Abstract
A functional abscisic acid (ABA)-induced protein phosphatase type 2C (PP2C) was previously isolated from beech (Fagus sylvatica) seeds (FsPP2C2). Because transgenic work is not possible in beech, in this study we overexpressed this gene in Arabidopsis (Arabidopsis thaliana) to provide genetic evidence on FsPP2C2 function in seed dormancy and other plant responses. In contrast with other PP2Cs described so far, constitutive expression of FsPP2C2 in Arabidopsis, under the cauliflower mosaic virus 35S promoter, produced enhanced sensitivity to ABA and abiotic stress in seeds and vegetative tissues, dwarf phenotype, and delayed flowering, and all these effects were reversed by gibberellic acid application. The levels of active gibberellins (GAs) were reduced in 35S:FsPP2C2 plants, although transcript levels of AtGA20ox1 and AtGA3ox1 increased, probably as a result of negative feedback regulation, whereas the expression of GASA1 was induced by GAs. Additionally, FsPP2C2-overexpressing plants showed a strong induction of the Responsive to ABA 18 (RAB18) gene. Interestingly, FsPP2C2 contains two nuclear targeting sequences, and transient expression assays revealed that ABA directed this protein to the nucleus. Whereas other plant PP2Cs have been shown to act as negative regulators, our results support the hypothesis that FsPP2C2 is a positive regulator of ABA. Moreover, our results indicate the existence of potential cross-talk between ABA signaling and GA biosynthesis.
Abscisic acid (ABA) and GAs regulate several aspects of plant growth showing antagonistic effects in different developmental processes, including seed dormancy or germination events (for review, see Kermode, 2005; Kucera et al., 2005).
Several components implicated in ABA signaling, mainly those regulating seed dormancy, have been identified in ABA-response mutants by isolating the affected genes and their corresponding proteins involving a complex network of positive and negative regulators, including kinases, phosphatases, and transcriptional regulators (for review, see Finkelstein et al., 2002; Abe et al., 2003). Protein phosphorylation/dephosphorylation events have been investigated in detail in ABA-mediated processes involving several well-known protein kinases and phosphatases (Leung and Giraudat, 1998; Finkelstein et al., 2002), such as PKABA1, an ABA-induced protein kinase that suppresses GA-inducible gene expression in barley (Hordeum vulgare) aleurone layers (Gómez-Cadenas et al., 1999, 2001); the guard cell-specific protein kinase AAPK, necessary for stomatal closure mediated by ABA (Li et al., 2000); and the protein kinase OST-1, an essential component mediating stomatal regulation in response to drought (Mustilli et al., 2002). Additionally, four Arabidopsis (Arabidopsis thaliana) Ser-Thr protein phosphatase 2Cs (PP2Cs; ABI1, ABI2, AtPP2CA, and HAB1) and one PP2C from beech (Fagus sylvatica; FsPP2C1) have been described as negative regulators of the ABA signal transduction cascade (Gosti et al., 1999; Merlot et al., 2001; Tahtiharju and Palva, 2001; González-García et al., 2003; Sáez et al., 2004; Kuhn et al., 2006; Yoshida et al., 2006).
In addition, analysis of mutants with reduced production of GAs has demonstrated the role of these hormones in several aspects of plant development, including stem elongation, fruit set, flower induction, or seed germination (for review, see Lange, 1998). For instance, Arabidopsis mutants blocked in GA biosynthesis fail to complete germination, this effect being reverted by exogenous addition of GAs (Koornneef and van der Veen, 1980). Three different phases can be considered within the biosynthetic pathway of GAs (Olszewski et al., 2002). The first one is the formation of ent-kaurene, involving geranylgeranyl diphosphate cyclation via copalyl diphosphate as an intermediate. Ent-kaurene is then converted to GA12 and GA53 (a C-13 hydroxylated GA) by oxidative reactions catalyzed by P450-dependent monooxygenases. Finally, during the last phase, GA 20-oxidases and GA 3-oxidases catalyze the last steps of the GA biosynthesis pathway. These are key enzymes controlling active GA biosynthesis from GA12 and GA53 (Xu et al., 1995; Williams et al., 1998) that regulate many developmental processes, such as promotion of seed germination. Thus, it has been shown that GAs and the expression of a GA 20-oxidase (FsGA20ox1) play an important role in breaking the dormancy of beech seeds (Calvo et al., 2004). Also, Pérez-Flores et al. (2003) have indicated that the level of seed dormancy presented by different lines of sorghum (Sorghum bicolor) correlates with the level of expression of two different GA 20-oxidases. Additionally, it has been shown that GA 3-oxidase genes also play an important role in the stimulation of seed germination in Arabidopsis (Yamauchi et al., 2004).
In a previous work, we reported the cloning of FsPP2C2, encoding a functional PP2C from beechnut, whose expression was induced by ABA in seeds and other tissues (Lorenzo et al., 2002). The alignment of the catalytic cores of several PP2Cs showed that this PP2C is included in cluster 3, where PP2Cs have not been studied in detail (Sáez et al., 2004). The common characteristic of all the phosphatases included in this cluster is the presence of two nuclear targeting sequences next to the C-terminal region of the gene, although there is no experimental evidence of its location or function. In this work, because there are no tools available to transform beech, we took advantage of the constitutive expression of FsPP2C2 in Arabidopsis to study the function of this protein.
We report here that Arabidopsis plants overexpressing FsPP2C2 showed enhanced sensitivity to ABA and abiotic stress and a deeper degree of seed dormancy compared to wild-type seeds and seedlings. Additionally, transgenic plants showed a dwarf phenotype, dark-green leaves, and late flowering, these effects being reversed by GA3. We have also confirmed the nuclear localization of this protein phosphatase and found that ABA is the signal that directs FsPP2C2 to the nucleus. The transgenic lines contain reduced levels of GAs associated with altered expression of genes involved in the late steps of GA biosynthesis (GA20ox and GA3ox). Taken together, these results are consistent with the role of FsPP2C2 as a positive regulator of ABA signaling by inhibiting GA biosynthesis.
