Abstract
It was investigated whether the methyl-erythritol phosphate (MEP) pathway that generates volatile isoprenoids and carotenoids also produces foliar abscisic acid (ABA) and controls stomatal opening. When the MEP pathway was blocked by fosmidomycin and volatile isoprenoid emission was largely suppressed, leaf ABA content decreased to about 50% and leaf stomatal conductance increased significantly. No effect of fosmidomycin was seen in leaves with constitutively high rates of stomatal conductance and in plant species with low foliar ABA concentration. In all other cases, isoprene emission was directly associated with foliar ABA, but ABA reduction upon MEP pathway inhibition was also observed in plant species that do not emit isoprenoids. Stomatal closure causing a midday depression of photosynthesis was also associated with a concurrent increase of isoprene emission and ABA content. It is suggested that the MEP pathway generates a labile pool of ABA that responds rapidly to environmental changes. This pool also regulates stomatal conductance, possibly when coping with frequent changes of water availability. MEP pathway inhibition by leaf darkening, and its down-regulation by exposure to elevated CO2, was also associated with a reduction of foliar ABA content. However, stomatal conductance was reduced, indicating that stomatal aperture is not regulated by the MEP-dependent foliar ABA pool, under these specific cases.
Volatile isoprenoids (isoprene and monoterpenes) are formed via a chloroplastic pathway (the methyl-erythritol phosphate [MEP] pathway; Lichtenthaler et al., 1997), predominantly by carbon shunted from the carbon fixation cycle, as indicated by the rapid and quasicomplete labeling of isoprene carbon atoms by 13C (Delwiche and Sharkey, 1993). The MEP pathway also generates more complex isoprenoids such as carotenoids, essential for chloroplast functioning. Xanthophylls, specialized carotenoids regulating excess energy through the well-described deepoxidation/epoxidation cycle (Demmig-Adams and Adams, 1996), also act as precursors of abscisic acid (ABA) through the intermediate xanthoxin (Milborrow, 2001; Schwartz et al., 2003). However, multiple ABA pools are present in plants, which may have different biosynthetic and regulatory steps. In leaves, ABA may be also produced after stress episodes, from a pool that is not blocked from carotenoid inhibitors (Li and Walton, 1987). ABA may also accumulate in roots (Cornish and Zeevaart, 1985) and be transported to leaves through the xylem, where it plays an important role in modulating stomatal responses to water stress (Tardieu et al., 1992). ABA has a general role in stress protection, inducing stomatal closure (Mittelheuser and van Steveninck, 1969) and reduction of leaf expansion rate (Zhang and Davies, 1990) under water stress, as well as under several other stress conditions (Milborrow, 2001).
Understanding the regulation of ABA biosynthesis and catabolism is key to developing an integrated perspective on plant stress and to linking processes acting at the chloroplast level to those acting at cellular and larger scales. Loreto and Velikova (2001) noted that fosmidomycin feeding to inhibit isoprene biosynthesis (Zeidler et al., 1998) could lead to an increase of stomatal conductance and to a stimulation of leaf transpiration. This could have been obtained by a direct effect of isoprene on conductance, but it might also reveal an impact of the inhibition of the MEP pathway on ABA production. If leaf levels of ABA were sensitive to MEP activity, then there may be a labile pool of ABA in leaves that could be regulated through processes that mediate the MEP pathway.
We explored whether a direct link between the MEP pathway functioning and ABA biosynthesis exists. Fosmidomycin and a range of environmental factors were used to modulate MEP pathway activity and the consequent emission of volatile isoprenoids, and ABA concentration was measured in all these conditions. It is shown that (1) The MEP pathway may generate a part of foliar ABA in both isoprenoid-emitting and -nonemitting plants; (2) Volatile isoprenoid emissions are directly associated to ABA content in leaves; and (3) The ABA pool formed by the MEP pathway regulates stomatal opening in response to rapid changes of water status while it does not control stomatal closure in the dark and at elevated CO2.
