Abstract
Detection of vector-borne pathogens is necessary for investigation of their association with vertebrate and invertebrate hosts. The ability to detect Ehrlichia spp. within individual experimentally infected ticks would be valuable for studies to evaluate the relative competence of different vector species and transmission scenarios. The purpose of this study was to develop a sensitive PCR assay based on oligonucleotide sequences from the unique Ehrlichia canis gene, p30, to facilitate studies that require monitoring this pathogen in canine and tick hosts during experimental transmission. Homologous sequences for Ehrlichia chaffeensis p28 were compared to sequences of primers derived from a sequence conserved among E. canis isolates. Criteria for primer selection included annealing scores, identity of the primers to homologous E. chaffeensis sequences, and the availability of similarly optimal primers that were nested within the target template sequence. The p30-based assay was at least 100-fold more sensitive than a previously reported nested 16S ribosomal DNA (rDNA)-based assay and did not amplify the 200-bp target amplicon from E. chaffeensis, the human granulocytic ehrlichiosis agent, or Ehrlichia muris DNA. The assay was used to detect E. canis in canine carrier blood and in experimentally infected Rhipicephalus sanguineus ticks. Optimized procedures for preparing tissues from these hosts for PCR assay are described. Our results indicated that this p30-based PCR assay will be useful for experimental investigations, that it has potential as a routine test, and that this approach to PCR assay design may be applicable to other pathogens that occur at low levels in affected hosts.
Canine monocytic ehrlichiosis (CME), sometimes known as tropical canine pancytopenia, is a cosmopolitan tick-borne disease of dogs that is primarily caused by the rickettsia Ehrlichia canis (10, 16-18, 31). This parasite invades and develops in monocytes and macrophages of dogs, resulting in leukopenia, thrombocytopenia, fever, depression, and anorexia, and this species has been reported in association with at least one human case (26). Three other ehrlichial organisms, E. chaffeensis, the human granulocytic ehrlichiosis (HGE) agent, and Ehrlichia ewingii, are zoonotic in the United States (5, 9), and several reports suggest that these organisms also occur naturally in dogs and deer (3, 4, 6-8, 11, 12, 15). Ixodid ticks transmit most of the Ehrlichia spp. characterized to date and appear to be the biological vectors of E. chaffeensis, the HGE agent, and E. ewingii. E. canis and E. chaffeensis are closely related, based on 16S rRNA gene (16S ribosomal DNA [rDNA]) sequences and antigenic cross-reactivity (2). These observations indicate that tick transmission and canine infection studies with E. canis can serve as a model for further work with E. chaffeensis in dogs and humans and lead to better understanding of both pathogens.
A sensitive, specific PCR assay would be useful for detection of Ehrlichia spp. that typically occur at low levels in peripheral blood and are often undetectable with conventional light microscopy. Serologic methods can be effective for detection of antibodies to Ehrlichia spp. in vertebrate hosts, but seroconversion is only indicative of exposure and not the presence of infection. In addition, a PCR assay could be applied to the detection of pathogens in ticks (32). A 16S rDNA-based PCR assay was previously developed for analysis of low levels of E. canis in canine blood after antibiotic therapy (36), but, in our hands, this assay did not provide the desired level of sensitivity for detection of E. canis in individual experimentally infected ticks. This may have resulted from the limited selection of unique primers that are specific to a highly conserved E. canis gene. Conversely, PCR assays based on species-specific DNA sequences have met with success. For example, PCR has been utilized for amplification of msp-1β and msp5 sequences from the ehrlichial parasite Anaplasma marginale and resulted in highly sensitive and specific assays for that parasite (14, 33, 35). Thus, another approach to a PCR assay for E. canis would be to utilize a gene sequence that is unique to Ehrlichia spp. to ensure specificity and to allow selection of optimally sensitive primer sequences.
The E. canis p30 outer membrane protein multigene family consists of tandem copies with orthologous yet species-specific genes found in E. canis and E. chaffeensis (21-25, 27, 37). The purpose of this work was to develop a PCR assay based on amplification of E. canis p30 to facilitate sensitive detection of the pathogen in experimentally exposed tick and canine hosts.
MATERIALS AND METHODS
Source of Ehrlichia spp.
