ratio | Expected No. | Observed No. | χ2 |
|---|---|---|---|---|
| gn1/gn1;xcl1/xcl1 | 1/16 | 5.8 | 8 | 0.5 |
| Gn1/?;xcl1/xcl1 | 3/16 | 17 | 10 | 2.8 |
| gn1/gn1;Xcl1/? | 3/16 | 17 | 18 | 0.1 |
| Gn1/?;Xcl1/? | 9/16 | 52 | 57 | 0.8 |
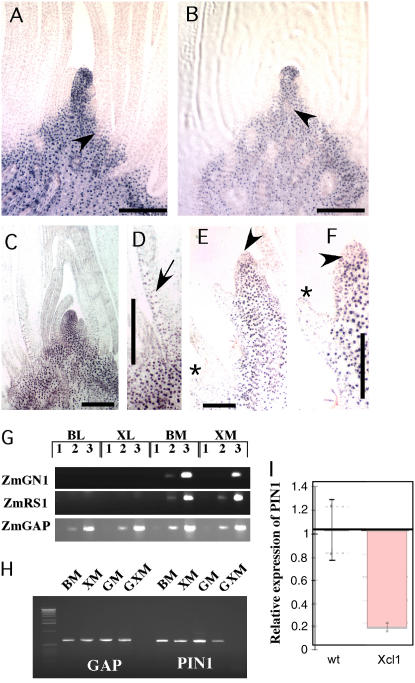
Polyclonal antibodies against KN1 and RS1 recognize multiple class 1 KNOX genes and have been used as markers for meristem identity in maize and other species (Smith et al., 1995; Scanlon et al., 1996; Bharathan et al., 2002). We performed immunolocalizations with a polyclonal KN1 antibody to analyze meristem identity in single and double mutants. In normal and Xcl1 meristems, KNOX proteins were localized to the stem and SAM and were not detected in developing leaves (Fig. 5, A and B). In Gn1;Xcl1 double mutants, the region of small, densely cytoplasmic cells expressing KNOX proteins normally associated with meristematic identity was nearly absent at the pointed shoot apex, and the internodes were greatly expanded (Fig. 5, E and F). In contrast to Gn1 single mutants (Fig. 5, C and D), KNOX protein was not observed in double mutant leaves (Fig. 5E), indicating that Xcl1 may suppress the ectopic expression of GN1 and other KNOX proteins in leaves. Thus, not only does Xcl1 reduce ectopic KNOX protein expression in Xcl1;Gn1 leaves, but it also suppresses KNOX expression in double mutant meristems, leading to a failure of meristem maintenance. Kn1-N2;Xcl1 double mutant meristems had no problems in maintaining meristem identity, and KNOX expression was detected in leaves (Fig. 3B).
Figure 5.
Analysis of gene expression in Gn1/Xcl1 double mutants. A to F, Immunolocalizations with anti-KN1 antibodies on seedling SAMs. A, Wild-type meristem showing normal KNOX expression in the SAM and unexpanded stem and down-regulation in the P0 and developing leaves. The provascular strand closest to the meristem (arrowhead) is near P3. B, Xcl1/Xcl1 meristem showing normal KNOX expression. The provascular strand closest to the meristem (arrowhead) is near P1. C, Gn1 meristem with KNOX localized in the SAM and in developing leaves. D, Higher magnification of P3 in C showing KNOX expression (arrow). E, Gn1;Xcl1 meristem at same magnification as A to C. The stem is greatly expanded between successive leaf primordia and the oldest leaf pictured (asterisks) is aborted and looks more like stem. F, Higher magnification of P2 in E. G, Semiquantitative RT-PCR of GN1 and RS1 in B73 versus Xcl1 meristems shows normal expression in Xcl1 meristems and no expression in Xcl1 leaves. Numbered lanes correspond to number of PCR cycles (1 = 30, 2 = 35, 3 = 40 for GN1 and RS1; 1 = 22, 2 = 26, 3 = 30 for GAP controls). H, PIN1 RT-PCR showing lower expression in Gn1;Xcl1 meristems. I, Detection of PIN1 RNA levels with QRT-PCR in wild-type (wt) and Xcl1 seedlings. The RNA levels were normalized to ZmGAPDH mRNA and are shown relative to wild-type levels. Bars represent ses over four technical replicates. B, B73 (wild type); X, Xcl1; G, Gn1; GX, Gn1;Xcl1; L, leaf; M, meristem. Scale bars = 200 μm.
GN1 and RS1 transcripts were absent from expanded Xcl1 seedling leaves and present at normal levels in Xcl1 meristems (Fig. 5G). This indicates that Xcl1 does not directly affect GN1 and RS1 transcript levels. The similarity of the Xcl1;Gn1 and Xcl1;Rs1 double mutant meristems to Arabidopsis mutations in the PIN1 auxin efflux carrier and to Arabidopsis and tomato meristems treated with polar auxin transport inhibitors (Galweiler et al., 1998; Reinhardt et al., 2000; Stieger et al., 2002) indicates that auxin signaling pathways may be compromised in these double mutant seedlings. The transcript levels of the PIN family of polar auxin transport genes have been shown to correlate with changes in protein levels in response to perturbations in auxin levels (Benkova et al., 2003; Furutani et al., 2004; Peer et al., 2004). We identified a maize PIN1 ortholog in the public databases and used RT-PCR to examine PIN1 levels in mutant leaves and meristems. ZmPIN1 transcript levels were reduced in Xcl1 plants (Fig. 5I) and in Xcl1;Gn1 meristems (Fig. 5H), indicating that auxin transport pathways may be compromised in the double mutants.
