Abstract
Bartonella koehlerae, a recently described feline Bartonella species, was isolated from two naturally infected cats in northern California. We experimentally infected domestic cats with B. koehlerae to establish the microbiological and immunological characteristics of this infection in cats and to compare it to infections with those caused by B. henselae and B. clarridgeiae. Four cats were inoculated intradermally with B. koehlerae (8.6 × 107 to 3.84 × 108 CFU/ml). None of the cats presented any obvious clinical signs, but all cats developed bacteremia, which peaked at 3.36 × 104 to 1.44 × 106 CFU/ml of blood between day 14 and day 36 postinoculation. B. koehlerae-inoculated cats had a bacteremia duration (mean, 74 days) shorter than did cats inoculated with B. clarridgeiae (mean, 324 days) (P = 0.03). None of the four cats inoculated with B. koehlerae had bacteremia relapse. As shown by enzyme-linked immunosorbent assay (ELISA) using B. koehlerae outer membrane protein (OMP) antigens, the four cats developed a species-specific antibody response, and ELISA testing using other feline Bartonella OMP antigens showed statistically lower optical density values. All four cats developed similar antibody reactivity patterns to B. koehlerae OMP antigens as seen by Western blotting, each with at least 20 seroreactive protein bands. Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, protein profile differences were observed for both whole-cell lysate and OMPs from B. koehlerae, compared with B. henselae and B. clarridgeiae. B. koehlerae was more closely related to B. henselae than to B. clarridgeiae by protein profile, and this relatedness was also confirmed by analysis of the genomic DNA profiles by pulsed-field gel electrophoresis.
Bartonellae are gram-negative fastidious bacteria, usually vector-borne and with a vertebrate animal reservoir, that cause various human diseases, which can be particularly severe in immunocompromised individuals (6, 38). The genus Bartonella is composed of at least 16 species, of which 4 (B. henselae, B. clarridgeiae, B. koehlerae, and B. weissii) have been isolated from cats (6, 14, 16; R. Regnery, M. Martin, and J. Olson, Letter, Lancet 340:557-558, 1992). B. henselae, which has been separated in two main variants or types (4, 15) is the zoonotic agent of cat scratch disease (27) and was isolated from the blood of a cat in 1992 (Regnery et al., Letter). Coinfection of cats with B. henselae and B. clarridgeiae or with the two variants of B. henselae has been reported (4, 26). B. koehlerae was isolated from the blood of two healthy cats from the same household in northern California (16, 28) and has different culture medium requirements than B. henselae or B. clarridgeiae (32). Finally, a fourth Bartonella species, designated B. weissii, was isolated from cats from Utah and Illinois (R. Regnery, N. Marano, P. Jameson, E. Marston, D. Jones, S. Handley, C. Goldsmith, and C. Greene, Abstr. 15th Meet. Am. Soc. Rickettsiol., abstr. 4, p. 15, 2000). This species is genetically identical to B. bovis isolated from domestic ruminants (5, 7).
Worldwide, domestic cats are the main reservoir for B. henselae and B. clarridgeiae (10, 28, 29, 33, 48, 51). Naturally infected cats do not usually exhibit specific clinical signs (11, 28, 42). However, in some B. henselae experimentally infected cats, clinical symptoms, such as fever, lethargy, neurological signs, and reproductive disorders, have been observed (24, 30, 40, 41). The presence or absence of clinical signs in experimentally infected cats may be explained by strain virulence differences (41). B. henselae is transmitted from cat to cat by the cat flea (Ctenocephalides felis) (13, 19), and no transmission of B. henselae by direct contact or from queens to kittens has been shown to occur in experimentally infected cats (1, 24).
Studies on experimental feline Bartonella infection have focused on B. henselae and B. clarridgeiae (1, 21-24, 31, 33, 40, 41, 42, 50). Bacteremia lasts from 7 weeks to up to 83 weeks (1, 21-24, 29, 41, 50) and cats inoculated with B. clarridgeiae show relapsing bacteremia (positive culture after two successive negative cultures) more frequently than those infected with B. henselae (30, 50).
Differences among feline Bartonella species are seen genotypically and phenotypically. Pulsed-field gel electrophoresis (PFGE) separation of SmaI endonuclease-digested genomic DNA fragments from feline B. henselae isolates reveals a wide diversity of profiles (2, 45), whereas such diversity is not observed for B. clarridgeiae (39). However, no PFGE genomic analysis has been performed for B. koehlerae. Additionally, characteristics of feline infection caused by B. koehlerae have not been investigated, and it is not known if domestic cats are a natural reservoir for B. koehlerae, as this species has only been isolated from two cats. Therefore, our objectives were to (i) determine if domestic cats experimentally inoculated with B. koehlerae manifest clinical signs of infection; (ii) evaluate time of bacteremia onset and duration, as well as presence of bacteremia relapses; (iii) characterize the serological immune response to experimental infection; (iv) characterize the genomic profile of B. koehlerae by PFGE; and (v) compare these characteristics to experimental infection of cats with either B. henselae or B. clarridgeiae.
MATERIALS AND METHODS
Animals.
Four 10- to 12-month-old, domestic cats (three males, one female) free of major feline viral infections (feline calicivirus and coronavirus, feline immunodeficiency virus, feline leukemia virus, and feline parvovirus), based on specific viral or antibody detection, and free of ringworm infection were enrolled. These cats were also confirmed to be culture negative and seronegative for Bartonella spp. For comparison of the characteristics of B. koehlerae infection in these four cats with cats infected with two other feline Bartonella species, six 8- to 12-month-old (one male, five females) domestic cats were inoculated with B. henselae type I (Houston I), and four 4- to 5-month-old cats (three males, one female) were inoculated with B. clarridgeiae. All cats were examined clinically each day for the first 2 weeks after inoculation and at least weekly thereafter. The cats were housed in groups of four to six in a controlled environment. Blood samples were collected weekly to every other week for blood culture and serological tests.
Inoculum preparation.
B. koehlerae (ATCC 700693), B. henselae Houston I (ATCC 49882), and B. clarridgeiae (ATCC 51734) were grown on chocolate agar (B. koehlerae) or 5% rabbit blood agar, as previously described (1, 16) and incubated at 35°C for 7 days. The harvested colonies were suspended in sterile saline, and 0.5 ml was inoculated intradermally, as previously described (1). The inoculum doses for B. koehlerae were 3.8 × 108 CFU/ml for two cats and 8.6 × 107 CFU/ml for the other two cats. The inoculum doses for B. henselae were 6.6 × 106 CFU/ml for four cats and 9.6 × 107 CFU/ml for the other two cats. The inoculum dose for B. clarridgeiae was 1.0 × 109 CFU/ml for the four cats. The inocula were confirmed to be B. koehlerae, B. henselae, or B. clarridgeiae by PCR-restriction fragment length polymorphism (PCR-RFLP) analysis (12, 16).