RESULTS
Generation and Characterization of Transgenic Arabidopsis Plants Overexpressing FsPP2C2
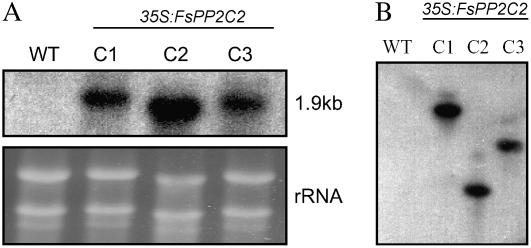
To investigate the role of FsPP2C2 in seed dormancy and other ABA responses, we used an overexpression approach in Arabidopsis. Transgenic plants were selected in the presence of kanamycin. Seventeen of them (26%) showed 3:1 segregation for kanamycin resistance in the T2 generation, possibly indicating a single insertion of the full-length 35S:FsPP2C2 transgene, and, finally, three independent T3 homozygous lines (from 65 independent lines; C1, C2, and C3) showing high levels of expression of the transgene were selected (Fig. 1A). Southern-blot analysis of these homozygous lines confirmed the presence of a single insertion of the 35S:FsPP2C2 transgene (Fig. 1B).
Figure 1.
Molecular analysis of 35S:FsPP2C2 transgenic lines. A, RNA-blot analysis of transgenic lines overexpressing FsPP2C2. Total RNA (10 μg/line) from wild-type plants and C1 to C3 transgenic plants was isolated and hybridized with a specific FsPP2C2 probe. Bottom, Ethidium bromide-stained gel showing rRNAs. B, Southern-blot analysis of wild-type (WT; Col-0 background) and transgenic lines (C1–C3) overexpressing FsPP2C2. Genomic DNA was digested with HindIII, blotted onto a nylon membrane, and hybridized with a FsPP2C2-specific probe.
Constitutive Expression of FsPP2C2 in Arabidopsis Increases Seed Dormancy and Confers Hypersensitivity to ABA and Osmotic Stress
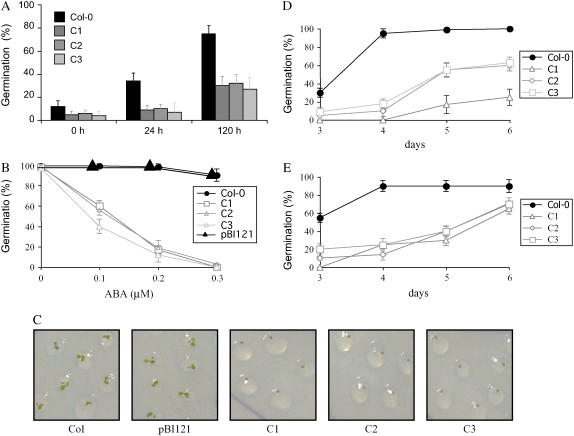
Wild-type and FsPP2C2 transgenic plants were plated on Murashige and Skoog (MS) media with ABA, NaCl, or mannitol included in the media to ascertain whether FsPP2C2 overexpression affected seed germination in response to ABA or osmotic stress treatment. To determine the degree of dormancy, we compared germination percentages at 5 d after seeding of 35S:FsPP2C2 and wild-type seeds collected at the same time after different cold treatment periods (0, 24, and 120 h). The three FsPP2C2 transgenic lines displayed a greater degree of dormancy compared with wild type after the three kinds of cold treatments assayed (Fig. 2A). In the presence of 0.1, 0.2, or 0.3 μm ABA, germination of FsPP2C2-overexpressing seeds was delayed (Fig. 2, B and C), whereas nearly all wild-type seeds completed germination after 8 d (even with 0.3 μm ABA). In contrast, only about 60% and 20% of FsPP2C2 transgenic seeds completed germination in the presence of 0.1 and 0.2 μm ABA, respectively, and no radicle protrusion was scored in 0.3 μm ABA. On the other hand, no differences in germination percentages were observed between wild-type and transgenic seeds in the absence of ABA after 8 d of treatment, although wild-type seeds completed germination to 100% at day 6 (data not shown). This delayed completion of germination in FsPP2C2 transgenic seeds, consistent with the deeper degree of dormancy of these seeds, may be due to increased sensitivity to endogenous ABA. Thus, all these results indicate that overexpression of FsPP2C2 in Arabidopsis confers ABA hypersensitivity.
Figure 2.
Effect of FsPP2C overexpression on dormancy and germination in Arabidopsis. A, Dormancy assay of FsPP2C2-overexpressing seeds. Germination percentage was determined 5 d after stratification at 4°C for 0, 24, and 120 h. B, Percentage of seeds that germinated and developed green cotyledons after 8 d following stratification at 4°C for 120 h, in 0.1, 0.2, and 0.3 μm ABA. C, Image showing differences in germination of FsPP2C2-overexpressing, wild-type seeds and seeds transformed with the empty vector pBI121 after 5 d on 0.1 μm ABA. D, Percentage of seeds that germinated and developed green cotyledons on 50 mm NaCl. E, Percentage of seeds that germinated and developed green cotyledons in 100 mm mannitol. C1, C2, and C3, Transgenic 35:FsPP2C2 lines; pBI121, transgenic line with the empty vector pBI121.
It seems unlikely that these phenotypes were due to mutations caused by random insertion of the transgene because many independent transformants were isolated and around 80% of the transgenic lines exhibited the phenotype. In addition, sensitivity to ABA in the pBI121 empty-vector control line did not differ significantly from those in untransformed wild-type plants, as shown in Figure 2, B and C, indicating that the phenotypes under study are due to the transgene and not to some peripheral endogenous gene altered during transformation.
Germination of FsPP2C2 transgenic seeds was also significantly more sensitive than the wild type to low concentrations of NaCl (50 mm; Fig. 2D) and mannitol (100 mm; Fig. 2E). Because it is known that osmotic shock blocks the completion of germination through ABA action (Leung and Giraudat, 1998), these results are consistent with the ABA-hypersensitive inhibition of germination described above.
Constitutive Expression of FsPP2C2 in Arabidopsis Confers Various Phenotypes in Vegetative Tissues
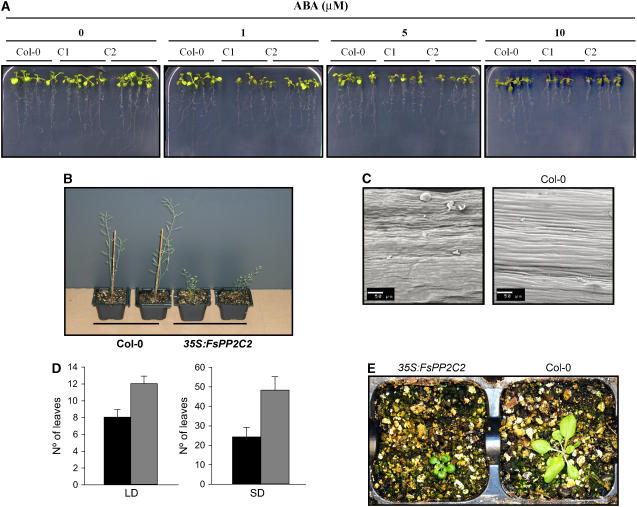
ABA sensitivity in vegetative tissues was also analyzed to determine whether FsPP2C2 overexpression affected other plant phenotypes. Thus, comparable wild-type and FsPP2C2 transgenic seedlings grown in vertical agar plates lacking ABA were transferred to vertical agar plates supplemented with this hormone to test the effect of the FsPP2C2 transgene on root sensitivity to ABA. As seen in Figure 3A, root elongation was significantly more sensitive to ABA in transgenic plants compared with the wild type.