Vascular Defects in Xcl1
The synergistic interactions between Xcl1 and Gn1 or Rs1 indicate that Xcl1 itself may exhibit auxin transport defects. Auxin flow is known to be involved in vascular patterning (Berleth et al., 2000). Wild-type maize leaves have up to 30 minor veins between the major lateral veins (Freeling and Lane, 1994). In contrast, Xcl1 leaves have significantly fewer minor veins between the major lateral veins when compared to wild type (Fig. 1I). When vascular development was examined in Xcl1 apices, departures from normal vascular patterning were seen. In normal maize apices, the most recently initiated vascular traces in the stem are between the second and third primordia (Figs. 1E and 5A; Sharman, 1942), while in Xcl1 apices, the most recently initiated vascular traces are between P1 and P2 (Figs. 1F and 5B). Since high levels of auxin are associated with vascular development, this indicates that higher levels of auxin may be present in this region of Xcl1 apices. Interestingly, KNOX protein is excluded from these developing vascular strands (Fig. 5, A and B), suggesting that high levels of auxin may be correlated with reduced KNOX expression.
Xcl1;cr4 Double Mutants
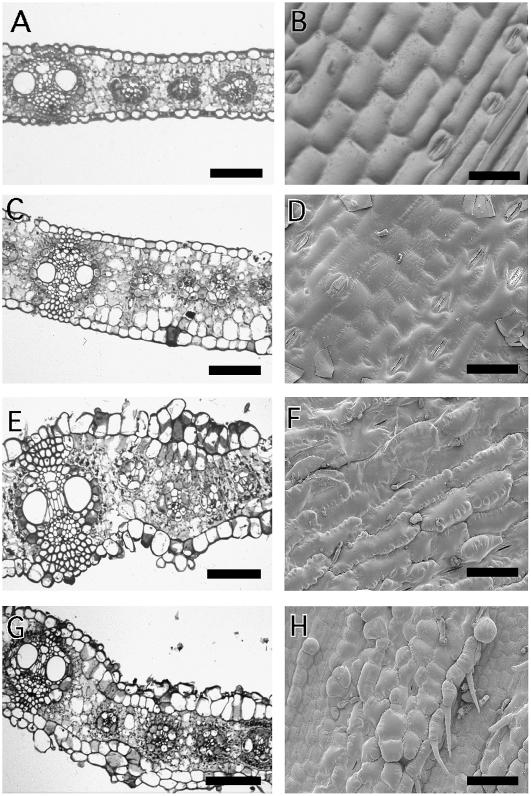
In Arabidopsis, the auxin efflux carrier PIN1 is localized in the L1 layer of the meristem, and auxin transport through the L1 has been proposed to play a role in determining phyllotaxy (Reinhardt et al., 2003b). Therefore, changes to the L1 layer of the meristem would be predicted to influence meristem function. To address this hypothesis, double mutants were made between Xcl1 and cr4. Mutations in CR4, a gene similar to the human tumor necrosis factor receptor, lead to aberrant epidermal differentiation and division patterns (Becraft et al., 1996, 2001; Jin et al., 2000). When an F2 population of Xcl1 crossed to the cr4-6143 allele of cr4 was grown in the field for observation, three mutant phenotypic classes were observed. In comparison to wild-type leaves with single epidermal layers (Fig. 6A) and rectangular, crenulated epidermal cells (Fig. 6B), Xcl1 single mutants had multiple epidermal layers and square, uncrenulated epidermal cells (Fig. 6, C and D). cr4 single mutant leaves had epidermal cells that divided in abnormal planes (Fig. 6E) and, when observed with SEM, had crenulations on the surface of cells instead of between cells (Fig. 6F). The third class of mutants seemed to be additive of the Xcl1 and cr4 phenotypes; a consistent extra epidermal layer was present below the abnormal outer epidermis (Fig. 6G), and SEM of the adaxial surface revealed cells that were square in dimension like Xcl1 but also had crenulations on the surface (Fig. 6H). F3 analysis of these individuals indicated that they were heterozygous for Xcl1 and homozygous for cr4. Thus, double homozygous mutants were predicted to be seedling lethal. The same F2 families were germinated in flats with a light soil. Representatives from each of the genotypic classes are shown in Figure 7. The segregation for this family was 3/16 xcl1/xcl1;Cr4/? (Fig. 7A); 1/16 xcl1/xcl1;cr4/cr4 (Fig. 7B); 9/16 Xcl1/?;Cr4/? (Fig. 7C); 2/16 Xcl/xcl;cr4/cr4; and 1/16 had reduced leaves or no leaves produced and were seedling lethal (Fig. 7, D–F) and therefore inferred to be double mutants (Xcl1/Xcl1;cr4/cr4; Table III).
Figure 6.
Phenotypes of adult leaves in a cr4;Xcl1 double mutant family. A, Transverse section of a wild-type leaf. B, Wild-type epidermis with rectangular pavement cells and evenly spaced stomata. C, Transverse section of an Xcl1 single mutant leaf shows multiple epidermal layers, but the outer epidermis is flat. D, SEM of the adaxial epidermis of Xcl1. Pavement cells are square in dimension. E, Transverse section of a cr4 single mutant leaf. Multiple epidermal-like layers are in smaller patches and extend out from the plane of the leaf. F, SEM of a cr4 adaxial epidermis. Pavement cells are rectangular in shape but have crenulations on the surface instead of between cells. G, Transverse section of a cr4/cr4;Xcl1/xcl1 leaf showing an additive phenotype. A complete extra epidermal layer is present under a cr4-like outer epidermis. H, SEM of a cr4/cr4;Xcl1/xcl1 adaxial epidermis. Cells are square in shape like Xcl1 but have crenulations on the surface like cr4. Scale bars = 100 μm.
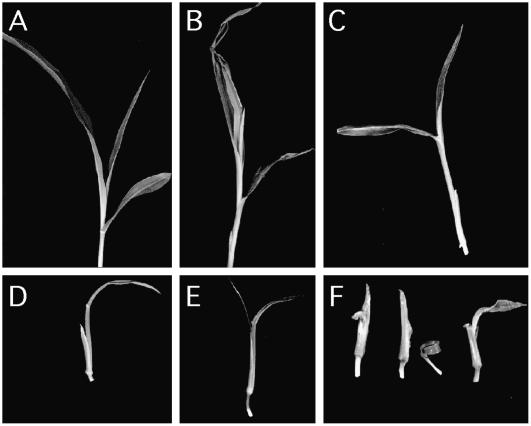
Figure 7.