Blood culture.
Blood was drawn from the jugular vein of each cat weekly for the first 2 months and every other week for the 6.5 subsequent months. Two milliliters of blood was placed into EDTA tubes (Becton Dickinson) for culture, and 1 ml was placed into serum separation tubes for serological tests. The EDTA tubes were frozen at −70°C and plated a few days later onto chocolate agar plates (two plates per cat), as previously described (16). The plates were incubated at 35°C with 5% CO2 for 4 weeks. Plates were examined two to three times a week for any bacterial growth. Isolated strains were confirmed to be B. koehlerae, B. henselae, or B. clarridgeiae by PCR-RFLP of the citrate synthase gene using HhaI and TaqI endonucleases (12, 16). The number of colonies on each plate was counted, and the number of CFU per milliliter of blood was calculated.
Serology. (i) IFA test.
Immunofluorescence antibody (IFA) immunoglobulin G (IgG) antibody titers were determined against whole organisms of B. koehlerae, B. henselae, and B. clarridgeiae, as previously described (9, 11).
(ii) ELISA.
The outer membrane proteins (OMPs) of three Bartonella strains were prepared as previously described (47). Bartonella organisms were grown on chocolate agar plates (B. koehlerae) and on rabbit blood agar plates (B. henselae and B. clarridgeiae) at 35°C in 5% CO2 and harvested from 50 plates, washed in sterile phosphate-buffered saline (PBS), and pelleted. Then enzyme-linked immunosorbent assay (ELISA) testing was performed as previously published (12). Serum samples were diluted at 1:50 for IgM and at 1:100 for IgG, and anti-cat-IgM (heavy and light chain peroxidase-labeled conjugate; Kirkegaard and Perry Laboratories [KPL], Gaithersburg, Md.) or anti-cat IgG phosphatase-labeled conjugate (KPL) were diluted at 1:100 dilution for IgM and at 1:2,000 for IgG conjugate, respectively. The optical density (OD) of each well was quantified for IgM at 450 nm, with a second wavelength at 570 nm as a reference, and for IgG at 410 nm, using a Spectra Max 340 device (Molecular Devices, Sunnyvale, Calif.).
SDS-PAGE and Western blotting.
As described above, each Bartonella species was cultured, harvested, and suspended in 0.5 ml of sterile PBS. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (0.5 ml) was added to the suspension. Similarly, OMPs of each Bartonella species were diluted with sample buffer and adjusted to 1.25 μg of protein/μl. Both preparations, whole organisms and OMPs of Bartonella, were then solubilized for 5 min at 100°C. Whole organisms and OMPs were electrophoresed through discontinuous SDS-polyacrylamide gel (4% stacking gel and 12% resolving gel) in an SE-600 (Hoefer Scientific Instruments, San Francisco, Calif.) apparatus at 8.5 mA for 16 h. Proteins, stained with SYPRO orange (Bio-Rad), were visualized under UV light by using a gel documentation system, Gel Doc 1000 (Bio-Rad).
The Western blotting strips were prepared by SDS-PAGE as described above. The electrophoretically separated proteins were transferred onto a sheet of nitrocellulose (0.2-μm pore size; Hoefer Scientific Instruments), using a TE 50× (Hoefer Scientific Instruments) unit at about 900 mA for 2 h at 10°C. The nitrocellulose sheet was dried and cut into strips (4.0 by 100 mm) and then soaked in blocking solution (20 mM Tris-HCl, 500 mM NaCl, 3% gelatin [pH 7.5]). Serum samples from cats inoculated with Bartonella, diluted at 1:100 in a buffer solution (20 mM Tris-HCl, 500 mM NaCl, 5% skim milk [pH 7.5]), were added to the strips and incubated for 1 h at room temperature. The strips were washed three times for 5 min, and peroxidase-labeled goat anti-cat IgG (heavy and light chain) conjugate diluted at 1:3,000 (KPL) was added and incubated for 1 h at room temperature. The strips were rinsed three times for 5 min, and then 3,3",5,5"-tetramethylbenzidine membrane peroxidase substrate (KPL) was added. Comparative analysis of the Bartonella species-specific protein profiles was performed to generate similarity values by the unweighted-pair group method with an arithmetic mean (UPGMA), using Molecular Analyst software (Fingerprinting Plus; Bio-Rad).
PFGE.
The PFGE procedure was performed as previously described (8, 43) with some very minor modifications. The harvested strain of each Bartonella species was cultured on permissive agar, scraped, and suspended in 1.0 ml of sterile PBS. Digestion of proteins was performed using proteinase K solution (10 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% SDS [Bio-Rad], 0.25% Triton X-100, proteinase K [1 mg/ml; GIBCO, BRL, Gaithersburg, Md.]), and DNA from each Bartonella species was digested with SmaI endonuclease. Cluster analysis using the Molecular Analyst software (Bio-Rad) was performed, and dendrograms based on results of the matrix of similarity values were created with UPGMA clustering.
Statistical tests.
To statistically analyze and compare duration of bacteremia, a nonparametric Mann-Whitney rank sum test was performed with statistical software (release 13.1; MINITAB Inc., State College, Pa.). For peak level of bacteremia and time to the maximum level of bacteremia, an equal-variance t test was performed with MINITAB software. For ELISA and IFA values postinoculation compared to the values at day 0, a paired t test was applied with MINITAB software. For univariate analysis, a nonparametric test (Fisher's exact test) was used to characterize the association between Bartonella species and bacteremia relapses (Epi-info, version 6.04b; Centers for Disease Control and Prevention, Atlanta, Ga.).
RESULTS
Clinical observations and bacteremia characteristics.