Figure 3.
A, Root growth assay to determine ABA sensitivity. The image was taken 7 d after transfer of 5- to 7-d-old seedlings from MS medium to plates containing different concentrations of ABA (0, 1, 5, and 10 μm). B, Adult wild-type (left) and FsPP2C2 transgenic plants (right). C, Scanning electron microscopic observation of the epidermal cells of the stems. D, Number of rosette leaves of Col and FsPP2C2-overexpressing lines at the flowering stage both in LD and SD. E, Image showing the size and greening of 35S:FsPP2C2 and wild-type seedlings.
Additionally, FsPP2C2 transgenic plants showed a dwarf phenotype. Adult FsPP2C2-overexpressing plants were approximately one-third as high as the wild type (Fig. 3B), and this dwarf phenotype was due to smaller length of cells of the stem, as observed by electron microscopy (Fig. 3C). These transgenic plants also exhibited a late-flowering phenotype under both long-day (LD) and short-day (SD) conditions (Fig. 3D), smaller, dark-green leaves (see Fig. 3E for a representative C1 transgenic line; a similar phenotype was also observed for the other 35S:FsPP2C2 lines), and a different distribution of trichomes (delayed formation of abaxial trichomes and abnormal distribution of adaxial trichomes, just on the edge of the leaf; data not shown). All these features are characteristic of GA-deficient and GA-insensitive mutants (Koornneef and van der Veen, 1980; Chien and Sussex, 1996; Magome et al., 2004).
Effect of Plant Hormones on 35S:FsPP2C2 Plants
In addition to ABA, the sensitivity of FsPP2C2 transgenic lines to brassinosteroid (BR), ethylene (using the ethylene precursor 1-aminocyclopropane-1-carboxylic acid [ACC]), and GA3 on germination and stem elongation was also investigated.
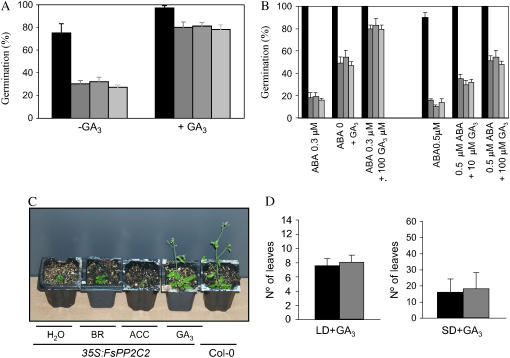
No differences between transgenic and wild-type plants were observed in response to BR and ACC treatments (data not shown). However, GA3 was able to counteract both seed dormancy (Fig. 4A) and seed hypersensitivity to ABA (Fig. 4B) of transgenic lines after 5 or 10 d of treatment, respectively. Furthermore, GA3 was also able to rescue the short stature of these transgenic plants (Fig. 4C) and the late-flowering phenotype (compare Figs. 4D and 3D). This effect of GA in reversing the phenotypes of 35S:FsPP2C2 suggests that overexpression of FsPP2C2 in Arabidopsis plants might affect GA biosynthesis in transgenic lines.
Figure 4.
Responses of FsPP2C2 transgenic plants to plant hormones. A, Germination percentage of moist, chilled (120 h at 4°C) seeds of wild-type and C1 to C3 transgenic lines 5 d following transfer to 25°C without or with 10 μm GA3. B, Reversion of the inhibitory effect of ABA on germination 10 d after addition of 10 or 100 μm GA3. C, Image showing the stature of wild-type plants and FsPP2C transgenic plants after the addition of BR (100 μm), ACC (100 μm), and GA3 (10 μm). D, Reversion by GA3 (100 μm) of late-flowering phenotype in LD and SD.
Cellular Expression Pattern of FsPP2C2:Green Fluorescent Protein
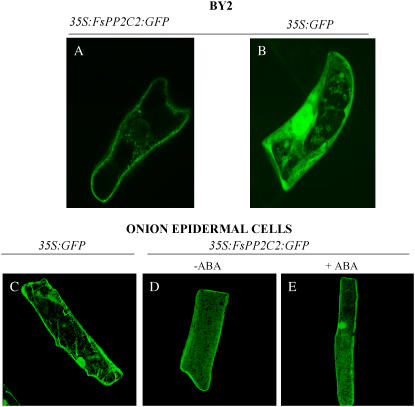
FsPP2C2, as all the members of cluster 3 of PP2Cs (Sáez et al., 2004), has two nuclear targeting sequences next to the C-terminal region of the gene product, an unusual feature among PP2Cs (Lorenzo et al., 2002). However, no experimental evidence on the subcellular localization of this group of PP2Cs has been described. Thus, to verify the possible nuclear localization of this phosphatase, the FsPP2C2 coding region was fused to the C- or N-terminal end of the green fluorescent protein (GFP) under the control of the cauliflower mosaic virus (CaMV) 35S promoter and transient expression assays were developed in BY2 tobacco (Nicotiana tabacum) cells and in onion (Allium cepa) epidermal cells. The results obtained with the two kinds of constructs were similar, so only pictures corresponding to the construct of the coding region of FsPP2C2 fused to the N-terminal end of the GFP are presented (Fig. 5). The results show that, in spite of the two nuclear targeting sequences present in FsPP2C2, this protein did not migrate to the nucleus in BY2 tobacco (Fig. 5A). This was confirmed in onion epidermal cells transformed with FsPP2C2:GFP, where, as in the case of tobacco cells, cytoplasmic distribution of the protein was observed in more than 90% of untreated cells (Fig. 5D). However, ABA application induced migration of FsPP2C2 to the nucleus in more than 85% of ABA-treated onion cells (Fig. 5E). On the other hand, when cells were treated with GA3, no nuclear localization was observed in any of the cells (data not shown). This indicates the involvement of FsPP2C2 in ABA signaling because of the absence or presence of this hormone is a signal that modulates the localization of this protein in the nucleus.