RESULTS
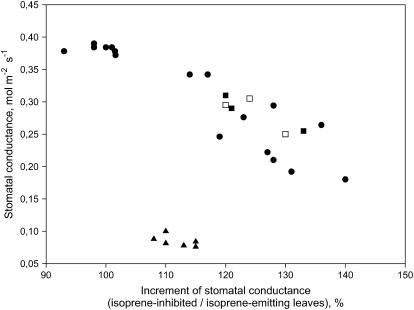
When the MEP pathway of the isoprene-emitting Phragmites australis leaves was inhibited by fosmidomycin to about 10% of the initial level, a simultaneous increase of stomatal conductance was observed (Fig. 1). The change in conductance was inversely proportional to the initial conductance and was absent in leaves that had very high initial rates of conductance. All plants were maintained under well-watered conditions (see “Materials and Methods”), and the range of initial conductances represents the natural variability observed in this species. The use of fosmidomycin caused no changes in photosynthesis in any of the leaves measured, even after several hours of feeding, suggesting that there were no toxic, direct effects of the inhibitor on carbon metabolism (data not shown). When MEP-inhibited leaves were supplied with exogenous isoprene to reconstitute the internal pool of this volatile metabolite, conductances remained high (Fig. 1). When the MEP pathway activity was blocked using fosmidomycin in Quercus ilex, a monoterpene-emitting oak, again a small increase of conductance was observed (Fig. 1).
Figure 1.
Stomatal conductance rates in leaves of P. australis (isoprene emitter, circles) and Q. ilex (monoterpene emitter, triangles). The increment of stomatal conductance after inhibiting isoprenoid formation (x axis, values expressed as percent of the stomatal conductance in the same leaves before isoprene inhibition [=100]) is plotted versus the stomatal conductance rates of each leaf, measured before isoprenoid inhibition (y axis). Isoprene was inhibited by feeding 20 μm fosmidomycin through petioles. In some leaves of P. australis (black squares) the increment of stomatal conductance after endogenous isoprene inhibition was again measured after reconstituting the internal pool with exogenous isoprene (3 ppm of gaseous isoprene in the air flowing over the leaf in the gas exchange cuvette) for 1 h (white squares).
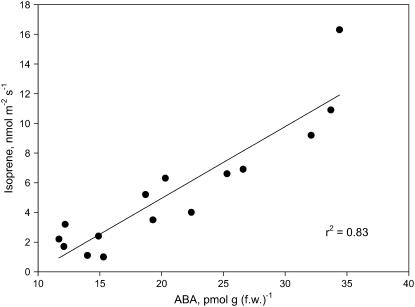
In P. australis leaves with conductances between 0.15 and 0.35 mol m−2 s−1 (i.e. those rates at which MEP inhibition was well correlated with an increase in conductance) a correlation (r2 = 0.83) was also found between isoprene emission and foliar ABA content (Fig. 2).
Figure 2.
Relationship between isoprene emission and ABA content in P. australis leaves characterized by stomatal conductances variable between 0.15 to 0.35 mol m−2 s−1. Also shown is the linear best fit generated by the Sigmaplot 2002 software (Systat; r2 = 0.83).
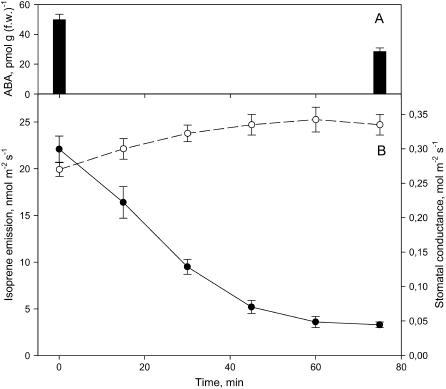
The effect of MEP inhibition on conductances and ABA content was also studied in a second isoprene-emitting plant, Populus alba. P. alba large leaves allowed us to investigate the temporal course of MEP inhibition on parameters measured in different areas of the same leaves, avoiding leaf-to-leaf variability and also studying the possible impact of wounding on ABA. The time course of stomatal conductance increase after fosmidomycin feeding was similar to that observed for isoprene emission inhibition, usually complete by 60 min after the fosmidomycin was added (Fig. 3). At this time the total ABA content of isoprene-inhibited leaves was significantly lower than in isoprene-emitting leaves, confirming that ABA levels and stomatal conductance are both influenced by MEP inhibition and do not respond specifically to wounding (Fig. 3).