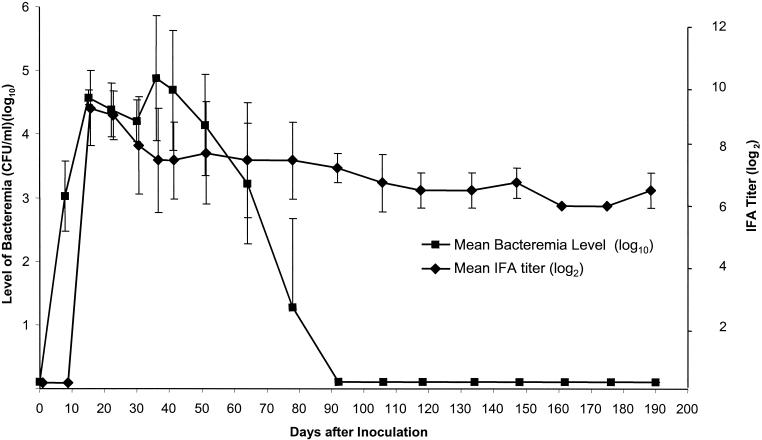
None of the fourteen cats, including the four B. koehlerae-inoculated cats, presented any quantifiable clinical symptoms, including fever. All four B. koehlerae-inoculated cats became bacteremic within 7 to 8 days after inoculation (Fig. 1). Bacteremia peaked between 14 and 36 days (mean, 30.3 days) after inoculation. The times at which bacteremia peaked in the cats inoculated with B. koehlerae were similar to those at which bacteremia peaked in the cats inoculated with the other Bartonella species, B. henselae (n = 6; mean, 28 days; range, 21 to 44 days; P = 0.76) and B. clarridgeae (n = 4; mean, 34.3 days; range, 29 to 36 days; P = 0.69). Bacteremia level in B. koehlerae-infected cats peaked at 3.36 × 104 to 1.44 × 106 CFU/ml (mean, 4 × 105 CFU/ml). There were no statistically significant differences compared to the cats inoculated with the other Bartonella species: B. henselae (n = 6; mean, 2.41 × 105 CFU/ml; range, 1.90 × 103 to 4.48 × 105 CFU/ml; P = 0.79) and B. clarridgeiae (n = 4; mean, 1.69 × 105 CFU/ml; range, 6.13 × 104 −3.20 × 105 CFU/ml; P = 0.91). Bacteremia lasted 70 to 78 days (mean, 74 days) (Fig. 1). For comparison, bacteremia lasted 37 to 77 days (mean, 60 days) for B. henselae (n = 6 cats) and 263 to 363 days (mean, 324 days) for B. clarridgeiae (n = 4 cats), respectively. The duration of bacteremia in cats inoculated with B. koehlerae was significantly shorter than that in cats inoculated with B. clarridgeiae (P = 0.03) but not significantly different from that in cats inoculated with B. henselae (P = 0.081).
FIG. 1.
Bacteremia level and IgG antibody response as determined by IFA in domestic cats (n = 4) experimentally inoculated with B. koehlerae. Results are expressed as means and standard deviations (error bars). On this figure, the bacteremia level is the mean of the four cats' bacteremia level for the day of blood collection, which is slightly lower than the overall mean peak of bacteremia for the four cats.
We defined a relapse as any positive culture occurring after two successive negative cultures. None of the four cats inoculated with B. koehlerae had relapsing bacteremia. Similarly, none of the six cats inoculated with B. henselae had relapses, whereas relapses occurred in 100% (4 of 4) of the cats inoculated with B. clarridgeiae. The difference in the number of cats having relapsing bacteremia was statistically significant between the cats inoculated with B. koehlerae and the cats inoculated with B. clarridgeiae (P = 0.03).
IFA.
B. koehlerae-specific IgG antibodies were detected by IFA within 1 to 2 weeks (mean, 12.6 days; range, 7 to 15 days) after inoculation (Fig. 1). IgG antibody titers peaked at day 15 postinfection (p.i.) (mean, 1:512; range, 1:256 to 1:2,048). All four cats remained seropositive (titer ≥ 1:64) during the entire study period (190 days p.i.), despite the absence of bacterial isolation after day 92 p.i. (Fig. 1). The IFA titers were significantly higher after day 15 p.i. compared to the titer at day 0 (P < 0.05). IFA cross-reactivity with antigens from other feline Bartonella species (B. henselae and B. clarridgeiae) was observed. However, titers were usually higher for B. koehlerae antigen than for the other Bartonella species antigens during the first month p.i., as shown for one representative cat (Table 1). For all four cats, the IgG cross-reacting antibody response to B. clarridgeiae was delayed for several weeks (mean, 60 days), compared to B. henselae responses, which were synchronous with the B. koehlerae response.
TABLE 1.
IgG antibody responsea to antigens from three Bartonella species in a B. koehlerae-infected domestic cat as determined by IFA
| Days after inoculation | Titer of antibody to antigen from (whole organisms):
|
||
|---|---|---|---|
| B. koehlerae | B. henselae type I (Houston I) | B. clarridgeiae | |
| 0 | 0 | 0 | 0 |
| 15 | 2,048 | 256 | 0 |
| 30 | 1,024 | 256 | 0 |
| 51 | 64 | 128 | 0 |
| 78 | 128 | 128 | 128 |
| 118 | 64 | 128 | 64 |
| 148 | 64 | 64 | 64 |
| 162 | 64 | 256 | 64 |
| 176 | 64 | 64 | 64 |
| 190 | 64 | 64 | 64 |
Data are expressed as reciprocal endpoint titers.
ELISA.
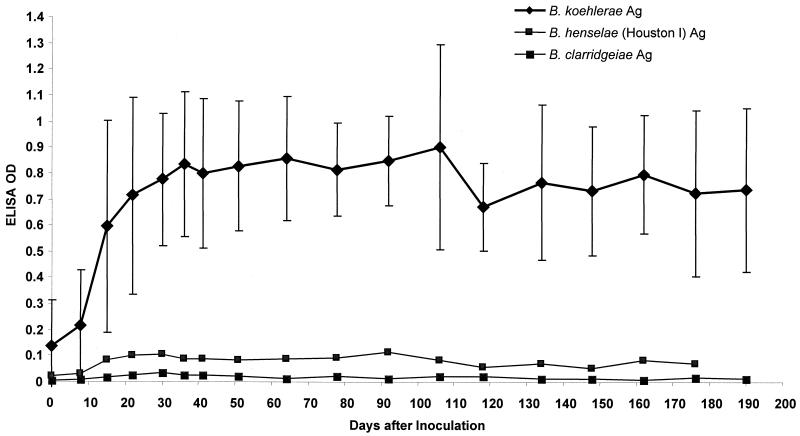
Bartonella-specific IgG (Fig. 2) and IgM antibodies against the OMP antigens from three Bartonella species (B. koehlerae, B. henselae, and B. clarridgeiae) were detected by ELISA in the four cats inoculated with B. koehlerae. The IgM antibody response detected with B. koehlerae OMP antigen peaked between day 7 and day 15 p.i. and decreased sharply by day 30 p.i. (data not shown). The IgM OD values, using B. koehlerae OMP antigen, were significantly higher between day 8 and day 41 p.i. compared to the OD values at day 0 (P < 0.05). The IgG antibody response to B. koehlerae OMP antigen began to rise 14 to 21 days after inoculation, peaked 1 month after inoculation, and persisted throughout the course of this study (Fig. 2). The OD values for IgG antibodies against B. koehlerae OMP antigen were significantly higher between day 15 and day 190 p.i. compared to the OD values at day 0 (P < 0.05). IgM and IgG antibody OD values against the OMP antigens from the other two Bartonella species were significantly lower than the OD values for IgM and IgG antibodies against B. koehlerae OMP antigens, between day 8 and 64 p.i. for IgM and between day 8 and 190 p.i. for IgG (Fig. 2). (P < 0.05).