Range of seedling phenotypes in a cr4;Xcl1 F2 family. A, Wild-type seedling. B, cr4/cr4 seedling showing leaf adherence. C, Xcl1/Xcl1 seedling with narrow, shiny leaves. D to F, Range of reduced leaf phenotypes seen in double mutants.
Table III.
Segregation of a representative cr4;Xcl1 F2 family
The double homozygotes cr4/cr4;Xcl1/Xcl1 were seedling lethal. The χ2 value fails to reject the segregation ratio/null hypothesis. Σχ2 = 3.06; critical value = 16.75 (P = 0.005, 5 degrees of freedom).
| Genotype | Expected Ratio | Expected No. | Observed No. | χ2 |
|---|---|---|---|---|
| Cr4/?;xcl1/xcl1 | 3/16 | 8.8 | 9 | 0.01 |
| Cr4/?;Xcl1/xcl1 | 6/16 | 17.6 | 12 | 1.78 |
| Cr4/?;Xcl1/Xcl1 | 3/16 | 8.8 | 11 | 0.55 |
| cr4/cr4;xcl1/xcl1 | 1/16 | 2.9 | 5 | 1.52 |
| cr4/cr4;Xcl1/+ | 2/16 | 5.9 | 7 | 0.20 |
| cr4/cr4;Xcl1/Xcl1 | 1/16 | 2.9 | 3 | 0.003 |
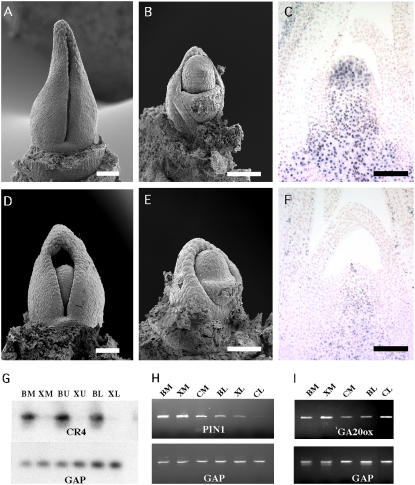
The reduction in leaves in the double mutants suggested that there was a problem in leaf initiation and/or meristem maintenance that may be similar to that seen in Xcl1;Gn1 and Xcl1;Rs1 double mutants. When compared to cr4 single mutants (Fig. 8, A and B), cr4;Xcl1 double mutants with reduced leaves (Fig. 7, D and F) showed a range of meristem phenotypes. The less severe double mutants had normal looking SAMs by SEM (Fig. 8, D and E) but reduced KNOX expression compared to normal, cr4, and Xcl1 siblings (Fig. 8, C and F compared to Fig. 5, A and B), indicating a gradual loss of meristem identity. Double mutant seedlings with the most severe phenotypes did not have a SAM (data not shown).
Figure 8.
Analysis of cr4 and cr4/Xcl1 meristems and gene expression. A, cr4/cr4 single mutant apex dissected to P3. B, cr4/cr4 apex in A with P3 removed. Leaf initiation appears normal. C, KNOX expression in cr4 mutants is similar to wild type and Xcl1 (Fig. 5). D, cr4/Xcl1 double homozygous mutant apex is very similar to A, except the P3 primordia are narrower. E, cr4;Xcl1 apex in B with P3 removed. Leaf primordia seem to be initiated normally, but the dome of the meristem is shorter than in cr4. F, KNOX protein levels are reduced in cr4;Xcl1 meristems. G, CR4 transcripts are reduced in Xcl1 meristems and leaves. H, PIN1 transcripts are decreased in Xcl1 and cr4 leaves. I, GA20 oxidase transcripts are increased in cr4 leaves. B, B73 (wild-type); X, Xcl1; C, cr4; M, meristem; U, unexpanded leaf; L, expanded leaf. Scale bars = 100 μm.
The extremely reduced seedling phenotypes seen in cr4;Xcl1 double mutants indicated that the two genes may act in the same pathway to influence both epidermal and meristem development. To address this, CR4 transcript levels were examined in wild-type and Xcl1 leaves and meristems by RT-PCR (Fig. 8G). When compared to B73, CR4 levels were greatly decreased in Xcl1 mutant SAMs and leaves, indicating that Xcl1 may regulate CR4 transcription. GA20ox and PIN1 levels were also examined in the mutants to determine if the effects of these mutations on meristem maintenance are linked to the hormones that have been shown to be involved in KNOX-mediated developmental pathways. PIN1 levels were decreased in cr4 leaves (Fig. 8H), while GA20 oxidase levels were increased in cr4 leaves (Fig. 8I).
DISCUSSION
The mutants described in this study all affect cell division and differentiation patterns in maize development. Kn1, Rs1, and Gn1 are all dominant KNOX mutations that show ectopic gene expression in leaves. This ectopic gene expression leads to periclinal divisions that produce knots and/or extra cell layers in the leaf blade and sheath (Vollbrecht et al., 1990; Smith et al., 1992). According to the maturation schedule hypothesis, ectopic KNOX expression in leaves retards the acquisition of competence for distal cell fates, leading to a proximalization of the leaf, or sheath identity in the blade (Freeling, 1992). The cr4 mutation also leads to changes in cell division and differentiation in the maize leaf. Loss-of-function cr4 mutants have problems in epidermal differentiation and also have periclinal divisions leading to multiple cell layers (Becraft et al., 1996; Jin et al., 2000), indicating that the CR4 protein is important for proper epidermal development. Finally, Xcl1 mutants have extra epidermal cell layers produced by aberrant oblique periclinal divisions that occur late in leaf development and lead to differentiation by lineage instead of position (Kessler et al., 2002). Because all of these mutants lead to cell proliferation through periclinal divisions, we constructed double mutants in the hopes of gaining insight into the pathways involved in controlling cell division during leaf development. The surprising meristem arrest phenotypes seen in some of the double mutant combinations point to a more basic role for these genes in meristem maintenance.