Figure 3.
Time course of changes in ABA content (A), isoprene emission (B; black circles), and stomatal conductances (white circles) upon feeding leaves of P. alba with fosmidomycin 20 μm. The inhibitor was fed at time 0. The measurements shown are means ± se (n = 3).
The inhibition of the MEP pathway regulated ABA and stomatal conductance not only in leaves emitting isoprene (as in P. australis and P. alba) but also in the nonemitting leaves of Prunus persica (Table I). In contrast, a reduction of ABA and stomatal conductance were not observed in Pawlonia fortunei leaves that do not emit isoprene and are characterized by constitutive low levels of ABA (Table I).
Table I.
Content of ABA and stomatal conductance of isoprene-emitting leaves of P. australis and P. alba and nonemitting leaves of P. fortunei and P. persica
In another batch of leaves the isoprenoid pathway leading to isoprene synthesis was inhibited by feeding 20 μm fosmidomycin. Asterisks (***) represent statistical separation between means of the two treatments (−/+ fosmidomycin) for each plant species at P ≤ 0.01 (n = 10 in P. australis, n = 5 for all other species; Tukey's test).
| Treatment | ABA Content | Stomatal Conductance | ||
|---|---|---|---|---|
| pmol g (fw)−1 | mol m−2 s−1 | |||
| Isoprene-emitting leaves | P. australis | P. alba | P. australis | P. alba |
| Isoprene emitting (−fosmidomycin) | 22.9 ± 2.6 | 44.4 ± 6.9 | 0.25 ± 0.04 | 0.24 ± 0.06 |
| Isoprene inhibited (+fosmidomycin) | 15.9 ± 3.1*** | 20.8 ± 8.1*** | 0.36 ± 0.09*** | 0.34 ± 0.07*** |
| Nonemitting leaves | P. fortunei | P. persica | P. fortunei | P. persica |
| −Fosmidomycin | 6.4 ± 0.7 | 51.2 ± 6.6 | 0.13 ± 0.03 | 0.08 ± 0.01 |
| +Fosmidomycin | 8.6 ± 1.7 | 34.4 ± 4.9*** | 0.09 ± 0.04 | 0.11 ± 0.01*** |
Three more treatments were used to induce changes in stomatal conductance and to study whether these changes were associated with changes in isoprene emission and ABA content in P. alba leaves.
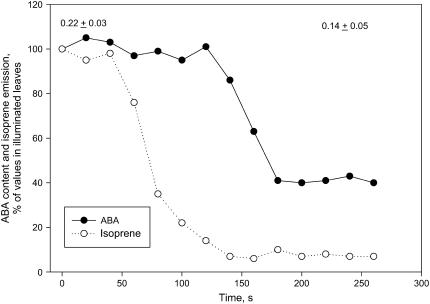
During a light-to-dark transition that caused a 30% reduction of stomatal opening, isoprene emission dropped rapidly and ABA leaf content was also reduced although more slowly than isoprene. ABA concentration after 200 s of darkness was about 50% of the value observed in illuminated leaves (Fig. 4). Stomatal closure in the dark therefore occurred independently of ABA accumulation in leaves.
Figure 4.
Time course of changes in ABA content and isoprene emission upon a light-to-dark transition in P. alba leaves. The illumination (1,000 μmol photons m−2 s−1) was switched off at time 0. The values are means of measurements on three leaves of different plants expressed as relative to isoprene emission and ABA content in illuminated leaves (=100). The se of these measurements were always <10% of the respective means. Also shown as means ± se are the values of stomatal conductance at the beginning and at the end of the experiment.