FIG. 2.
Mean ELISA IgG antibody response against OMP antigens (Ag) of three different Bartonella spp. (B. koehlerae, B. henselae, and B. clarridgeiae) in sera from domestic cats (n = 4) inoculated with B. koehlerae. Results are expressed as means and standard deviations (error bars).
SDS-PAGE analysis.
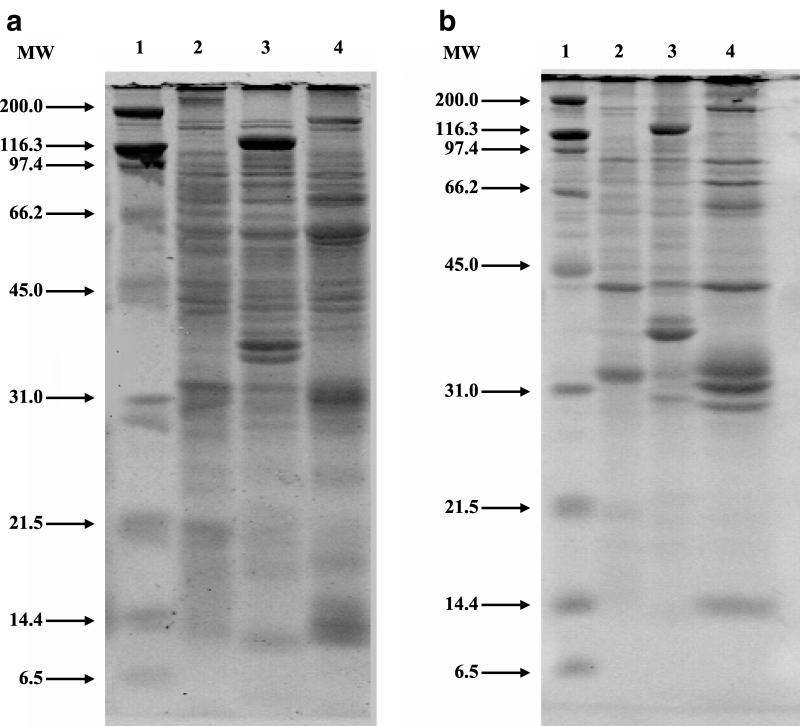
The SDS-PAGE analysis of whole organisms (Fig. 3a) and OMPs (Fig. 3b) for B. koehlerae showed a unique protein profile, different from the protein profiles of B. henselae and B. clarridgeiae. For B. koehlerae whole-organism lysate, at least 25 protein bands were identified between 14 and 180 kDa, with prominent bands at approximately 14, 25, 29, 31, 40, 45, 55, 65, and 178 kDa. For B. koehlerae OMPs, protein bands (at least 14) were identified between 14 and 180 kDa, with prominent bands at approximately 14, 30, 31, 33, 37, 50, 63, 75, 92, and 178 kDa. Pairwise comparison of the protein profiles between B. koehlerae, B. henselae, and B. clarridgeiae by UPGMA showed that B. koehlerae and B. henselae had an 83.9 and a 69.2% similarity for whole organisms and OMPs, respectively. Similarly, B. koehlerae and B. clarridgeiae had a 68.2 and a 54.7% similarity for whole organisms and OMPs, respectively.
FIG. 3.
(a) Comparison of protein profiles from whole-organism lysates of three Bartonella species separated in a 12% gel by SDS-PAGE. Lane 1, molecular weight (MW) standard (in thousands); lane 2, B. henselae (Houston I strain; ATCC 49882); lane 3, B. clarridgeiae (ATCC 51734); lane 4, B. koehlerae (ATCC 700693). (b) Comparison of OMP profiles from three Bartonella species separated in a 12% gel by SDS-PAGE. Lane 1, MW standard (in thousands); lane 2, B. henselae (Houston I strain; ATCC 49882); lane 3, B. clarridgeiae (ATCC 51734); lane 4, B. koehlerae (ATCC 700693).
Western blotting.
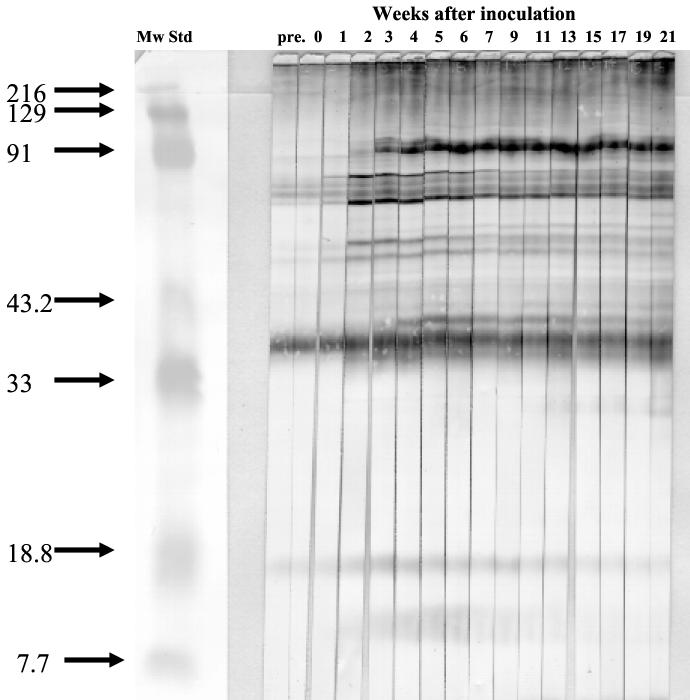
Serum samples from the four cats inoculated with B. koehlerae were examined by Western blotting to identify specific IgG seroreactivity patterns to OMP antigens and showed a similar Western blotting profile for sera from all four cats. At least 20 seroreactive bands were identified between 14 and 180 kDa (Fig. 4). IgG seroreactivity specific to B. koehlerae OMP antigens was not detected until 2 weeks p.i., and prominent specific bands were noted at 14, 30, 40, 45, 50, 53, 58, 60, 65, 73, 78, and 97 kDa. Bands at 40, 53, 58, 65, and 78 kDa were detected by week 2 p.i., and other positive bands appeared by week 5 p.i. and persisted until the end of the experiment. Cross-reactivity with other feline Bartonella species was examined using OMP antigens from B. henselae and from B. clarridgeiae. Protein bands were detected 2 to 3 weeks after the inoculation at 60 and 75 kDa by using B. henselae OMP antigens and at 37, 60, and 75 kDa by using B. clarridgeiae OMP antigens (data not shown).