Figure 5.
Expression pattern of FsPP2C2 fused to the N-terminal end of GFP in BY2 tobacco cells (A); ABA-untreated onion epidermal cells (D); ABA-treated onion epidermal cells (E); and 35S:GFP as controls (B and C).
Quantification of GAs
Because many of the effects observed in 35S:FsPP2C2 plants were reversed by GAs, we quantified the levels of endogenous GAs in 4-week-old transgenic and wild-type plants to determine whether GA metabolism was altered in FsPP2C2-overexpressing lines. As shown in Table I, the levels of the intermediate GA19 and the end products GA20 (in the early C-13 hydroxylation pathway) and GA9 (in the non-C-13 hydroxylation pathway) of GA 20-oxidase activity were not significantly altered in our transgenic lines with respect to wild-type plants. However, the levels of GA4 (the main active GA in Arabidopsis) and of GA34 (the inactive metabolite of GA4) were reduced to about 60% in the transgenic lines. Interestingly, the levels of GA1 and GA8 (the active GA and its inactive metabolite in the early C-13 hydroxylation pathway) were also reduced in the transgenic lines, as well as GA29 (the inactive metabolite of GA20). These results show that the decrease of bioactive GAs in FsPP2C2 transgenic plants was probably due to lower GA 3-oxidase activity.
Table I.
GA amount (ng g−1 fresh weight) in Arabidopsis plants from wild-type and FsPP2C2 transgenic lines (C1 and C2)
Levels of active GAs (GA1 and GA4) are in bold. Values are means of three biological replicates ±se, except in the case of GA8 (only two replicates).
| GA19 | GA20 | GA29 | GA1 | GA8 | GA9 | GA4 | GA34 | |
|---|---|---|---|---|---|---|---|---|
| Wild type | 0.47 ± 0.13 | 0.23 ± 0.03 | 0.20 ± 0.07 | 1.06 ± 0.26 | 1.00 | 0.22 ± 0.07 | 1.25 ± 0.18 | 0.68 ± 0.14 |
| C1 | 0.51 ± 0.11 | 0.17 ± 0.03 | 0.09 ± 0.07 | 0.83 ± 0.04 | 0.08 | 0.29 ± 0.10 | 0.71 ± 0.10 | 0.43 ± 0.10 |
| C2 | 0.40 ± 0.12 | 0.13 ± 0.02 | 0.04 ± 0.02 | 0.45 ± 0.14 | 0.10 | 0.15 ± 0.07 | 0.73 ± 0.13 | 0.42 ± 0.06 |
Expression of GA20ox, GA3ox, and a GASA1 Gene
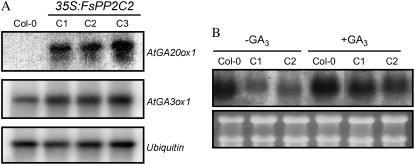
Expression of two genes involved in the last steps of GA biosynthesis, GA5 (AtGA20ox1, encoding a GA 20-oxidase; Xu et al., 1995) and GA4 (AtGA3ox1, encoding a GA 3-oxidase; Williams et al., 1998), key enzymes in controlling active GA biosynthesis, were also analyzed. Transcripts of AtGA20ox1 were not detected in the wild type but were very abundant in transgenic seedlings (Fig. 6A). Slightly higher levels of AtGA3ox1 transcripts were also observed in transgenic seedlings, as compared with the wild type (Fig. 6A). Expression of many GA20ox and GA3ox genes in different species is subjected to negative feedback regulation (for review, see Olszewski et al., 2002). Thus, the increase of AtGA20ox1 and AtGA3ox1 transcripts found in our transgenic lines is probably a consequence of the reduction in the level of active GAs described before. To check whether the GA signal transduction pathway was affected in FsPP2C2-overexpressing lines, we also analyzed the expression of GASA1, a GA-induced gene (Herzog et al., 1995), in both GA-treated and untreated transgenic and wild-type plants. As seen in Figure 6B, expression of this gene is up-regulated in our transgenic lines after GA treatment, although such up-regulation was not so clear in wild-type plants, as previously described (Herzog et al., 1995; Aubert et al., 1998). This result indicates that the mechanism of GA action is not affected in transgenic lines overexpressing FsPP2C2.
Figure 6.
A, Expression of AtGA20ox1 (GA5) and AtGA3ox1 (GA4) genes. B, GASA1 gene expression in FsPP2C2-overexpressing plants (C1 and C2) compared to wild type. mRNA levels of the indicated genes were determined by semiquantitative RT-PCR (A) or northern-blot analysis (B) using total RNAs (10 μg/line) isolated from 7-d-old seedlings. Bottom, Ubiquitin (A) and ethidium bromide-stained gel showing rRNAs (B) as controls.
Expression of an ABA-Responsive Gene
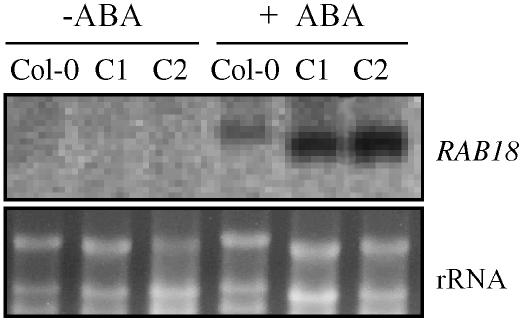
We compared the expression of the RAB18 gene, a known ABA-responsive gene (Lang and Palva, 1992) in 35S:FsPP2C2 plants, with that of Columbia (Col-0) to examine whether the enhanced ABA sensitivity in transgenic plants was accompanied by altered expression of ABA-responsive genes. The hyperinduction of this ABA-responsive gene further confirms that the PP2C from beech is involved in ABA signal transduction. RAB18 transcripts were not detected in wild-type and untreated transgenic lines (Fig. 7, lanes 1–3), but were induced by ABA treatment, much more so in transgenic that in wild-type lines (Fig. 7, lanes 4–6).
Figure 7.
Expression of the RAB18 ABA-regulated gene in FsPP2C2-overexpressing plants (C1 and C2) compared to wild type. mRNA levels of the indicated genes were determined by northern-blot analysis using total RNAs (10 μg/line) isolated from mock-treated (−) or ABA-treated (+50 μm ABA for 24 h) plants. Bottom, Ethidium bromide-stained gel showing rRNAs.