Heparinized blood from a donor dog infected with E. canis (Ebony isolate) (18) served as a source of buffy coat for PCR optimization and as an intravenous (i.v.) inoculum for two colony-reared Beagle dogs that were seronegative for exposure to the pathogen by indirect fluorescent antibody assay. All vertebrate animals were cared for in accordance with a protocol approved by and on file with The Ohio State University Institutional Laboratory Animal Care and Use Committee. E. muris, E. chaffeensis, and the HGE agent were cultivated as previously described (28, 29, 36).
Primer design.
Seven E. canis p30 and five homologous E. chaffeensis p28 open reading frame (ORF) sequences, which are reported elsewhere (21, 37), were analyzed to identify candidate primer sequences. Development of a sensitive assay for experimental conditions was the highest priority regarding primer design. Specific amplification of E. canis DNA for diagnosis and for field studies in which infection with mixed Ehrlichia spp. might exist was beyond our current objectives, but was still of secondary importance.
Prospective primer sequences (Prime, Wisconsin Package V. 10.2; Genetics Computer Group [GCG], Madison, Wis.) were analyzed by multiple sequence alignment (Pileup, GCG V. 10.2, and Boxshade V. 3.2) (http://www.ch.embnet.org/software) with seven E. canis and five E. chaffeensis isolate ORF sequences to determine the identity of the primers to the corresponding sequences of each p28 group. Both E. canis p30 multiple gene clusters and the E. chaffeensis p28 multiple gene cluster were searched for sequences homologous to primers chosen for experimental evaluation with PCR (Findpatterns, GCG V.10.2). It was expected that primers that would specifically amplify the target sequence of multiple E. canis isolates, but not the E. chaffeensis isolates, would likely be species universal and species specific for E. canis, because E. chaffeensis is one of the most closely related known species to E. canis (1).
Optimization of PCR assays.
E. canis DNA template was isolated from the buffy coat of an experimentally infected dog by using DNAzol (Molecular Research Center, Cincinnati, Ohio) according to the manufacturer's instructions. The samples containing E. canis DNA were diluted empirically throughout the optimization process. PCR was performed with a Perkin-Elmer 2400 thermal cycler. Master mixes, made with the PE Biosystems Reagents (Foster City, Calif.), were divided into 50- or 25-μl final reaction volumes containing PCR Gold buffer, 0.8 mM deoxynucleoside triphosphate (dNTP) mix, and specified amounts of MgCl2, primers, Amplitaq-Gold DNA polymerase, and 10% (vol/vol) template. The reaction profile, except for stated exceptions, consisted of 95°C for 10 min; followed by 35 cycles of 94°C for 1.0 min, 60°C for 0.5 min, and 72°C for 0.5 min; followed by a final extension at 72°C for 7.0 min.
The following PCR parameters were progressively optimized: (i) annealing temperature (37, 50, 55, 60, 65, and 70°C with 1.5 mM MgCl2, 0.5 μM primers, and 0.025 U of Amplitaq-Gold per μl), (ii) MgCl2 concentration (1.0 to 4.0 mM MgCl2 in 0.5 mM increments), (iii) primer concentration (0.1 to 1.0 μM in increments of 0.1 μM), (iv) Amplitaq-Gold concentration (0.01 to 0.1 U/μl in 0.01-U/μl increments), and (v) cycle number (25 to 65 cycles in 5-cycle increments).
The PCR product (20 μl) was added to 5 μl of loading buffer (40% [wt/vol] sucrose, 89 mM Tris, 89 mM boric acid, 2 mM EDTA) and separated by electrophoresis on a 1.5% (wt/vol) agarose gel with 0.5 μg of ethidium bromide per ml in 1× TBE (89 mM Tris, 89 mM boric acid, 2 mM EDTA) at 60 to 80 V for 1 to 1.5 h. A 100-bp ladder (Life Technologies, Rockville, Md.) was used as the molecular weight standard. DNA bands were visualized with UV light and documented with an Alpha Innotech 2000 gel imaging system.
To confirm that the 200-bp amplicon associated with E. canis infection originated from the target DNA sequence, several p30-based nested PCR assays of buffy coat DNA from an E. canis-infected dog were pooled, and the amplicons were isolated with the QIAquik PCR purification kit (Qiagen, Valencia, Calif.) according to the manufacturer's instructions. The purified amplicon was submitted to The Ohio State University Neurobiotechnology DNA Sequencing Facility for cycle sequencing in the presence of the primer ECAF1384S.