FIG. 4.
Immunoblotting with serum from a cat inoculated with B. koehlerae against antigen from B. koehlerae. Colonies of B. koehlerae were scraped from agar, OMP fractions were prepared, and the proteins were separated in a 12% gel by PAGE. The proteins were transferred and blotted with a 1:100 dilution of serum from a domestic cat taken at different time points after experimental inoculation with B. koehlerae. Mw Std, molecular weight standard (in thousands).
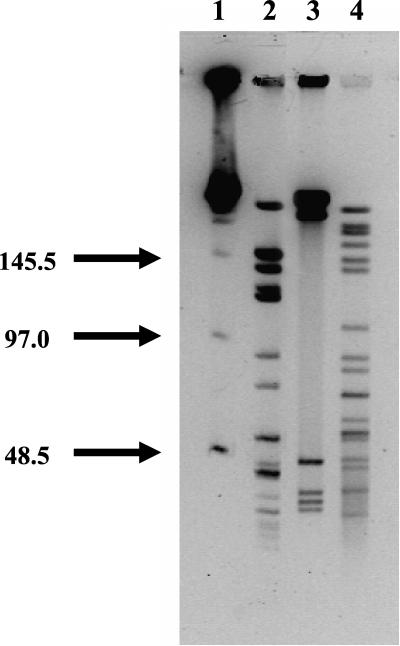
PFGE.
DNA from B. koehlerae, B. henselae, and B. clarridgeiae was digested with SmaI endonuclease. The PFGE profiles showed a range of fragment sizes between 4 and 230 kb (Fig. 5). B. koehlerae showed a wide range of bands (at least 17 bands for B. koehlerae) as B. henselae did, whereas B. clarridgeiae was digested into fewer fragments. Similarity among the profiles of restriction fragments from these Bartonella species showed that B. koehlerae was more closely related to B. henselae than to B. clarridgeiae.
FIG. 5.
PFGE analysis of SmaI-digested genomic DNA from three Bartonella species. Lane 1, molecular size standards (48.5 to 970 kbp); lane 2, B. henselae genomic DNA (Houston I strain; ATCC 49882); lane 3, B. clarridgeiae genomic DNA (ATCC 51734); lane 4, B. koehlerae genomic DNA (ATCC 700693).
DISCUSSION
B. henselae and B. clarridgeiae have been isolated from domestic cats in many parts of the world (10). In contrast, B. koehlerae has been isolated only from two asymptomatic and naturally infected cats from northern California (16), perhaps in part because B. koehlerae is even more fastidious than B. henselae and B. clarridgeiae. Numerous Bartonella species infect an equally numerous variety of domestic and wild mammalian hosts. With the exception of rodents, often only one or a few mammalian species are permissive hosts for productive infection with a given Bartonella species. For instance, persistent bacteremia in dogs experimentally infected with B. henselae has not been documented, but bacteremia is readily established in virtually all cats inoculated with B. henselae. We experimentally inoculated four domestic cats with B. koehlerae and found that all four cats became bacteremic for at least 70 days, indicating that domestic cats can readily become infected with B. koehlerae. However, none of these four cats presented any noticeable clinical signs. This is similar to B. henselae or B. clarridgeiae naturally or experimentally infected cats, in which obvious clinical symptoms have not been consistently observed (1, 28, 33, 42).
Previous studies have demonstrated that the intradermal route is the most efficient route for cat infection with B. henselae (1) and that cat fleas (C. felis) are responsible for the transmission of B. henselae from cat to cat (13, 19). We successfully infected cats with B. koehlerae, also using the intradermal route. Interestingly, the two kittens naturally infected with B. koehlerae were living outdoors on a farm and were flea infested (16). It is possible that B. koehlerae could also be flea-borne from cat to cat, as for B. henselae.
As previously observed for B. henselae or B. clarridgeiae experimental infections, all four cats inoculated with B. koehlerae became bacteremic within 7 to 8 days after inoculation and bacteremia peaked at 104 to 106 CFU/ml in 5 weeks (21, 23, 24, 42). However, the duration of bacteremia in the four B. koehlerae-inoculated cats was significantly shorter than that in those inoculated with B. clarridgeiae. In contrast, no statistically significant differences in duration of bacteremia were observed between cats inoculated with B. koehlerae and those inoculated with B. henselae type I (Houston I).
Relapsing bacteremia in cats naturally or experimentally infected with B. henselae or B. clarridgeiae has been reported (1, 21, 23, 33, 50). Cats infected with B. clarridgeiae show more frequent relapses than do cats infected with B. henselae (50). In this study, none of the four cats inoculated with B. koehlerae had relapsing bacteremia, nor did any of the six cats inoculated with B. henselae (Houston I). In contrast, all four cats inoculated with B. clarridgeiae had relapses of bacteremia. It will be important to investigate the factors resulting in bacteremia relapse.
Bartonella IgG antibodies are usually detected by IFA within 1 to 3 weeks p.i. and last for several months in cats experimentally inoculated with either B. henselae or B. clarridgeiae (1, 21, 33, 42, 50). Similarly, the IFA IgG antibody titers in all four cats inoculated with B. koehlerae increased significantly 1 to 2 weeks after inoculation, peaked at 14 days p.i., and then gradually decreased and persisted despite the absence of bacteremia. Unfortunately, reproducible detection of B. henselae-specific IgM antibodies has not been possible by IFA (30). However, detection of B. henselae-specific IgM and IgG in experimentally infected cats has been successfully performed by ELISA (22, 23, 25, 40, 41). In cats experimentally infected with B. henselae, IgM antibodies are detected by ELISA within 1 to 2 weeks p.i., peaking between 2 to 5 weeks p.i. and quickly declining, but do not return to the baseline value (23, 40, 41). As shown by ELISA, IgG antibodies begin to rise by week 2 p.i. and persist for more than 12 months (24, 41). Because of the long-lasting IgG response in Bartonella-infected cats, detection of Bartonella-specific IgM antibodies may be a better positive predictor of bacteremia than a high titer of IgG antibodies, as previously reported for bacteremic cats (3, 11). In our cats, B. koehlerae IgM antibody detected by ELISA sharply declined by week 6 p.i., whereas bacteremia disappeared by week 11 p.i. Similar results have been observed for cats experimentally infected with B. henselae (23, 40).