DISCUSSION
Only six PP2Cs are found in the yeast (Saccharomyces cerevisiae) genome (Stark, 1996) and no more than 15 in the human genome (Cheng et al., 2000). In contrast, the high number of PP2C genes (at least 69) present in the Arabidopsis genome denotes the importance of the PP2C class of Ser-Thr phosphatases in plants (Kerk et al., 2002) and suggests that plant PP2Cs have a narrower substrate specificity than other organisms (Merlot et al., 2001).
One plant PP2C family (cluster 5; Sáez et al., 2004) has been identified as constituted by components of ABA signaling. For instance, ABI1, ABI2, AtPP2CA, HAB1, and FsPP2C1 act as negative regulators of the ABA signal transduction cascade (Gosti et al., 1999; Merlot et al., 2001; Tahtiharju and Palva, 2001; González-García et al., 2003; Sáez et al., 2004; Kuhn et al., 2006; Yoshida et al., 2006). Other members of PP2C, such as MP2C (cluster 6; Sáez et al., 2004) from ice plant (Mesembryanthemum crystallinum), act as negative regulators of the mitogen-activated protein kinase pathway activated by salt stress or wounding (Meskiene et al., 1998, 2003). Interestingly, all these PP2Cs act as negative regulators in different signal transduction cascades. In fact, except for the recent controversy on the role of ABI1 from the work of Wu et al. (2003), all the PP2Cs described and characterized so far would act as negative regulators in different signal transduction cascades.
We have previously isolated FsPP2C2, a PP2C from beech that is up-regulated by ABA in seeds and vegetative tissues (Lorenzo et al., 2002), making it a reasonable candidate as a regulator of ABA signaling. However, genetic evidence of the role of this phosphatase was lacking. Studies of orthologous genes and functional tests in heterologous systems have shown that the ABA signal transduction cascade is essentially conserved among evolutionarily distant plant species (for review, see Finkelstein et al., 2002). Therefore, in this study, we used an overexpression approach in Arabidopsis to investigate FsPP2C2 function. It was expected that if FsPP2C2 is a negative regulator of ABA responses, as is another PP2C described in beech (FsPP2C1; Lorenzo et al., 2001; González-García et al., 2003), the overexpression of FsPP2C2 in Arabidopsis would lead to ABA insensitivity in seeds and, consequently, to reduced dormancy. Surprisingly, 35S:FsPP2C2 plants exhibited an enhanced response to ABA and osmotic stress (Figs. 2 and 3) together with other phenotypes, such as dwarfism, late flowering, smaller and dark-green leaves (Fig. 3), and abnormal trichome distribution (data not shown), all these typical characteristics of GA-deficient and/or insensitive mutants (Koornneef and van der Veen, 1980; Magome et al., 2004). Furthermore, these phenotypes, including enhanced response to ABA, were reversed by GA3 application (Fig. 4).
In addition to the genes ABI1, ABI2, PP2CA, HAB1, and FsPP2C1 (Gosti et al., 1999; Merlot et al., 2001; Tahtiharju and Palva, 2001; González-García et al., 2003; Sáez et al., 2004; Kuhn et al., 2006; Yoshida et al., 2006) encoding PP2C, other genes have been identified as negative regulators of the ABA-signaling pathway, such as the farnesyl transferase β-subunit (ERA1; Cutler et al., 1996), the mRNA cap-binding protein (ABH1; Hugouvieux et al., 2001), the homeodomain protein 6 (ATHB6; Himmelbach et al., 2002), and the calcium sensor CBL-9 (Pandey et al., 2004). Moreover, ABI3, ABI4, and ABI5 encode different types of transcription factors (Giraudat et al., 1992; Finkelstein et al., 1998; Finkelstein and Lynch, 2000) known to act as positive regulators of ABA-mediated regulation of seed development, germination, and early seedling growth (Finkelstein et al., 2002; Arroyo et al., 2003). However, FsPP2C2 is described as a positive regulator of the ABA signal transduction pathway in seeds and other vegetative tissues. Evidence of the role of this PP2C on ABA action was obtained from the observation that plants overexpressing FsPP2C2 had hypersensitivity to ABA and osmotic stress and a deeper degree of dormancy compared with wild-type plants (Figs. 2 and 3). Expression of the ABA-responsive gene RAB18 was also increased in 35S:FsPP2C2 plants (Fig. 7). Moreover, the fact that FsPP2C2 is localized in the nucleus only in the presence of ABA (Fig. 5) strongly supports its involvement in ABA signaling.
Evidence of the mode of action of FsPP2C2 in ABA responses was initially obtained from the observation that GA3 was able to rescue the poor germination, dwarfism, and delay in flowering of transgenic plants overexpressing FsPP2C2 (Fig. 4). Because GA action did not seem to be affected in these plants (Fig. 6B), this indicated that FsPP2C2 might exert its action by modulating GA metabolism. Quantification of endogenous GAs showed that the levels of active GAs (GA4 and GA1) were significantly reduced in transgenic lines, whereas the contents of their immediate metabolic precursors (GA9 and GA20, respectively) were not affected (Table I). The low reduction of GA4 content (about 50%) in transgenic lines displaying phenotype agrees with the low increase of active GA4 (2- to 3-fold) in transgenic Arabidopsis overexpressing GA20ox. This is in contrast with the 10-fold increases in applied GA3 needed to get partial reversal of germination phenotypes in the presence of ABA (Fig. 4B). However, this is similar to what happens in pea (Pisum sativum) ovary, where small differences in active GA content (within 1 order of magnitude) have very significant physiological effects, whereas higher doses of GA1 (orders of magnitude) have to be applied exogenously to produce a similar effect probably due to transport and/or metabolism problems (Rodrigo et al., 1997).
Our results suggest that overexpression of FsPP2C2 decreased GA 3-oxidase activity. The lower content of inactive metabolites of GA4 and GA1 (GA34 and GA8, respectively) also supports this conclusion, while showing that the decrease of active GA content was not the result of higher inactivating metabolism. Interestingly, transcript levels of AtGA3ox1 (gene GA4) were not reduced, but rather increased in transgenic lines (Fig. 6A). An even more dramatic effect was observed on AtGA20ox1 (gene GA5) transcript levels (Fig. 6A). It is known that expression of many GA20ox and GA3ox genes is controlled by active GAs through negative feedback regulation (Olszewski et al., 2002). Therefore, the increase of GA5 and GA4 transcripts is probably a result of this kind of regulation and we can postulate that the regulation of GA 3-oxidase activity by FsPP2C2 is probably carried out at the posttranslational level.