Xcl1 Suppresses the Kn1 Phenotype
KNOX genes play an important role in the maintenance of meristem identity. In simple-leafed model species such as maize and Arabidopsis, KNOX genes are down-regulated in the P0 and remain off throughout leaf development (Hake et al., 2004). However, tomato, which has compound leaves, displays KNOX expression in developing leaf primordia (Hareven et al., 1996; Chen et al., 1997). This expression pattern is seen in compound and secondarily simple-leafed species with independent origins throughout nature, and thus represents an evolutionarily conserved developmental pathway (Bharathan et al., 2002). How KNOX gene expression is regulated to bring about different developmental programs is an unanswered question. In recent years, the PHANTASTICA group of Myb-domain transcription factors has been shown to maintain KNOX suppression in developing leaves in Antirrhinum (PHANTASTICA), maize (rs2), and Arabidopsis (ASYMMETRIC LEAVES1; Timmermans et al., 1999; Tsiantis et al., 1999b; Byrne et al., 2000; Ori et al., 2000). In maize, rs2 mutants phenocopy dominant, gain-of-function mutations in several KNOX genes, including Kn1, Rs1, and Gn1, indicating that RS2 functions to down-regulate these genes in leaves (Schneeberger et al., 1998; Timmermans et al., 1999; Tsiantis et al., 1999b).
Suppressors of the dominant KNOX phenotypes would be expected to represent downstream elements necessary for KNOX activity. Xcl1 is a semidominant mutation that partially suppresses the Kn1 phenotype in leaves. Xcl1 could act either directly or indirectly on the KN1 pathway to suppress Kn1 mutant phenotypes and thus presents us with a way to further dissect the KNOX pathway.
Dosage analysis revealed that Xcl1 is a hypermorphic mutation resulting from increased expression of a normal gene product (Kessler et al., 2002). Kn1-O and Kn1-N2 are neomorphic dominant mutations in which KN1 is expressed ectopically in developing leaves. One model for the suppression of Kn1 phenotypes by Xcl1 is a biophysical model. Xcl1 leaves have aberrant oblique, periclinal divisions that lead to the production of multiple epidermal layers. In Kn1, knot formation requires signals from underlying mesophyll cells (Hake and Freeling, 1986; Sinha and Hake, 1990). In Xcl1;Kn1 double mutant leaves, it is possible that the suppression of knots is merely a reflection of decreased signaling through the extra epidermal layers conditioned by Xcl1. This hypothesis seems unlikely, because knots in Kn1 mutants form primarily over lateral veins (Gelinas et al., 1969; Hake and Freeling, 1986; Sinha and Hake, 1990). In Xcl1, multiple epidermal layers arise after the initiation of lateral veins, and therefore, the epidermis over lateral veins is always single layered (Kessler et al., 2002).
A second model for regulation of the KN1 pathway by Xcl1 is that in normal development, XCL1 acts to reduce KN1 function in the P0 and causes periclinal divisions that lead to leaf primordium outgrowth. When Xcl1 is expressed at higher levels than normal, KN1 activity would be down-regulated even more, and more cells would be recruited into the P0, thereby affecting meristem maintenance. This model is supported by the fact that Xcl1 meristems are shorter than normal and recruit more cells into P0 (Kessler et al., 2002). Additional evidence for XCL1 regulation of KN1 activity comes from the other double mutants. Even though Gn1 or Rs1 gain-of-function mutants ectopically express the proteins only in leaves, Xcl1 suppresses KNOX expression in Gn1;Xcl1 SAMs, leading to loss of meristem integrity. Construction of double mutant between Xcl1 and loss-of-function kn1 mutants (Vollbrecht et al., 2000) may also help to define the role of XCL1 in regulating KN1 activity.
The suppression of knot formation in Kn1 leaves by Xcl1, even though ectopic KN1 transcript was present in leaves, suggests that XCL1 might regulate KN1 posttranscriptionally or could differentially regulate genes downstream of KN1. One candidate pathway for regulation by both XCL1 and KN1 is the GA signaling pathway. When KN1 or tobacco KNOX genes are overexpressed in tobacco, leaf shape is altered and ectopic meristems form on leaves. GA levels have been shown to be reduced in tobacco leaves that overexpress NTH15 (Tamaoki et al., 1997), and NTH15 was subsequently shown to directly suppress the GA biosynthesis gene, GA20 oxidase (Sakamoto et al., 2001). In addition, GA20 oxidase in tobacco is expressed in P0, complementary to KNOX expression patterns (Sakamoto et al., 2001). Our experiments showed that GA treatment suppressed the Kn1 phenotype, and inhibiting GA biosynthesis with uniconazole enhanced the Kn1 phenotype. While GA20 oxidase levels are not appreciably altered in Xcl1;Kn1-N2 double mutants, GA2 oxidase levels are decreased, indicating that XCL1 overexpression may lead to higher GA levels, which in turn suppresses the formation of knots in double mutant leaves. Thus, decreased GA levels seem to be necessary for knot formation in dominant neomorphic Kn1 mutants. Increased XCL1 product may bypass KNOX regulation of the GA pathway by independently decreasing the GA metabolizing enzyme GA2 oxidase.
Xcl1 Interactions with Gn1, Rs1, and cr4 Lead to Loss of Meristem Function
Since dominant Gn1 and Rs1 mutants have very similar phenotypes to Kn1 mutants, we expected a similar suppression of the KNOX overexpression phenotype by Xcl1. Instead, both Gn1;Xcl1 and Rs1;Xcl1 double mutants displayed synergistic phenotypes that led to meristem arrest at early stages of seedling development. The double mutant seedlings had agravitropic roots and shoots, reduced vasculature in the developing stem, and did not initiate lateral roots, all phenotypes that are hallmarks of disrupted auxin transport and/or signaling (Vogler and Kuhlemeier, 2003).