Western blotting has been used both in humans (18, 35, 36) and cats (20, 31) for detection of Bartonella species-specific antibodies. In the present study, at least 12 prominent immunoreactive protein bands were identified when sera from B. koehlerae-infected cats were blotted against B. koehlerae OMPs. Some of these bands appeared or gave a stronger reaction 2 to 5 weeks p.i., as previously observed for B. henselae infection in cats (20).
In humans, serological cross-reactivity within the genus Bartonella, especially between B. henselae and B. quintana, has been reported (17, 49). However, little is known about cross-reactivity of antibodies developing in response to feline infection with Bartonella species. In this study, we compared immunoreactivity of serum from cats infected with B. koehlerae against homologous and heterologous Bartonella species OMP antigens. By IFA, IgG antibodies cross-reactive against B. henselae antigens were detected between day 15 and day 51 p.i. in the sera of the four cats infected with B. koehlerae. However, the development of IgG cross-reacting antibodies to B. clarridgeiae was delayed for several weeks, appearing for most of the cats in week 10 p.i., when bacteremia had already resolved. On the contrary, only limited cross-reactivity of either B. koehlerae IgM or IgG antibodies with heterologous Bartonella antigens was observed using an ELISA technique. Similarly, only a few cross-reactive protein bands were detected by Western blotting using serum from these four B. koehlerae-infected cats against heterologous Bartonella species OMP antigens. These findings may be explained by the fact that purified antigens improve the sensitivity and specificity of these serological tests, as previously described (18, 37). ELISA and Western blotting appear to be more-specific serological diagnostic tools than IFA for B. koehlerae infection in cats because of the extensive cross-reactivity by IFA.
SDS-PAGE analysis has been performed to differentiate and classify Bartonella species and types (31, 35, 44). Distinctive protein profiles, especially among proteins smaller than 54 kDa, were observed among nine different Bartonella species (35). More recently, a common Bartonella antigenic protein (at approximately 32 to 33 kDa) was reported (34). Major differences among Bartonella species, mainly B. koehlerae versus B. clarridgeiae, were observed for proteins <45 kDa, especially between 14 and 35 kDa. B. clarridgeiae has a major flagellar protein (flagellin A) at 41 kDa (46), but no similar protein band was observed at 41 kDa for B. koehlerae. The absence of flagella and a specific band at 41 kDa have also been reported for B. henselae (6, 20). The results obtained from pairwise comparisons of protein profiles either for whole organisms or for OMPs indicated that B. koehlerae is phenotypically more closely related to B. henselae (Houston I strain) than to B. clarridgeiae. OMPs had better discriminatory values than proteins from whole organisms. These findings suggest that purification of antigens improves the test specificity of ELISA or Western blotting, as previously reported (18, 37).
For genetic comparison, PCR-RFLP has been commonly used for differentiation and identification of Bartonella species (6). As indicated by Droz et al. (16), the citrate synthase gene PCR-RFLP analysis, using HhaI, TaqI, or MseI digestion, showed that B. koehlerae has a unique pattern, different from those of B. henselae and B. quintana. However, PCR-RFLP usually amplifies a specific gene or region on the chromosomal DNA. In contrast, PFGE analyzes the whole genome and has been utilized to identify intraspecies genomic diversity of B. henselae and B. clarridgeiae (2, 39, 43, 45). Feline B. henselae isolates, using PFGE with SmaI endonuclease digestion, show a number of different profiles, but only a single pattern has been observed for the feline B. clarridgeiae isolates (2, 39, 45). We performed PFGE using B. koehlerae genomic DNA; using SmaI endonuclease, B. koehlerae, like B. henselae, showed at least 17 fragments, but B. clarridgeiae DNA was only separated into approximately 6 fragments. The genome size of B. koehlerae, obtained from PFGE with SmaI digestion, was estimated to be approximately 1.5 Mbp, which corresponds to the lower range of B. henselae (1.5 and 2.9 Mbp) genome size (39, 43, 45). The similarity of the digested DNA profiles of these three Bartonella species was determined by UPGMA clustering. Similar to the SDS-PAGE protein profiles, the dendrogram showed that B. koehlerae is more closely related to B. henselae than to B. clarridgeiae. Thus, this consistent relationship among these Bartonella species was observed both phenotypically by SDS-PAGE and genotypically by PFGE.
Domestic cats infected with B. koehlerae developed bacteremia of several months' duration, without obvious clinical signs of infection. Our results indicate that cats can serve as a reservoir for B. koehlerae, in addition to B. henselae and B. clarridgeiae. Specific antibody responses (IgM and IgG) to B. koehlerae infection were observed within the predicted time for a bacterial infection. Detection of IgM antibodies to B. koehlerae OMP antigens may be more specific for identification of bacteremic cats than IgG antibodies. Furthermore, B. koehlerae-specific IgG antibodies detected by ELISA were not cross-reactive with OMP antigens from other Bartonella species. It will be important to determine if cats that have been infected with B. koehlerae are protected against infections caused by other feline Bartonella species. Epidemiological studies on infection prevalence in domestic cats and the geographical distribution of B. koehlerae are still necessary.
Acknowledgments
We thank Carol Cardona from the School of Veterinary Medicine, University of California, Davis, for helpful discussions on SDS-PAGE and Western blotting and Palma Lower, University of California, Davis, for reviewing the manuscript.
This research was supported by a grant from the Center for Companion Animal Health (George and Phyllis Miller Feline Research Fund), School of Veterinary Medicine, University of California, Davis. Jane E. Koehler received support from the National Institutes of Health, R01 AI43703, and from the Universitywide AIDS Research Program.