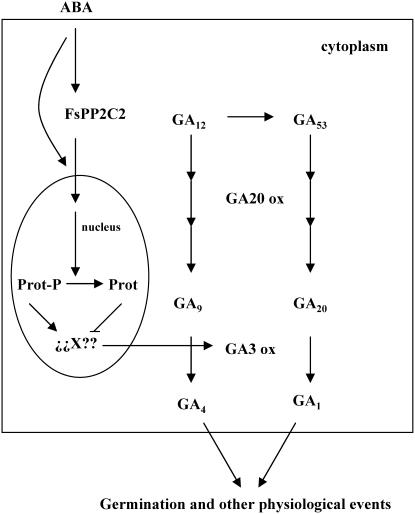
In conclusion, the results presented in this work are consistent with the role of the ABA-induced FsPP2C2 as a positive regulator of ABA signaling through a reduction of GA biosynthesis, probably affecting GA 3-oxidase activity (Fig. 8). In this proposed model, we hypothesize that a phosphorylated protein in the nucleus may activate an unknown element responsible for the activation of GA 3-oxidase, but ABA action through FsPP2C2 would dephosphorylate this protein, leading to inactivation of this key enzyme in GA biosynthesis. Thus, a potential interaction between ABA action and GA biosynthesis is described, showing another junction in the complex mechanism of hormone signaling. The data presents new insights into future research about the antagonistic effect of ABA and GAs in plant development.
Figure 8.
Proposed model for the role of FsPP2C2 in ABA signaling through a reduction of GA biosynthesis.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana) plants, ecotype Col-0, were used in this research. They were normally grown in a growth chamber with 40% humidity, at 22°C, under SD (8 h light/16 h dark) or LD (16 h light/8 h dark; light intensity of 80–100 μE m−2 s−1) in pots containing a 1:3 vermiculite:soil mixture, as described previously (González-García et al., 2003). For in vitro culture, seeds were surface sterilized in 70% (v/v) ethanol solution containing 1% (v/v) Triton X-100 and washed four times in sterile distilled water. Stratification of seeds was conducted during 3 d at 4°C, unless otherwise indicated. Afterward, seeds were sowed on MS plates (Murashige and Skoog, 1962) containing solid medium composed of MS basal salts and 1% (w/v) Suc, pH 5.7, solidified with 1% (w/v) agar. Plates were sealed and incubated in a controlled-environment growth chamber.
Vector Construction and Plant Transformation
To produce transgenic plants, the coding region of the FsPP2C2 cDNA was cloned into the pBIN121 vector, which contains the modified CaMV 35S promoter. 5′-GGATCCATGTTTTCGGA-3′ (sense) and 5′- GAGCTCTTAGGTGCTGCCAAC-3′ (antisense), containing the BamHI and SacI cloning sites, were used as primers to amplify FsPP2C2 cDNA and subcloned in the corresponding sites of the pBIN121 vector. The pBIN121-FsPP2C2 construct, after confirmation of the nucleotide sequence, was introduced into Agrobacterium tumefaciens C58C1 (pGV2260; Deblaere et al., 1985) by heat shock. Arabidopsis plants (Col-0 ecotype) were transformed by the floral-dip method (Clough and Bent, 1998) and transgenic seedlings were selected on kanamycin medium (50 μg mL−1). T2 plants that produced 100% kanamycin-resistant plants in the T3 generation were considered homozygous for the selection marker and used for further studies. Nontransformed plants and transgenic plants transformed with the empty vector pBI121 were used as controls.
From 65 kanamycin-resistant independent lines rescued, around 80% showed an ABA-hypersensitive phenotype in seed germination compared with controls that did not display any phenotypes related to ABA responses.
Germination Assays
Seeds were plated on solid medium composed of MS basal salts, 1% (w/v) Suc, and different concentrations of ABA (Sigma-Aldrich; 0.1, 0.2, 0.3 μm) to determine ABA sensitivity. For the dormancy assay, seed lots to be compared were harvested on the same day from individual plants grown in identical conditions and were stratified during 0, 1, and 5 d at 4°C.
To determine sensitivity to inhibition of germination by osmotic stress, the medium was complemented with low concentrations of mannitol (100 mm) or NaCl (50 mm).
Reversion of the inhibitory effect of ABA in seed germination was carried out using media containing 10 and 100 μm GA3 (Sigma-Aldrich).
Seed germination was scored by determining daily the percentage of seeds that had germinated and developed green cotyledons for 5 to10 d. At least three replicates of each germination assay were performed.
Root Growth and Flowering Time
Root growth assay for scoring ABA sensitivity was carried out by measuring root growth 7 d after transferring 5- to 7-d-old seedlings onto vertical MS plates containing different concentrations of ABA (0, 1, 5, and 10 μm). The leaf number used to determine flowering phenotype was performed as described by Wilson et al. (1992).
Hormone Dose Response Experiments
Ten-day-old seedlings were transferred to pots containing a 1:3 vermiculite:soil mixture. The seedlings were sprayed every 2 d with GA3 (100 μm), BR (Epibrassinolide; 10 μm), or ACC (100 μm; Sigma-Aldrich).
Northern-Blot Analysis
Total RNA was extracted using the RNA wiz kit (Ambion) following the manufacturer's protocol, separated on formaldehyde-agarose gels, and blotted onto a nylon membrane. Blots were hybridized with [32P]-labeled specific probes. All RNA gel-blot experiments were repeated at least three times, and results from one representative experiment are shown in the figures. RAB18 and GASA1 probes were prepared by reverse transcription (RT)-PCR with the primers described elsewhere (Herzog et al., 1995; González-Guzmán et al., 2002). Blots were exposed for 24 h in a phosphor imager screen (Fujitsu).
Semiquantitative RT-PCR
cDNA was synthesized from total RNA using the first-strand cDNA synthesis kit for RT-PCR of Avian myeloblastosis virus (Roche Diagnostics) with oligo-p(dT) as primer, following the manufacturer's instruction. Two microliters were used for each PCR consisting of 1-min cycles at 94°C, 50°C, and 72°C. AtGA20ox1, AtGA3ox1, and UBQ14 primers were those used by Oka et al. (2001), Williams et al. (1998), and Vriezen et al. (2004), respectively. Each PCR was performed for 15, 20, 25, and 30 cycles following the procedure described by Vriezen et al. (2004). After Southern blotting and hybridizing with the purified PCR products, the signals were quantified by phosphor imager analysis (Fujitsu). The amplification reactions were exponential between 15 and 25 cycles, and the products of the reactions after 20 cycles were used for quantification. The experiment was performed twice and produced reproducible patterns.