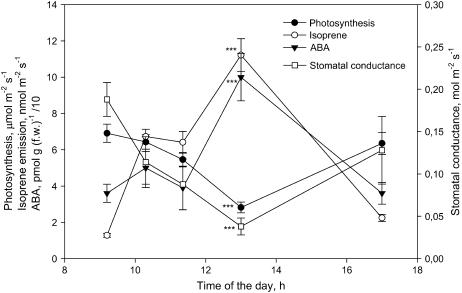
ABA leaf content and isoprene emission increased in pair during the day, while poplar leaves experienced a midday depression of photosynthesis consequent to stomatal closure (Fig. 5).
Figure 5.
Time course of changes in photosynthesis, isoprene emission, ABA content, and stomatal conductance during a day in P. alba leaves. The measurements shown are means ± se (n = 4). Asterisks (***) represent statistical separation between means of each parameter measured at 9:30 am and at 1:00 pm (P ≤ 0.01, Tukey's test).
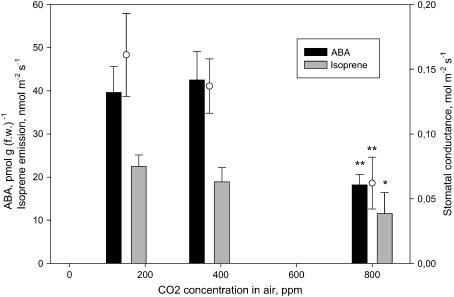
Finally, stomata of poplar leaves closed at elevated CO2, but again this was not a consequence of ABA accumulation (Fig. 6). Both ABA level and isoprene emission at elevated CO2 were lower than at ambient CO2 level, while at low CO2 there were no significant effects on any parameter. Fosmidomycin feeding after exposing plants at elevated CO2 did not significantly change ABA content and did not induce stomatal opening (data not shown).
Figure 6.
Isoprene emission (gray bars), ABA content (black bars), and stomatal conductance (white circles) of P. alba leaves in response to exposure to different concentrations of CO2. Asterisks represent statistical separation between means of each parameter under the three CO2 concentrations (n = 3; * = P ≤ 0.10, ** = P ≤ 0.05; Tukey's test).
DISCUSSION
In all plants where the inhibition of the MEP pathway caused a decrease in ABA content, a significant increase of stomatal conductance was also observed. In those leaves of P. australis and P. fortunei where the inhibition of the MEP pathway did not reduce ABA content, there was also no effect on conductance. In the two isoprene-emitting species, the inhibition of the MEP pathway also caused the well-known sharp decline in isoprene emission (Loreto and Velikova, 2001). However, two lines of evidence support the contention that stomatal conductance is not affected directly by changes in isoprene emission. First, addition of exogenous isoprene had no effect on stomatal conductance, and second, stomatal conductance increased upon inhibition of the MEP pathway in P. persica, a species that does not emit isoprene. It is therefore suggested that the increase in stomatal conductance associated with inhibition of the MEP pathway is caused by a decrease in a labile pool of ABA that is highly sensitive to current biosynthesis.
To our knowledge, this result is the first to demonstrate a link between MEP pathway activity, ABA concentration, and stomatal conductance over short time scales. The biosynthesis of ABA has been widely discussed, and several pathways suggested. Recent advances reviewed by Schwartz et al. (2003) have, however, confirmed that one pathway for ABA biosynthesis is the oxidative cleavage of xanthophylls in the cytosol, as hypothesized by Taylor and Smith (1967). Xanthophylls are formed in chloroplasts from the MEP pathway, the same pathway that leads to volatile isoprenoid production (Lichtenthaler et al., 1997). Our findings of a firm relationship between MEP pathway activity and leaf-level ABA concentrations support the hypothesis that at least one component of leaf ABA can be tightly linked to metabolism in chloroplasts rather than to cytosolic metabolism alone.