REFERENCES
- 1.Abbott, R. C., B. B. Chomel, R. W. Kasten, K. A. Floyd-Hawkins, Y. Kikuchi, J. E. Koehler, and N. C. Pedersen. 1997. Experimental and natural infection with Bartonella henselae in domestic cats. Comp. Immunol. Microbiol. Infect. Dis. 20:41-51. [DOI] [PubMed] [Google Scholar]
- 2.Arvand, M., A. J. Klose, D. Schwartz-Porsche, H. Hahn, and C. Wendt. 2001. Genetic variability and prevalence of Bartonella henselae in cats in Berlin, Germany, and analysis of its genetic relatedness to a strain from Berlin that is pathogenic for humans. J. Clin. Microbiol. 39:743-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmans, A. M., C. M. de Jong, G. van Amerongen, C. S. Schot, and L. M. Schouls. 1997. Prevalence of Bartonella species in domestic cats in The Netherlands. J. Clin. Microbiol. 35:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmans, A. M., J. F. Schellekens, J. D. van Embden, and L. M. Schouls. 1996. Predominance of two Bartonella henselae variants among cat-scratch disease patients in The Netherlands. J. Clin. Microbiol. 34:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermond, D., H. J. Boulouis, R. Heller, G. Van Laere, H. Monteil, B. B. Chomel, A. Sander, C. Dehio, and Y. Piemont. Bartonella bovis Bermond et al. sp. nov. and B. capreoli sp. nov., isolated from European ruminants. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 6.Breitschwerdt, E. B., and D. L. Kordick. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, C. C., B. B. Chomel, R. W. Kasten, R. Heller, K. M. Kocan, H. Ueno, K. Yamamoto, V. C. Bleich, B. M. Pierce, B. J. Gonzales, P. K. Swift, W. M. Boyce, S. S. Jang, H. J. Boulouis, and Y. Piemont. 2000. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg. Infect. Dis. 6:306-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, C. C., R. W. Kasten, B. B. Chomel, D. C. Simpson, C. M. Hew, D. L. Kordick, R. Heller, Y. Piemont, and E. B. Breitschwerdt. 2000. Coyotes (Canis latrans) as the reservoir for a human pathogenic Bartonella sp.: molecular epidemiology of Bartonella vinsonii subsp berkhoffii infection in coyotes from central coastal California. J. Clin. Microbiol. 38:4193-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Childs, J. E., J. A. Rooney, J. L. Cooper, J. G. Olson, and R. L. Regnery. 1994. Epidemiologic observations on infection with Rochalimaea species among cats living in Baltimore, Md. J. Am. Vet. Med. Assoc. 204:1775-1778. [PubMed] [Google Scholar]
- 10.Chomel, B. B. 2000. Cat-scratch disease. Rev. Sci. Tech. Off. Int. Epizoot. 19:136-150. [DOI] [PubMed] [Google Scholar]
- 11.Chomel, B. B., R. C. Abbott, R. W. Kasten, K. A. Floyd-Hawkins, P. H. Kass, C. A. Glaser, N. C. Pedersen, and J. E. Koehler. 1995. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J. Clin. Microbiol. 33:2445-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomel, B. B., E. T. Carlos, R. W. Kasten, K. Yamamoto, C. C. Chang, R. S. Carlos, M. V. Abenes, and C. M. Pajares. 1999. Bartonella henselae and Bartonella clarridgeiae infection in domestic cats from The Philippines. Am. J. Trop. Med. Hyg. 60:593-597. [DOI] [PubMed] [Google Scholar]
- 13.Chomel, B. B., R. W. Kasten, K. Floyd-Hawkins, B. Chi, K. Yamamoto, J. Roberts-Wilson, A. N. Gurfield, R. C. Abbott, N. C. Pedersen, and J. E. Koehler. 1996. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 34:1952-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarridge, J. E., T. J. Raich, D. Pirwani, B. Simon, L. Tsai, M. C. Rodriguez-Barradas, R. Regnery, A. Zollo, D. C. Jones, and C. Rambo. 1995. Strategy to detect and identify Bartonella species in routine clinical laboratory yields Bartonella henselae from human immunodeficiency virus-positive patient and unique Bartonella strain from his cat. J. Clin. Microbiol. 33:2107-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drancourt, M., R. Birtles, G. Chaumentin, F. Vandenesch, J. Etienne, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441-443. [DOI] [PubMed] [Google Scholar]
- 16.Droz, S., B. H. Chi, E. Horn, A. G. Steigerwalt, A. M. Whitney, and D. J. Brenner. 1999. Bartonella koehlerae sp. nov., isolated from cats. J. Clin. Microbiol. 37:1117-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engbaek, K., and C. Koch. 1994. Immunoelectrophoretic characterization and cross-reactivity of Rochalimaea henselae, Rochalimaea quintana and Afipia felis. Acta Pathol. Microbiol. Immunol. Scand. 102:931-942. [PubMed] [Google Scholar]
- 18.Engbaek, K., L. O. Uttenthal, and C. Koch. 1997. Immunopurified extracellular Bartonella henselae antigen for detecting specific antibodies by enzyme immunoassay. Acta Pathol. Microbiol. Immunol. Scand. 105:941-950. [PubMed] [Google Scholar]
- 19.Foil, L., E. Andress, R. L. Freeland, A. F. Roy, R. Rutledge, P. C. Triche, and K. L. O'Reilly. 1998. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J. Med. Entomol. 35:625-628. [DOI] [PubMed] [Google Scholar]
- 20.Freeland, R. L., D. T. Scholl, K. R. Rohde, L. J. Shelton, and K. L. O'Reilly. 1999. Identification of Bartonella-specific immunodominant antigens recognized by the feline humoral immune system. Clin. Diagn. Lab. Immunol. 6:558-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene, C. E., M. McDermott, P. H. Jameson, C. L. Atkins, and A. M. Marks. 1996. Bartonella henselae infection in cats: evaluation during primary infection, treatment, and rechallenge infection. J. Clin. Microbiol. 34:1682-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guptill, L., L. Slater, C. C. Wu, L. T. Glickman, T. L. Lin, D. F. Welch, J. T. Crippen, and H. HogenEsch. 1999. Immune response of neonatal specific pathogen-free cats to experimental infection with Bartonella henselae. Vet. Immunol. Immunopathol. 71:233-243. [DOI] [PubMed] [Google Scholar]
- 23.Guptill, L., L. Slater, C. C. Wu, T. L. Lin, L. T. Glickman, D. F. Welch, and H. HogenEsch. 1997. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J. Infect. Dis. 176:206-216. [DOI] [PubMed] [Google Scholar]
- 24.Guptill, L., L. N. Slater, C. C. Wu, T. L. Lin, L. T. Glickman, D. F. Welch, J. Tobolski, and H. HogenEsch. 1998. Evidence of reproductive failure and lack of perinatal transmission of Bartonella henselae in experimentally infected cats. Vet. Immunol. Immunopathol. 65:177-189. [DOI] [PubMed] [Google Scholar]
- 25.Guptill, L., C. C. Wu, L. Glickman, J. Turek, L. Slater, and H. HogenEsch. 2000. Extracellular Bartonella henselae and artifactual intraerythrocytic pseudoinclusions in experimentally infected cats. Vet. Microbiol. 76:283-290. [DOI] [PubMed] [Google Scholar]
- 26.Gurfield, A. N., H. J. Boulouis, B. B. Chomel, R. Heller, R. W. Kasten, K. Yamamoto, and Y. Piemont. 1997. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J. Clin. Microbiol. 35:2120-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehler, J. E. 2000. Bartonella species, p. 339-353. In J. P. Nataro, M. J. Blaser, and S. Cunningham-Rundles (ed.), Persistent bacterial infections. ASM Press, Washington, D.C.