Both Gn1;Xcl1 and Rs1;Xcl1 meristems have a striking similarity to severe rs2-R mutants described by Schneeberger et al. (1998). Severe rs2-R meristems have narrow leaf primordia that are often bifurcated. In addition, bladeless leaves similar to those in Figure 5E were also reported in rs2. RS1, LIGULELESS 3 (LG3), and KN1 are all ectopically expressed in rs2 leaves, but single dominant mutants in these genes have, to our knowledge, never been shown to have these severe meristem abnormalities, indicating that misexpression of more than one KNOX gene or overexpression of KNOX genes in specific domains is necessary for meristem abnormalities. A polyclonal antibody raised against KN1 also recognizes other class 1 KNOX proteins, including RS1, GN1, and LG3. Immunolocalizations with this antibody revealed no KNOX expression in developing leaves of Xcl1 single mutants (Kessler et al., 2002), indicating that Xcl1 must be enhancing Gn1 to give an rs2-like phenotype and ultimate meristem arrest by a mechanism other than misexpression of multiple KNOX genes.
Layers, Hormones, and Meristem Maintenance
The arrested seedling development seen in Xcl1;cr4 double mutants indicates that Xcl1 may act through CR4 to exert its effect on KNOX genes in meristem maintenance. As leaves are initiated from the flanks of the meristem, the L1 must expand to accommodate periclinal divisions in internal layers, and the corpus of the meristem must replenish the cells that become the new leaf primordia. Thus, communication between the cell layers is necessary for proper meristem maintenance and organ production (Ingram, 2004). The importance of the L1 in meristem function was illustrated by mechanical removal of the L1 in tomato meristems, which led to terminal differentiation of the underlying cells (Reinhardt et al., 2003a). The loss of meristem maintenance in the most severe Xcl1;cr4 double mutants indicates that there may be a relationship between proper protoderm specification and SAM maintenance. Xcl1 mutants have a shorter apical meristem than normal siblings in three different backgrounds, indicating that Xcl1 may play a role in regulating KNOX activity in the SAM (Kessler et al., 2002). CR4 has been shown to be expressed throughout the SAM and leaf primordia early in development and is later restricted to the epidermis and vascular traces (Becraft et al., 2001), but cr4 loss-of-function mutants have not been reported to affect meristem maintenance. Therefore, it seems that when XCL1 is expressed at normal levels, CR4 is not necessary for meristem maintenance, but when XCL1 is overproduced and there is no functional CR4, KNOX expression is reduced leading to loss of SAM identity. There are at least two possible explanations for the loss of meristem identity in Xcl1;cr4 double mutants. The first is that physical abnormalities in the L1 layer of the double mutant meristems disrupt signaling processes (such as KN1 intercellular trafficking or hormonal signaling) necessary for meristem maintenance. The second hypothesis is that XCL1 and CR4 are involved more directly in regulating genes that play a role in meristem maintenance. Since CR4 expression is reduced in Xcl1 mutants, it is possible that XCL1 also regulates genes with meristem maintenance functions redundant to that of CR4.
Hormonal Balance in Double Mutants
While the meristem abnormalities seen in Xcl1;cr4 double mutants are different than those seen in Gn1;Xcl1 and Rs1;Xcl1 mutants, it is clear that the presence of the Xcl1 mutation creates an environment in which the meristem is highly sensitive to perturbations in other pathways (such as KNOX and CR4). Hormonal pathways are the most likely candidates for this altered environment, as GA, auxin, and cytokinin have all been shown to be involved in KNOX signaling pathways (Hay et al., 2004). As discussed previously, the suppression of the Kn1 phenotype by Xcl1 seems to be mediated through the GA pathway. The loss of meristem function in the other double mutants seems more likely to involve auxin signaling pathways.
Auxin has been implicated in the initiation of leaf primordia from the flanks of SAMs. Tomato meristems that are treated with polar auxin transport inhibitors do not form leaves, but when auxin is applied to the flanks of these naked meristems, leaf primordia are initiated (Reinhardt et al., 2000). In Arabidopsis, polar auxin transport through the L1 layer is necessary for leaf initiation, and the distribution of auxin is thought to be a primary determinate of phyllotaxy (Reinhardt et al., 2003b). This indicates that foci of concentrated auxin are sufficient for specifying leaf initiation, possibly by influencing the down-regulation of KNOX genes.
Connections between KNOX genes and auxin have also been reported in maize. Seedlings grown on the polar auxin transport inhibitor, 2,3,5-triiodobenzoic acid, had leaf phenotypes similar to rs2 and dominant KNOX mutants. In the same study, rs2 mutants were shown to have defective polar auxin transport (Tsiantis et al., 1999a). Treatment of cultured maize meristems with another polar auxin transport inhibitor, napthylphthalamic acid, leads to a cessation of leaf primordia initiation correlated with a failure to down-regulate KNOX genes in P0 (Scanlon, 2003). These results indicate that changes in the distribution of auxin (or in auxin to cytokinin ratios) phenocopy KNOX mutations in maize and that high auxin levels correlate with reduction in KNOX expression (Hay et al., 2004). The vascular defects seen in Xcl1 mutants and the various auxin-related phenotypes observed in Gn1;Xcl1 and Rs1;Xcl1 double mutants indicate that the Xcl1 mutation may change auxin distribution in seedlings, leading to meristem arrest in double mutants. The altered levels of PIN1 transcripts also suggest that auxin transport or distribution is perturbed, as PIN transcript levels have been shown to correlate with changes in auxin (Benkova et al., 2003; Furutani et al., 2004; Peer et al., 2004). Further proof of this hypothesis awaits the development of tools such as the Arabidopsis auxin responsive reporter DR5∷β-glucuronidase (Ulmasov et al., 1997) for analyzing auxin localization in maize. The semaphore1 mutation leads to misexpression of RS1, GN1, and LG3 (but not KN1) in leaves independent of the RS2 pathway and also disrupts polar auxin transport (Scanlon et al., 2002). This indicates that KN1 expression has a different sensitivity to changes in auxin distribution than RS1 and GN1 and could explain the lack of meristem abnormalities seen in Kn1-N2;Xcl1 double mutants when compared to Gn1;Xcl1 and Rs1;Xcl1 double mutants.