The very similar time constants of the response to fosmidomycin of isoprene emission, ABA concentration, and stomatal conductance also indicate a direct link between MEP pathway activity and ABA formation. However, while isoprene was completely inhibited 60 min after fosmidomycin feeding, ABA concentrations only dropped from 50% to 70% of the initial values in the different species (Table I), suggesting that only one component of the leaf ABA pool depends closely upon activity of the MEP pathway. As noted in “Materials and Methods,” these experiments were all performed with excised leaves, and no contribution of root or xylem ABA was possible (Tardieu et al., 1992). We speculate that leaves contain at least two pools of ABA, one that turns over slowly and is independent of MEP activity and another that is much more labile and that depends directly on MEP activity in the chloroplast. These two pools may correspond to the slowly turning over pool described by Zeevaart et al. (1989) on the basis of 18O2-labeling experiments with ABA carotenoid precursors, and to the direct synthetic pathway recently discovered in fungi (Hirai et al., 2000), respectively.
These results might suggest that changes in MEP pathway activity should also lead to changes in xanthophylls, the carotenoids from which ABA is formed (Schwartz et al., 2003). However, no evidence of changes in total carotenoids (Loreto et al., 2004) and in xanthophylls deepoxidation status (Loreto et al., 2004; Scholefield et al., 2004) were previously found upon fosmidomycin feeding for short periods (up to 1 h). Similar results were found in these experiments, being the deepoxidation status of xanthophylls independent of fosmidomycin, while the total amount of xanthophylls was slightly but not significantly reduced following fosmidomycin feeding (data not shown). We explain this finding with the slow turnover of carotenoid large pools. This explains why fosmidomycin often does not affect the photochemistry of photosynthesis within hours of feeding (Loreto and Velikova, 2001). It also corroborates evidence that the pool of ABA inhibited by fosmidomycin is different than the larger pool deriving from xanthoxin. However, our experiments also demonstrate that fosmidomycin is not a selective inhibitor of isoprene biosynthesis as previously maintained (Zeidler et al., 1998), and indicate that leaf physiology may be strongly perturbed by fosmidomycin feeding, starting with changes in ABA concentration over short-term scales.
The labile pool of ABA directly linked to the MEP pathway seems to regulate stomatal opening in well-watered leaves, except in conditions where stomata are wide open and ABA concentration is very low (e.g. in P. fortunei and some P. australis leaves). However, as shown in Figure 5, isoprene emission and ABA concentration were found to increase simultaneously during the day, when stomatal closure induced a midday depression of photosynthesis (Raschke and Resemann, 1986). It is therefore suggested that the ABA pool generated by the MEP pathway is also involved in the regulation of stomatal closure under stress conditions. ABA inhibition of stomatal opening is a widely recognized ecological mechanism of defense against stress, especially drought (Wright and Hiron, 1969).
The other two environmental factors used to reduce stomatal conductance, darkening and elevated CO2, also caused a reduction in isoprene emission and leaf ABA concentration. The inhibition of isoprene following a light-to-dark transition is very rapid (Loreto and Sharkey, 1990), probably reflecting immediate inhibition of its photosynthetic intermediate in the dark. Interestingly, however, as also observed in the case of MEP inhibition by fosmidomycin, ABA concentration after darkening only declined to approximately 50% of the value measured in illuminated leaves. This again supports the hypothesis that only a part of foliar ABA is tightly related to the MEP pathway while the other is independent, at least over a short time. Dark-induced ABA reduction was slightly delayed with respect to isoprene inhibition. This may simply reflect the time needed to deplete the pool of ABA formed in the light. Stomata close in response to darkness but this occurs in patches and after several minutes (Cardon et al., 1994). We did not record the time course of stomatal closure in the light-to-dark transition, but conductances measured at the end of the experiment (after about 4 min of darkness) were 30% lower than those measured in the illuminated leaves at the beginning of the experiment. It is therefore suggested that the dark-induced stomatal closure is not caused by the accumulation of foliar ABA, although it may be possible that other sources of ABA that we have not examined in our experiment with cut leaves contribute to stomatal closure upon darkening in intact leaves.