Specificity and sensitivity.
The sensitivity of this p30-based assay was compared to that of the previously reported 16S rDNA-based assay (36). The buffy coat fraction was removed from 5 ml of heparinized blood from an experimentally infected dog during acute CME and subjected to DNA isolation with DNAzol (Molecular Research Center) according to the manufacturer's instructions. A 10-fold dilution series of the buffy coat DNA was then tested with both the p30- and the 16S rDNA-based PCR assays. Specificity of the p30-based assay was tested by attempts to amplify 50-ng samples of DNA isolated from E. muris, E. chaffeensis, and the HGE agent in experimentally infected host cells.
Preparation of canine blood for PCR assay.
For detection of E. canis during acute CME (dogs W11 and W90), blood was collected in heparinized tubes and prepared with DNAzol BD (Molecular Research Center, Cincinnati, Ohio) according to the manufacturer's instructions.
An optimized template preparation procedure was used for assay of blood from a subclinical carrier (dog A72) of E. canis (Ebony isolate). Buffy coats were isolated from heparinized whole blood after centrifugation at 1,000 × g for 20 min, and transferred to 1.5-ml microcentrifuge tubes. Erythrocytes remaining in the buffy coat were lysed with two volumes of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA), and hemoglobin was removed by washing pelleted cells (13,000 × g for 1 min) three times in TE. The final cell suspension was in 400 μl of RPMI 1640 containing 100 μg of proteinase K per ml and 0.45% (vol/vol) NP-40 and 0.45% (vol/vol) Tween 20, and the protein was digested for 1 to 2 h at 55°C. Digests were extracted one time each with equal volumes of buffer-saturated phenol (pH >7.5) (Life Technologies), phenol-chloroform-isoamyl alcohol (25:24:1), and chloroform-isoamyl alcohol (24:1). DNA was precipitated by adding 1/10 volume of 3 M sodium acetate and 2.5 volumes of absolute ethanol (−20°C). The DNA samples were centrifuged at 14,000 × g for 20 min at 4°C, allowed to air dry in a sterile, bleach-treated cabinet, and resuspended in 25 μl of high-performance liquid chromatography (HPLC)-grade H2O.
Preparation of ticks for PCR assay.
A seronegative dog (W90) was infected by i.v. injection with blood infected with the Ebony isolate of E. canis (provided by S. A. Ewing, Oklahoma State University), and once the dog displayed signs of patency, Rhipicephalus sanguineus nymphs (purchased from the Oklahoma State University Medical Entomology Laboratory) were allowed to acquisition feed in tick feeding cells until engorged. Engorged nymphs were held at room temperature at 95 to100% relative humidity with a 12-h:12-h photoperiod. After they had molted into adults, 10 male ticks were divided into two groups, and half were incubated for 80 h at room temperature or 37°C prior to bisection under aseptic conditions with flame-sterilized forceps and razor blades. Cohorts of these trans-stadially infected R. sanguineus ticks (30 pairs of adult-stage males and females) were allowed to transmission feed on dog A72 until all of the females had engorged and detached from the host, to confirm infection and transmissibility of E. canis by these ticks.
Each individual tick half was incubated overnight at 37°C in 50 μl of digestion buffer containing 100 μg of proteinase K per ml, 0.45% NP-40, and 0.45% Tween 20 in RPMI 1640, and then overlaid with mineral oil (Perkin-Elmer), heated to 105°C for 10 min, and placed on ice ≥1 min prior to further use. Aliquots (15 μl) were removed from each digested tick sample and then pooled according to the preincubation temperature group. Another aliquot (25 μl) was removed from each digest pool for PCR assay, and the remainder was subjected to protein extraction and DNA precipitation. Protein was extracted one time each with equal volumes of buffer-saturated phenol (pH >7.5) (Life Technologies), phenol-chloroform-isoamyl alcohol (25:24:1), and chloroform-isoamyl alcohol (24:1). DNA was precipitated by adding 1/10 volume of 3 M sodium acetate and 2.5 volumes of absolute ethanol (−20°C), followed by storage overnight at −20°C. The DNA samples were centrifuged at 14,000 × g for 20 min at 4°C, allowed to air dry in a sterile, bleach-treated cabinet, and resuspended in 25 μl of HPLC-grade H2O.