- 28.Koehler, J. E., C. A. Glaser, and J. W. Tappero. 1994. Rochalimaea henselae infection: a new zoonosis with the domestic cat as reservoir. JAMA 271:531-535. [DOI] [PubMed] [Google Scholar]
- 29.Kordick, D. L., and E. B. Breitschwerdt. 1998. Persistent infection of pets within a household with three Bartonella species. Emerg. Infect. Dis. 4:325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kordick, D. L., and E. B. Breitschwerdt. 1997. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am. J. Vet. Res. 58:492-497. [PubMed] [Google Scholar]
- 31.Kordick, D. L., T. T. Brown, K. Shin, and E. B. Breitschwerdt. 1999. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J. Clin. Microbiol. 37:1536-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kordick, D. L., E. J. Hilyard, T. L. Hadfield, K. H. Wilson, A. G. Steigerwalt, D. J. Brenner, and E. B. Breitschwerdt. 1997. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease). J. Clin. Microbiol. 35:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kordick, D. L., M. G. Papich, and E. B. Breitschwerdt. 1997. Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats. Antimicrob. Agents Chemother. 41:2448-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang, Z. X., B. La Scola, H. Lepidi, and D. Raoult. 2001. Production of Bartonella genus-specific monoclonal antibodies. Clin. Diagn. Lab. Immunol. 8:847-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang, Z. X., and D. Raoult. 2000. Species-specific monoclonal antibodies for rapid identification of Bartonella quintana. Clin. Diagn. Lab. Immunol. 7:21-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litwin, C. M., T. B. Martins, and H. R. Hill. 1997. Immunologic response to Bartonella henselae as determined by enzyme immunoassay and western blot analysis. Am. J. Clin. Pathol. 108:202-209. [DOI] [PubMed] [Google Scholar]
- 37.Mallqui, V., E. C. Speelmon, M. Veraastegui, C. Maguiana-Vargas, P. Pinell-Salles, R. Lavarello, J. Delgado, M. Kosek, S. Romero, Y. Arana, and R. H. Gilman. 2000. Sonicated diagnostic immunoblot for bartonellosis. Clin. Diagn. Lab. Immunol. 7:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margileth, A. M., and D. F. Baehren. 1998. Chest-wall abscess due to cat-scratch disease (CSD) in an adult with antibodies to Bartonella clarridgeiae: case report and review of the thoracopulmonary manifestations of CSD. Clin. Infect. Dis. 27:353-357. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama, S., R. W. Kasten, H. J. Boulouis, N. A. Gurfield, Y. Katsube, and B. B. Chomel. 2001. Genomic diversity of Bartonella henselae isolates from domestic cats from Japan, the USA and France by pulsed-field gel electrophoresis. Vet. Microbiol. 79:337-349. [DOI] [PubMed] [Google Scholar]
- 40.Mikolajczyk, M. G., and K. L. O'Reilly. 2000. Clinical disease in kittens inoculated with a pathogenic strain of Bartonella henselae. Am. J. Vet. Res. 61:375-379. [DOI] [PubMed] [Google Scholar]
- 41.O'Reilly, K. L., R. W. Bauer, R. L. Freeland, L. D. Foil, K. J. Hughes, K. R. Rohde, A. F. Roy, R. W. Stout, and P. C. Triche. 1999. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU16). Infect. Immun. 67:3066-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regnery, R. L., J. A. Rooney, A. M. Johnson, S. L. Nesby, P. Manzewitsch, K. Beaver, and J. G. Olson. 1996. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am. J. Vet. Res. 57:1714-1719. [PubMed] [Google Scholar]
- 43.Roux, V., and D. Raoult. 1995. Inter- and intraspecies identification of Bartonella (Rochalimaea) species. J. Clin. Microbiol. 33:1573-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sander, A., C. Buhler, K. Pelz, E. von Cramm, and W. Bredt. 1997. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J. Clin. Microbiol. 35:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sander, A., M. Ruess, S. Bereswill, M. Schuppler, and B. Steinbrueckner. 1998. Comparison of different DNA fingerprinting techniques for molecular typing of Bartonella henselae isolates. J. Clin. Microbiol. 36:2973-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sander, A., A. Zagrosek, W. Bredt, E. Schiltz, Y. Piemont, C. Lanz, and C. Dehio. 2000. Characterization of Bartonella clarridgeiae flagellin (FlaA) and detection of antiflagellin antibodies in patients with lymphadenopathy. J. Clin. Microbiol. 38:2943-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slater, L. N., D. F. Welch, D. Hensel, and D. W. Coody. 1990. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N. Engl. J. Med. 323:1587-1593. [DOI] [PubMed] [Google Scholar]
- 48.Tappero, J. W., J. Mohle-Boetani, J. E. Koehler, B. Swaminathan, T. G. Berger, P. E. LeBoit, L. L. Smith, J. D. Wenger, R. W. Pinner, C. A. Kemper, and A. L. Reingold. 1993. The epidemiology of bacillary angiomatosis and bacillary peliosis. JAMA 269:770-775. [PubMed] [Google Scholar]
- 49.Tsuneoka, H., R. Fujii, K. Fujisawa, H. Iino, C. Isida, K. Murakami, and M. Tsukahara. 2000. Clinical evaluation of commercial serological test for Bartonella infection. J. Jpn Assoc. Infect. Dis. 74:387-391. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto, K., B. B. Chomel, R. W. Kasten, C. C. Chang, T. Tseggai, P. R. Decker, M. Mackowiak, K. A. Floyd-Hawkins, and N. C. Pedersen. 1998. Homologous protection but lack of heterologous-protection by various species and types of Bartonella in specific pathogen-free cats. Vet. Immunol. Immunopathol. 65:191-204. [DOI] [PubMed] [Google Scholar]
- 51.Zangwill, K. M., D. H. Hamilton, B. A. Perkins, R. L. Regnery, B. D. Plikaytis, J. L. Hadler, M. L. Cartter, and J. D. Wenger. 1993. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N. Engl. J. Med. 329:8-13. [DOI] [PubMed] [Google Scholar]