Isoprene is often inhibited by exposure (Loreto and Sharkey, 1990) or growth (Scholefield et al., 2004) at elevated CO2, perhaps because of insufficient cytosolic phosphoenolpyruvate availability (Rosenstiel et al., 2003). We show that this elevated CO2 may also reduce ABA content in leaves. While the elevated CO2 experiment brings further evidence in support of the hypothesized tight relationship between the MEP pathway and ABA content, it also shows that as with darkened leaves, the MEP-dependent ABA pool is not involved in CO2-induced stomatal closure. Whether CO2 activates stomatal closure by a mechanism independent of ABA is still an unresolved issue (Vavasseur and Raghavendra, 2004), as evidence in favor and against this have been presented. Our experiments indicate that CO2 activates stomatal closure without a concurrent ABA increase.
In an additional experiment, it was also found that ABA concentration did not decrease and stomata did not open when the MEP pathway was inhibited by fosmidomycin in leaves exposed to elevated CO2. This result is interpreted as confirming that elevated CO2 may inhibit the formation of the MEP-dependent labile pool of ABA by a yet unknown mechanism.
In conclusion, these experiments indicate that the MEP pathway is involved in ABA biosynthesis. Changes in the activity of the MEP pathway may modulate a labile ABA pool and, consequently, stomatal conductance in leaves, although stomatal closure under darkness and elevated CO2 does not depend on leaf ABA content. For those taxa that have evolved the capability to emit isoprenoids, changes in isoprenoid emission can be used as a proxy of MEP pathway activity and changes in the ABA labile pool. For those plants with inherently high stomatal conductance (as observed in some cases with P. australis) or constitutively low ABA content (P. fortunei), this rapid modulation of leaf ABA does not appear to occur. A stimulation of isoprene emission has been observed after water stress occurrence (Sharkey and Loreto, 1993). This observation may imply increasing ABA biosynthesis, in turn regulating stomatal aperture to cope with frequent and often strong changes of water availability. We speculate that plants growing in periodically stressful environments may have evolved the possibility to form the labile pool of ABA observed in this work.
MATERIALS AND METHODS
Plant Material and Experiment Planning
Potted plants of Phragmites australis and Populus alba (isoprene-emitting species), Quercus ilex (monoterpene-emitting species), and Prunus persica and Pawlonia fortunei (species not emitting isoprenoids) were used. Plants were maintained under optimal water and nutrient conditions by regular fertilization and irrigation. P. australis plants were maintained with the pot immersed in water to recreate the living conditions of this aquatic plant species. Experiments were carried out on fully developed, healthy leaves. Experiments were replicated at least three times on different leaves of different plants. Statistical significance of differences between means were assessed by Tukey's test and differences significant at P < 0.01, 0.05, and 0.10 levels are represented by ***, **, and *, respectively. The time course of changes of the measured parameters in response to fosmidomycin and leaf darkening was followed in three leaves of different P. alba plants. P. alba large leaves allowed multiple sampling on the same leaf, minimizing leaf-to-leaf variability, especially when isoprene emission and ABA concentration changed rapidly, as in response to darkening. In the experiment with fosmidomycin, a part of the leaf was punched away before feeding the inhibitor and another part of the same leaf was sampled when the inhibitor suppressed isoprene emission to a steady-state minimum value. In the light-to-dark transition experiment, samples from the same leaf were punched away every 20 s after switching off the light to determine ABA content. The daily trend of the ABA concentration, isoprene emission, photosynthesis, and stomatal conductance was also measured on the same leaf of P. alba, punching away five different areas (away from edges and midrib) at 9:30, 10:30, and 11:30 am, and 1:00 and 5:00 pm. This experiment was replicated on four different leaves.