CONCLUSION
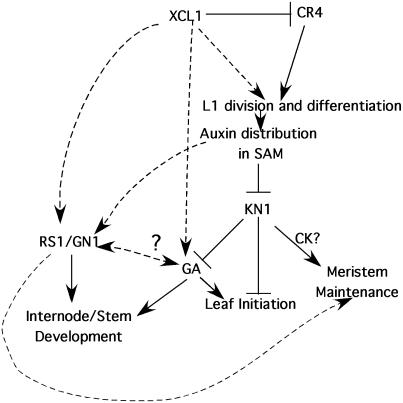
Our double mutant analyses between Xcl1, dominant KNOX mutants, and cr4 provide evidence for a link between division patterns in the L1 and meristem maintenance and development. The multiple epidermal layers found in Xcl1 may disrupt auxin flow from older leaves through perturbations in the differentiation of the L1 layer, altering hormonal balance at the shoot apex and leading to meristem abnormalities that are enhanced by Gn1 and Rs1 (Fig. 9). The identity of the XCL1 gene has yet to be determined, but its effects on CR4, GA2 oxidase, and PIN1 transcript levels indicate that it regulates a network of other developmental genes and likely affects hormone biosynthesis, metabolism, and/or transport during shoot development.
Figure 9.
Model for the genetic interactions involved in maize meristem maintenance and shoot development. XCL1 and CR4 are both involved in L1 division and differentiation processes that influence hormonal balance and meristem function. Solid lines represent data confirmed in our experiments or published results, and dashed lines represent inferred relationships.
MATERIALS AND METHODS
Genetic Stocks
The original Xcl1 allele introgressed five to six generations into the appropriate genetic background was used for all crosses. Kn1-N2 and Gn1-R were generously provided by Dr. Sarah Hake (USDA-Plant Gene Expression Center). Kn1-O (118B), Rs1-O (727G), and cr4-6143 (X28F) were provided by the Maize Genetics Stock Center (accession numbers are listed after stock name). Xcl1 genotypes were inferred by hand sectioning leaves to look for extra cell layers.
Histology, Immunolocalizations, and Microscopy
Tissue fixation, sectioning, staining, and KN1 immunolocalizations were performed as previously described (Kessler et al., 2002). For SEM, approximately 5-mm sections containing the SAM were fixed in formaldehyde-acetic acid (10% formaldehyde, 5% acetic acid, 50% ethanol) for 4 h to overnight. Next, meristems were transferred to 70% ethanol for 1 h, then 85% ethanol. Older leaves (down to P3–P5) were dissected away from the meristems (under 85% ethanol) using fine forceps and hypodermic needles. Meristems were then transferred to 95% ethanol for 1 h and 100% ethanol for 15 min to 1 h. Critical point drying, sputter coating, and microscopy were performed as previously described (Kessler et al., 2002).
Vein Counts
Two-centimeter pieces of leaf were taken from leaf 10 of five Xcl1 mutants and five wild-type plants at three positions (base, middle, and tip) of the leaf, midway between the margin and midrib. Leaves were cleared by autoclaving in 85% lactic acid for 20 min, washed three times in water, and stained overnight in 0.05% Toluidine Blue O. The number of veins between major lateral veins were counted and averages were compared by Student's t test (at each position the difference was significant at P = 0.05).
Hormone Treatments
Heterozygous maize (Zea mays) Kn1-N2 seedlings were grown and treated with external sprays of hormones after the first leaf unfolded. Treatments continued every second day until the third leaf reached maximum size at which point leaves 2 and 3 were collected and measurements taken. Hormones were diluted into 250 mL water with five drops TWEEN-20 to aid leaf surface adhesion. GA treatment was at 100 μm GA3 (Sigma). Uniconizole-P (Sumagic, Valent) treatment was at 20 μm as higher concentrations prevented further growth. Control was water with Tween-20 and no hormones.
RT-PCR Analysis
Total RNA was extracted from seedling leaves (leaf 1 or 2) and meristems with either RNAwiz (Ambion) or Qiagen RNeasy Miniprep kit according to the manufacturer's recommendations. For semiquantitative RT-PCR the Promega PolyA tract kit was used to isolate mRNA, which was then reverse transcribed with SuperscriptII (Invitrogen) or Thermoscript (Invitrogen) with random hexamers according to the manufacturer's recommendations. GAPDH primers were used as the constitutively expressed control for semiquantitative RT-PCR and QRT-PCR. The QRT-PCR was performed according to published protocols (Yamagishi et al., 2005). Primer specificity was determined by sequencing the RT-PCR product from each primer pair. For semiquantitative RT-PCR each primer pair was tested with different numbers of PCR cycles to determine the linear range, and subsequent reactions were performed with the following number of cycles: GAPDH (25), KN1 (35), GA20ox (32), CR4 (35), or as indicated in figure legends. Experiments shown in the figures are representatives of two biological replicates (RNA preparations from different sets of tissue) and at least three PCR replicates from each template for the semiquantitative RT-PCR and several replicates for the QRT-PCR. In the QRT-PCR experiments RNA levels were normalized to an internal control (ZmGAPDH), and the results are reported as relative RNA levels compared to wild type. The primers used for semiquantitative RT-PCR were: ZmGAPDH-1, AGGGTGGTGCCAAGAAGGTTG; ZmGAPDH-2, GTAGCCCCACTCGTTGTCGTA; ZmKn1-C, GGAGGAGATCACCCAACACTTTGG; ZmKn1-D, TTGATCCTCCTCAGAAGAGCCAGATGAAAG; ZmGA20ox-1, CCTGGAAGGAGACGCTGTCG; ZmGA20ox-2, GCTGAGCCAGTTGGAGAAGG; ZmCR4-1, GCAGTACCTCTGATCAAAG; ZmCR4-2, CATCTCCTGAATTTCTTCAG; ZmRS1-A, GAGAACTACAAGCCATGCATAGACGCTAC; ZmRS1-B, TTCTGAAGATGACATGGACCCGAATGGTC; ZmGn1-B1, TACGCAGAAACACTCCGACACGGTCG; and ZmGn1-I, GGCCGTACCCTTCGAGACG. Primers used for QRT-PCR were: ZmQPin1-1, CGCTGATGCTGTTCATGTTC; ZmQPin1-2, GCCGTCCTCCTTCACCTC; ZmQGa2ox-1, ACCTACCTAAGCCTCCAACATAAACTCTCTG; ZmQGa20x-2, AGCCGCCTCAACCTCTTCCACATC; ZmQGAPDH-1, CGCTCTGAACGACCACTTC; and ZmQGAPDH-2, ACACAAGCAGCAACCATCC.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: GA20 oxidase, AY105651; PIN1, AY110024; and GA2 oxidase, DN228214.