FsPP2C2:GFP Constructs
The FsPP2C2 coding region was fused to the N-terminal end of GFP in the vector pMON30063 (Pang et al., 1996), under the control of the CaMV 35S promoter. A fragment corresponding to the FsPP2C2 coding region was amplified by PCR using synthetic anchored primers that contained a NcoI-compatible restriction site at their 3′ and 5′ ends. In addition, the coding region of FsPP2C2 was also fused to the C-terminal end of GFP in the same vector, although in this case the subsequent cloning required a different strategy. The pMON vector was digested with EcoRI restriction enzyme, whereas FsPP2C2 was digested with NdeI and XhoI restriction enzymes because its coding region contained an EcoRI restriction site. Both the linearized vector and the DNA fragment containing FsPP2C2 coding regions were treated with the Klenow fragment of DNA polymerase in order to obtain blunt ends before ligation. Following sequence verification of the inserts, GFP fusion and control constructs were transiently expressed by particle bombardment.
Transient Expression Assays
Transformation was carried out following the procedure described by Varagona et al. (1992). DNA absorption to gold particles and bombardment using a helium-driven particle accelerator (PDS-1000/He; Bio-Rad) was performed according to the manufacturer's recommendations. One microgram of plasmid was used for transformation, and the target material (BY2 tobacco [Nicotiana tabacum] and onion [Allium cepa] epidermal cells) were bombarded three times. After bombardment, onion epidermal cells were transferred to MS medium supplemented or not with 100 μm ABA and kept at 28°C in the dark for 18 h before analysis by fluorescence microscopy. Cells were examined using a Zeiss Axiovert 200 microscope equipped with epifluorescence optics. GFP-specific fluorescence was detected using Zeiss filters 515 to 530 nm. Confocal images were obtained on a Zeiss LSM 510 confocal laser-scanning microscope with Zeiss LSM version 2.8 software.
Scanning Electron Microscopy
Stems were harvested from mature plants and observed directly by a scanning microscope (Zeiss DSM 940) equipped with a cooling stage.
Quantification of GAs
Endogenous GAs were quantified by gas chromatography-mass spectrometry as described elsewhere (García-Martínez et al., 1997). The material (about 10 g fresh weight of rosette leaves from 4-week-old plants grown at 22°C under LD conditions, at the vegetative state, and collected 3 h after starting the light period) was spiked at the time of extraction with [3H]GA20 (1.41 TBq mmol−1) and [3H]GA9 (1.41 TBq mmol−1) to check recoveries during the purification procedure, and with [17-2H2]GA1, [17-2H2]GA4, [17-2H2]GA8, [17-2H2]GA9, [17-2H2]GA19, [17-2H2]GA20, [17-2H2]GA29, and [17-2H2]GA34 (purchased from Prof. L. Mander, Research School of Chemistry, Australian National University, Canberra, Australia) as internal standards for quantification. Fractions of HPLC containing the GAs of interest were methylated and trimethylsilylated before analysis by gas chromatography-mass spectrometry using a Hewlett-Packard 5890 gas chromatograph coupled to a 5971A mass selective detector. The peak-area ratios of the following ion pairs, in the appropriate HPLC fractions and having a retention time similar to that of the corresponding GAs, were determined to calculate the concentrations of endogenous GAs by reference to calibration curves: 506/508 (GA1), 284/286 (GA4), 594/596 (GA8), 298/300 (GA9), 434/436 (GA19), 418/420 (GA20), 506/508 (GA29), and 506/508 (GA34).
We only measured GA amounts in the transgenic C1 and C2 lines because the size and production of seeds of the C3 line were not sufficient to perform this set of experiments.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AJ277744.
Acknowledgments
We thank Dr. P.L. Rodriguez (Instituto de Biología Molecular y Celular de Plantas-Consejo Superior de Investigaciones Cientificas, Valencia), for providing us with Arabidopsis seeds transformed with the empty vector pBI121, used as a control in this study.
This work was supported by the Ministerio de Ciencia y Tecnología, Spain (grant nos. BFI2003–01755 and BIO2003–00151), by Junta de Castilla y León (grant no. SA046A05), and by a “Ramón y Cajal” research contract (to O.L.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Carlos Nicolás (cnicolas@usal.es).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.084681.
References
- Abe H, Urao T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo A, Bossi F, Finkelstein RR, Leon P (2003) Three genes that affect sugar sensing (abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiol 133: 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert D, Chevillard M, Dorne AM, Arlaud G, Herzog M (1998) Expression patterns of GASA genes in Arabidopsis thaliana: The GASA4 gene is up-regulated by gibberellins in meristematic regions. Plant Mol Biol 36: 871–883 [DOI] [PubMed] [Google Scholar]
- Calvo AP, Nicolás C, Nicolás G, Rodríguez D (2004) Evidence of a cross-talk regulation of a GA 20-oxidase (FsGA20ox1) by gibberellins and ethylene during the breaking of dormancy in Fagus sylvatica seeds. Physiol Plant 120: 623–630 [DOI] [PubMed] [Google Scholar]
- Cheng A, Kaldis P, Solomon MJ (2000) Dephosphorylation of human cyclin-dependent kinases by protein phosphatase type 2C alpha and beta isoforms. J Biol Chem 275: 34744–34749 [DOI] [PubMed] [Google Scholar]
- Chien JC, Sussex IM (1996) Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol 111: 1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P (1996) A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273: 1239–1241 [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J (1985) Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res 13: 4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid in seeds and seedlings. Plant Cell 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ (2000) The abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12: 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell 10: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez JL, Santes C, Croker SJ, Hedden P (1997) Identification, quantification and distribution of gibberellins in fruits of Pisum sativum L. cv Alaska during pod development. Planta 184: 53–60 [DOI] [PubMed] [Google Scholar]
- Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM (1992) Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4: 1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho THD, Walker-Simmons MK (1999) An abscisic acid induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone. Proc Natl Acad Sci USA 96: 1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Zentella R, Walker-Simmons MK, Ho THD (2001) Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. Plant Cell 13: 667–679 [PMC free article] [PubMed] [Google Scholar]