Gas-Exchange Measurements
Single leaves were cut from the plants and the petiole was recut again under water to avoid embolism. The petiole was placed in an Eppendorf vial filled with water, while a portion of the leaf lamina was enclosed in a gas-exchange cuvette to determine photosynthesis, stomatal conductance, and water transpiration. The gas-exchange system was described by Loreto and Velikova (2001) and allowed to control singularly the environmental parameters including temperature, light intensity, air humidity, and the concentration of CO2 in air. The leaf was exposed to a light intensity of 1,000 μmol photons m−2 s−1 at a leaf temperature of 30°C and under a relative humidity of 40%. The ambient CO2 concentration (380 ppm) in air was generally set, but, on a different experiment, CO2 was reduced (150 ppm) or increased (800 ppm) to artificially induce stomatal opening or closure, respectively. Exchange of water between the leaf and air was measured with an infrared gas analyzer (Li 6262, Licor) and transpiration and stomatal conductance were therefore calculated as in Loreto and Velikova (2001). Isoprene emission was detected by gas chromatography online with the gas-exchange system, as described in Loreto and Velikova (2001), using a Syntech Spectras BTX analyzer GC 855 (Syntech Spectras). In the experiment investigating the impact of a light-to-dark transition, fast detection of isoprene was achieved using a Proton Transfer Reaction Mass Spectrometer (PTR-MS, Ionicon) following the protoned mass (mass-to-charge ratio 69) of isoprene.
Fosmidomycin Feeding and Exogenous Isoprene Fumigation
When photosynthesis was steady for at least 30 min, fosmidomycin was fed to the leaf through the petiole. Fosmidomycin was dissolved in the water of the vial to get a 20 μm concentration. Fosmidomycin causes a quasicomplete and rapid inhibition of volatile isoprenoid emissions (Loreto and Velikova, 2001). We followed gas exchange of the fosmidomycin-fed leaves for 1 h after feeding.
In some leaves of P. australis the increment of transpiration after endogenous isoprene inhibition was again measured after reconstituting the internal pool with exogenous isoprene (3 ppm of gaseous isoprene in the air flowing in the chamber, see Loreto et al., 2001) for 1 h.
ABA Measurements
Leaf discs (2 cm2) were cut from the same leaves exposed to gas exchange, immediately frozen in liquid nitrogen, and homogenized in a precooled mortar with pestle. Twenty milligrams of leaf tissue (free of midrib) were extracted overnight in 1.5 mL distilled water in dark, at 4°C on a shaker. The extracts were centrifuged at 10,000g for 25 min, and the ABA content of the supernatants was quantified in an ELISA using the Phytodetek-ABA kit (AGDIA) according to the indications of the manufacturer. The monoclonal antibody raised against ABA (ABA-15-I-C-5) was previously shown to have high specificity for 2-cis-(S)-ABA and cross-reactivity of less than 1 or 0 against 12 different structurally ABA-related compounds (Weiler, 1982).
Acknowledgments
The authors would like to thank Dr. Violeta Velikova and Prof. Ulo Niinemets for discussions about data interpretation, Prof. Gabriel Cornic for critical reading of the manuscript, and Prof. Manuel Lerdau for discussions and help with the revision of the manuscript.
This work was supported by the European Commission Marie Curie project “Ecological and physiological functions of biogenic isoprenoids and their impact on the environment” (ISONET, MRTN-CT–2003–504720) and by the European Science Foundation scientific programme Volatile Organic Compounds in the Biosphere-Atmosphere System.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Francesco Loreto (francesco.loreto@ibaf.cnr.it).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.083063.