Acknowledgments
We thank Sarah Hake for providing the Kn1 and Gn1 stocks, Angela Hay for providing GN1 primer sequences, Dan Koenig for help with the statistical analyses, Siobhan Braybrook for help with the QRT-PCR experiments, and John Harada, Naoyuki Uchida, Julie Kang, and Connie Champagne for critical reading of this manuscript.
This work was supported by the National Science Foundation (Plant Cell Biology Training Grant and NSF-IBN 0316877 and 0344743 to N.S.) and by the University of California at Davis (Jastro Shields, Elsie Stocking, and Rosalind Russell fellowships to S.K.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Neelima Sinha (nrsinha@ucdavis.edu).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.076075.
References
- Abe M, Katsumata H, Komeda Y, Takahashi T (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643 [DOI] [PubMed] [Google Scholar]
- Barton M, Poethig RS (1993) Formation of the shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development 119: 823–831 [Google Scholar]
- Becraft PW, Freeling M (1994) Genetic analysis of rough sheath1 developmental mutants of maize. Genetics 136: 295–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft PW, Kang S-H, Suh S-G (2001) The maize CRINKLY4 receptor kinase controls a cell-autonomous differentiation response. Plant Physiol 127: 486–496 [PMC free article] [PubMed] [Google Scholar]
- Becraft PW, Stinard PS, McCarty DR (1996) CRINKLY4: a TNFR-like receptor kinase involved in maize epidermal differentiation. Science 273: 1406–1409 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Berleth T, Mattsson J, Hardtke CS (2000) Vascular continuity and auxin signals. Trends Plant Sci 5: 387–393 [DOI] [PubMed] [Google Scholar]
- Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR (2002) Homologies in leaf form inferred from KNOXI gene expression during development. Science 296: 1858–1860 [DOI] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408: 967–971 [DOI] [PubMed] [Google Scholar]
- Chen J-J, Janssen B-J, Williams A, Sinha N (1997) A gene fusion at a homeobox locus: alternations in leaf shape and implications for morphological evolution. Plant Cell 9: 1289–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G, Lincoln C, Hake S (1996) KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell 8: 1277–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T, Yamaguchi J, Wong BC, Veit B, Hake S (1999) Gnarley1 is a dominant mutation in the knox4 homeobox gene affecting cell shape and identity. Plant Cell 11: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M (1992) A conceptual framework for maize leaf development. Dev Biol 153: 44–58 [DOI] [PubMed] [Google Scholar]
- Freeling M, Lane B (1994) The maize leaf. In M Freeling, V Walbot, eds, The Maize Handbook. Springer-Verlag, New York, pp 17–28
- Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M (2004) PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131: 5021–5030 [DOI] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Gelinas D, Postlethwait SN, Nelson OE (1969) Characterization of development in maize through the use of mutants. II. The abnormal growth conditioned by the Knotted mutant. Am J Bot 56: 671–678 [Google Scholar]
- Hake S, Freeling M (1986) Analysis of genetic mosaics shows that the extra epidermal divisions in Knotted mutant maize plants are induced by adjacent mesophyll cells. Nature 320: 621–623 [Google Scholar]
- Hake S, Smith HM, Holtan H, Magnani E, Mele G, Ramirez J (2004) The role of knox genes in plant development. Annu Rev Cell Dev Biol 20: 125–151 [DOI] [PubMed] [Google Scholar]
- Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E (1996) The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell 84: 735–744 [DOI] [PubMed] [Google Scholar]
- Hay A, Craft J, Tsiantis M (2004) Plant hormones and homeoboxes: bridging the gap? Bioessays 26: 395–404 [DOI] [PubMed] [Google Scholar]
- Hay A, Jackson D, Ori N, Hake S (2003) Analysis of the competence to respond to KNOTTED1 activity in Arabidopsis leaves using a steroid induction system. Plant Physiol 131: 1671–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram GC (2004) Between the sheets: inter-cell-layer communication in plant development. Philos Trans R Soc Lond B Biol Sci 359: 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Veit B, Hake S (1994) Expression of maize KNOTTED 1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120: 405–413 [Google Scholar]
- Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M (2005) KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol 15: 1560–1565 [DOI] [PubMed] [Google Scholar]
- Jin P, Guo T, Becraft PW (2000) The maize CR4 receptor-like kinase mediates a growth factor-like differentiation response. Genes J Genet Dev 27: 104–116 [DOI] [PubMed] [Google Scholar]
- Kessler S, Seiki S, Sinha N (2002) Xcl1 causes delayed oblique periclinal cell divisions in developing maize leaves, leading to cellular differentiation by lineage instead of position. Development 129: 1859–1869 [DOI] [PubMed] [Google Scholar]
- Kim JY, Yuan Z, Cilia M, Khalfan-Jagani Z, Jackson D (2002) Intercellular trafficking of a knotted1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc Natl Acad Sci USA 99: 4103–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Yuan Z, Jackson D (2003) Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development 130: 4351–4362 [DOI] [PubMed] [Google Scholar]
- Kusaba S, Kano-Murakami Y, Matsuoka M, Tamaoki M, Sakamoto T, Yamaguchi I, Fukumoto M (1998) Alteration of hormone levels in transgenic tobacco plants overexpressing the rice homeobox gene OSH1. Plant Physiol 116: 471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69 [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Bouche-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S (1995) Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270: 1980–1983 [DOI] [PubMed] [Google Scholar]