- González-García MP, Rodríguez D, Nicolás C, Rodríguez PL, Nicolás G, Lorenzo O (2003) Negative regulation of abscisic acid signaling by the Fagus sylvatica FsPP2C1 plays a role in seed dormancy regulation and promotion of seed germination. Plant Physiol 133: 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guzmán M, Apostolova N, Belles JM, Barrero JM, Piqueras P, Ponce MR, Micol JL, Serrano R, Rodríguez PL (2002) The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 14: 1833–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AA, Vartanian N, Giraudat J (1999) ABI1 protein phosphatase 2C is a negative regulator of abscisic acid. Plant Cell 11: 1897–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M, Dorne AM, Grellet F (1995) GASA, a gibberellin regulated gene family from Arabidopsis thaliana related to the tomato GAST1 gene. Plant Mol Biol 27: 743–752 [DOI] [PubMed] [Google Scholar]
- Himmelbach A, Hoffmann T, Leube M, Hohener B, Grill E (2002) Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J 21: 3029–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux V, Kwak JM, Schroeder JI (2001) An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106: 477–487 [DOI] [PubMed] [Google Scholar]
- Kerk D, Bulgrien J, Smith DW, Barsam B, Veretnik S, Gribskov M (2002) The complement of protein phosphatase catalytic subunits encoded in the genome of Arabidopsis. Plant Physiol 129: 908–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermode AR (2005) Role of abscisic acid in seed dormancy. J Plant Growth Regul 24: 319–344 [Google Scholar]
- Koornneef M, van der Veen JH (1980) Induction and analysis of gibberellin-sensitive mutants in Arabidopsis thaliana L. Theor Appl Genet 58: 257–263 [DOI] [PubMed] [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15: 281–307 [Google Scholar]
- Kuhn JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI (2006) The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol 140: 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang V, Palva ET (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20: 951–962 [DOI] [PubMed] [Google Scholar]
- Lange T (1998) Molecular biology of gibberellin synthesis. Planta 204: 404–419 [DOI] [PubMed] [Google Scholar]
- Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 19: 199–222 [DOI] [PubMed] [Google Scholar]
- Li J, Wang XQ, Watson MB, Assmann SM (2000) Regulation of abscisic acid-induced stomatal closure and anion channels by guard cells AAPK kinase. Science 287: 300–303 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Nicolás C, Nicolás G, Rodríguez D (2002) Molecular cloning of a functional protein phosphatase 2C (FsPP2C2) with unusual features and synergistically up-regulated by ABA and calcium in dormant seeds of Fagus sylvatica. Physiol Plant 114: 482–490 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Rodríguez D, Nicolás G, Rodríguez PL, Nicolás C (2001) A new protein phosphatase 2C (FsPP2C1) induced by abscisic acid is specifically expressed in dormant beechnut seeds. Plant Physiol 125: 1949–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2004) dwarf and delayed flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J 37: 720–729 [DOI] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid pathway. Plant J 25: 295–303 [DOI] [PubMed] [Google Scholar]
- Meskiene I, Beaudoin E, Schweighofer A, Liwosz A, Jonak C, Rodríguez PL, Jelinek H, Hirt H (2003) The stress-induced protein phosphatase 2C is a negative regulator of a mitogen-activated protein kinase. J Biol Chem 278: 18945–18952 [DOI] [PubMed] [Google Scholar]
- Meskiene I, Bogre L, Glaser W, Balog J, Brandstotter M, Zwerger K, Ammerer G, Hirt H (1998) MP2C, a plant protein phosphatase 2C, functions as a negative regulator of mitogen-activated protein kinase pathways in yeast and plants. Proc Natl Acad Sci USA 95: 1938–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco. Physiol Plant 15: 473–497 [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Tasaka Y, Iwabuchi M, Masanobu M (2001) Elevated sensitivity to gibberellin by vernalization in the vegetative rosette plants of Eustoma grandiflorum and Arabidopsis thaliana. Plant Sci 160: 1237–1245 [DOI] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F (2002) Gibberellin: biosynthesis, catabolism and response pathways. Plant Cell 14: S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GK, Cheong YH, Kim N, Grant JJ, Li L, Hung W, D'Angelo C, Weinl S, Kudla J, Luan S (2004) The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell 16: 1912–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang SZ, DeBoer D, Wan Y, Ye C, Layton JC, Neher MK, Armstrong C, Fry JE (1996) An improved green fluorescent protein gene as a vital marker in plants. Plant Physiol 112: 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Flores L, Carrari F, Osuna-Fernández R, Rodríguez MV, Enciso S, Stanelloni R, Sánchez RA, Bottini R, Iusem ND, Benech-Arnold RL (2003) Expression analysis of a GA 20-oxidase in embryos from two sorghum lines with contrasting dormancy: possible participation of this gene in the hormonal control of germination. J Exp Bot 54: 2071–2079 [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, García-Martínez JL, Santes CM, Gaskin P, Hedden P (1997) The role of gibberellins A1 and A3 in fruit growth of Pisum sativum L., and the identification of gibberellins A4 and A7 in young seeds. Planta 201: 446–455 [Google Scholar]
- Sáez A, Apostolova N, González-Guzmán M, González-García MP, Nicolás C, Lorenzo O, Rodríguez PL (2004) Gain of function and loss of function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid. Plant J 37: 354–369 [DOI] [PubMed] [Google Scholar]
- Stark MJ (1996) Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast 12: 1647–1675 [DOI] [PubMed] [Google Scholar]
- Tahtiharju S, Palva T (2001) Antisense inhibition of protein phosphatase 2C accelerates cold acclimation in Arabidopsis thaliana. Plant J 26: 461–470 [DOI] [PubMed] [Google Scholar]
- Varagona MJ, Schmidt RJ, Raikhel NV (1992) Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein Opaque-2. Plant Cell 4: 1213–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriezen WH, Achard P, Harberd NP, van der Straeten D (2004) Ethylene-mediated enhancement of apical hook formation in etiolated Arabidopsis thaliana seedlings is gibberellin dependent. Plant J 37: 505–516 [DOI] [PubMed] [Google Scholar]
- Williams J, Phillips AL, Gaskin P, Hedden P (1998) Function and substrate specificity of the gibberellin 3β-hydroxylase encoded by the Arabidopsis GA4 gene. Plant Physiol 117: 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR (1992) Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol 100: 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sánchez JP, López-Molina L, Himmelbach A, Grill E, Chua NH (2003) The abi1-1 mutation blocks ABA downstream cADPR action. Plant J 34: 307–315 [DOI] [PubMed] [Google Scholar]
- Xu YL, Li L, Wu K, Peeters AJ, Gage DA, Zeevaart JAD (1995) The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression. Proc Natl Acad Sci USA 92: 6640–6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S (2004) Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16: 367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, Shinozaki K, Hirayama T (2006) ABA-Hypersensitive Germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol 140: 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]