References
- Cardon ZG, Mott KA, Berry JA (1994) Dynamics of patchy stomatal movements and their contribution to steady-state and oscillating stomatal conductance calculated using gas-exchange techniques. Plant Cell Environ 17: 995–1007 [Google Scholar]
- Cornish K, Zeevaart JAD (1985) Abscisic acid accumulation by roots of Xanthium strumarium L. and Lycopersicon esculentum Mill. in relation to water stress. Plant Physiol 79: 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche CD, Sharkey TD (1993) Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ 16: 587–591 [Google Scholar]
- Demmig-Adams B, Adams WW III (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1: 21–26 [Google Scholar]
- Hirai N, Yoshida R, Todoroki Y, Ohigashi H (2000) Biosynthesis of abscisic acid by the non-mevalonate pathway in plants, and by the mevalonate pathway in fungi. Biosci Biotechnol Biochem 64: 1448–1458 [DOI] [PubMed] [Google Scholar]
- Li Y, Walton DC (1987) Xanthophylls and abscisic acid biosynthesis in water-stressed bean leaves. Plant Physiol 85: 910–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Schwendler J, Disch A, Rohmer M (1997) Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett 400: 271–274 [DOI] [PubMed] [Google Scholar]
- Loreto F, Ferranti F, Mannozzi M, Maris C, Nascetti P, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in plants. Plant Physiol 126: 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Pinelli P, Manes F, Kollist H (2004) Impact of ozone on monoterpene emissions and evidences for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex (L.) leaves. Tree Physiol 24: 361–367 [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD (1990) A gas exchange study of photosynthesis and isoprene emission in red oak (Quercus rubra L.). Planta 182: 523–531 [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127: 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Milborrow BV (2001) The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. J Exp Bot 52: 1145–1164 [PubMed] [Google Scholar]
- Mittelheuser CJ, van Steveninck RFM (1969) Stomatal closure and inhibition of transpiration induced by (RS)-abscisic acid. Nature 221: 281–282 [Google Scholar]
- Raschke K, Resemann A (1986) The midday depression of CO2 assimilation in leaves of Arbutus unedo L.: diurnal changes in photosynthetic capacity related to changes in temperature and humidity. Planta 168: 546–558 [DOI] [PubMed] [Google Scholar]
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK (2003) Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421: 256–259 [DOI] [PubMed] [Google Scholar]
- Scholefield PA, Doick KJ, Herbert B, Hewitt CN, SchnitzlerJ-P, Pinelli P, Loreto F (2004) Impact of rising CO2 on emissions of volatile organic compounds: isoprene emission from Phragmites australis growing at elevated CO2 on a natural carbon dioxide spring. Plant Cell Environ 27: 393–401 [Google Scholar]
- Schwartz SH, Qin X, Zeevaart JAD (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131: 1591–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F (1993) Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 95: 328–333 [DOI] [PubMed] [Google Scholar]
- Tardieu F, Zhang J, Katerji N, Bethenod O, Palmer S, Davies WJ (1992) Xylem ABA controls stomatal conductance of field-grown maize subjected to soil compaction or soil drying. Plant Cell Environ 15: 187–193 [Google Scholar]
- Taylor HF, Smith TA (1967) Production of plant growth inhibitors from xanthophylls: a possible source of dormin. Nature 215: 1513–1514 [DOI] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS (2004) Guard cell metabolism and CO2 sensing. New Phytol 165: 665–682 [DOI] [PubMed] [Google Scholar]
- Weiler EW (1982) An enzyme-immunoassay for cis-(+)-abscisic acid. Physiol Plant 54: 510–514 [Google Scholar]
- Wright STC, Hiron RWP (1969) (+)-Abscisic acid, the growth inhibitor induced in detached wheat leaves by a period of wilting. Nature 224: 719–720 [Google Scholar]
- Zeevaart JAD, Heath TG, Gage DA (1989) Evidence for a universal pathway of abscisic acid biosynthesis in higher plants from 18O incorporation patterns. Plant Physiol 91: 1594–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler J, Schwender J, Mueller C, Wiesner J, Weidemeyer C, Back E, Jomaa H, Lichtenthaler HK (1998) Inhibition of the nonmevalonate 1-deoxy-D-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z Naturforsch 53c: 980–986 [Google Scholar]
- Zhang J, Davies WI (1990) Does ABA in the xylem control the rate of leaf growth in soil-dried maize and sunflower plants? J Exp Bot 41: 1125–1132 [Google Scholar]