- Mele G, Ori N, Sato Y, Hake S (2003) The knotted1-like homeobox gene BREVIPEDICELLUS regulates cell differentiation by modulating metabolic pathways. Genes Dev 17: 2088–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Eshed Y, Chuck G, Bowman JL, Hake S (2000) Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 127: 5523–5532 [DOI] [PubMed] [Google Scholar]
- Ori N, Juarez MT, Jackson D, Yamaguchi J, Banowetz GM, Hake S (1999) Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence-activated promoter. Plant Cell 11: 1073–1080 [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Frenz M, Mandel T, Kuhlemeier C (2003. a) Microsurgical and laser ablation analysis of interactions between the zones and layers of the tomato shoot apical meristem. Development 130: 4073–4083 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003. b) Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Richert J, Kranz E, Lorz H, Dresselhaus T (1996) A reverse transcriptase-polymerase chain reaction assay for gene expression studies at the single cell level. Plant Sci 114: 93–99 [Google Scholar]
- Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M (2001) KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes Dev 15: 581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Sentoku N, Miura Y, Hirochika H, Kitano H, Matsuoka M (1999) Loss-of-function mutations in the rice homeobox gene OSH15 affect the architecture of internodes resulting in dwarf plants. EMBO J 18: 992–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon MJ (2003) The polar auxin transport inhibitor N-1-naphthylphthalamic acid disrupts leaf initiation, KNOX protein regulation, and formation of leaf margins in maize. Plant Physiol 133: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon MJ, Henderson DC, Bernstein B (2002) SEMAPHORE1 functions during the regulation of ancestrally duplicated knox genes and polar auxin transport in maize. Development 129: 2663–2673 [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Schneeberger RG, Freeling M (1996) The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 122: 1683–1691 [DOI] [PubMed] [Google Scholar]
- Schneeberger R, Tsiantis M, Freeling M, Langdale JA (1998) The rough sheath2 gene negatively regulates homeobox gene expression during maize leaf development. Development 125: 2857–2865 [DOI] [PubMed] [Google Scholar]
- Schneeberger RG, Becraft PW, Hake S, Freeling M (1995) Ectopic expression of the knox homeobox gene rough sheath 1 alters cell fate in the maize leaf. Genes Dev 9: 2292–2304 [DOI] [PubMed] [Google Scholar]
- Sharman BC (1942) Developmental anatomy of the shoot of Zea mays L. Ann Bot 6: 245–284 [Google Scholar]
- Sinha N, Hake S (1990) Mutant characters of Knotted maize leaves are determined in the innermost tissue layers. Dev Biol 141: 203–210 [DOI] [PubMed] [Google Scholar]
- Sinha N, Hake S (1994) The Knotted leaf blade is a mosaic of blade, sheath, and auricle identities. Dev Genet 15: 401–414 [Google Scholar]
- Sinha N, Williams RE, Hake S (1993) Overexpression of the maize homeobox gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Genes Dev 7: 787–795 [DOI] [PubMed] [Google Scholar]
- Sinha NR (1990) Developmental analysis of the Knotted-1 mutant in Zea mays. PhD thesis. University of California, Berkeley, CA
- Smith LG, Greene B, Veit B, Hake S (1992) A dominant mutation in the maize homeobox gene, Knotted-1, cause its ectopic expression in leaf cells with altered fates. Development 116: 21–30 [DOI] [PubMed] [Google Scholar]
- Smith LG, Jackson D, Hake S (1995) Expression of knotted1 marks shoot meristem formation during maize embryogenesis. Dev Genet 16: 344–348 [Google Scholar]
- Stieger PA, Reinhardt D, Kuhlemeier C (2002) The auxin influx carrier is essential for correct leaf positioning. Plant J 32: 509–517 [DOI] [PubMed] [Google Scholar]
- Tamaoki M, Kusaba S, Kano-Murakami Y, Matsuoka M (1997) Ectopic expression of a tobacco homeobox gene, NTH15, dramatically alters leaf morphology and hormone levels in transgenic tobacco. Plant Cell Physiol 38: 917–927 [DOI] [PubMed] [Google Scholar]
- Timmermans MCP, Hudson A, Becraft PW, Nelson T (1999) Rough sheath2: a Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science 284: 151–153 [DOI] [PubMed] [Google Scholar]
- Tsiantis M, Brown MIN, Skibinski G, Langdale JA (1999. a) Disruption of auxin transport is associated with aberrant leaf development in maize. Plant Physiol 121: 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA (1999. b) The maize rough sheath2 gene and leaf development programs in monocot and dicot plants. Science 284: 154–156 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit B, Vollbrecht E, Mathern J, Hake S (1990) A tandem duplication causes the Kn1-O allele of Knotted, a dominant morphological mutant of maize. Genetics 125: 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler H, Kuhlemeier C (2003) Simple hormones but complex signalling. Curr Opin Plant Biol 6: 51–56 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Reiser L, Hake S (2000) Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development 127: 3161–3172 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S (1990) The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350: 241–243 [DOI] [PubMed] [Google Scholar]
- Yamagishi K, Nagata N, Yee KM, Braybrook S, Pelletier J, Fujioka S, Yoshida S, Fischer R, Goldberg R, Harada J (2005) TANMEI/EMB2757 encodes a WD repeat protein required for embryo development in Arabidopsis. Plant Physiol 139: 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, Samach A, Ori N (2005) Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol 15: 1566–1571 [DOI] [PubMed] [Google Scholar]