Nucleotide sequence accession number.
E. canis p30 and the homologous E. chaffeensis p28 ORF sequences were acquired from the GenBank database. Sequences for the Jake (accession no. AF082744), Florida (accession no. AF082750), Fuzzy (accession no. AF082749), DJ (accession no. AF082748), Demon (accession no. AF082747), Oklahoma (accession no. AF082746), and Louisiana (accession no. AF082745) isolates of E. canis and the St. Vincent (accession no. AF077735), Sapulpa (accession no. AF077734), Jax (accession no. AF077733), 91HE17 (accession no. AF077732), and Arkansas (accession no. AF068234) isolates of E. chaffeensis were used in this study. Multiple gene clusters for E. canis p30 (accession no. AF078553 and AF324792) and E. chaffeensis p28 (accession no. AF021338) were used to search for additional sequences complementary to those of the primers chosen for experimental evaluation.
RESULTS
Primer design.
As previously reported, E. canis p30 ORF sequences of the same paralog from seven isolates tested were identical (21), while five E. chaffeensis p28 isolate sequences of the orthologous gene fell into three groups that were all different from the E. canis homologue (37). Thirteen primer sets predicted to provide optimum amplification of the E. canis p30 ORF were identified for further analysis (Table 1). The mean identity of each primer set to the corresponding E. chaffeensis p28 sequence was determined, and the products of identities and total annealing scores were used to rank potential primer sets for use in an E. canis PCR assay (Table 2). The two top-ranked primer sets were synthesized for further development, and primer set 12 was synthesized because it was the highest-ranked set that flanked sets 1 and 2. Primers chosen for experimental evaluation were not identical to any other sequences within E. canis p30 or E. chaffeensis p28 multigene clusters, indicating that a sequence from a single gene is probably targeted for amplification with these primers. The forward primer sequences ranged from 21 to 25 nucleotides in length and were all located between nucleotides 278 and 412 of the ORF sense strand. The reverse primer sequences ranged from 20 to 23 nucleotides in length and comprised inverse complements of sequences that were all located between nucleotides 465 and 597 of the ORF sense strand.
TABLE 1.
Oligonucleotide sequences of E. canis p30 primer sets
| Primer set | Primer (sequence)
|
|
|---|---|---|
| Forward | Reverse | |
| 1 | ECA30-384S (ATAAACACGCTGACTTTACTGTTCC) | ECA30-583A (GTGATGAGATAGAGCGCAGTACC) |
| 2 | ECA30-387S (AACACGCTGACTTTACTGTTCC) | ECA30-597A (ATGGCTGCCGATGTGTGATG) |
| 3 | ECA30-390S (ACGCTGACTTTACTGTTCCAAAC) | ECA30-597A |
| 4 | ECA30-351S (AACATGATTGGGATGGAAGTC) | ECA30-591A (GCCGATGTGTGATGAGATAG) |
| 5 | ECA30-350S (AAACATGATTGGGATGGAAGTC) | ECA30-591bA (GCCGATGTGTGATGAGATAGAG) |
| 6 | ECA30-356S (GATTGGGATGGAAGTCCAATAC) | ECA30-591A |
| 7 | ECA30-356S | ECA30-591bA |
| 8 | ECA30-388S (ACACGCTGACTTTACTGTTCCAAAC) | ECA30-597bA (ATGGCTGCCGATGTGTGATGAG) |
| 9 | ECA30-282S (GTGTCTCACATTTTGGTAGCTTCTC) | ECA30-486A (CTTGGGCCACCCATTGAGTAAC) |
| 10 | ECA30-356S | ECA30-589A (CGATGTGTGATGAGATAGAGC) |
| 11 | ECA30-355S (TGATTGGGATGGAAGTCCAATAC) | ECA30-589bA (CGATGTGTGATGAGATAGAGCG) |
| 12 | ECA30-353S (CATGATTGGGATGGAAGTCCAATAC) | ECA30-597A |
| 13 | ECA30-278S (CCAAGTGTCTCACATTTTGGTAGC) | ECA30-484A (TGGGCCACCCATTGAGTAAC) |
TABLE 2.
Ranks of E. canis p30 primer sets with predicted annealing and specificity values
| Primer set | Identity scores vs E. chaffeensisa
|
Total annealing scorec | Rankd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group I
|
Group II
|
Group III
|
Meanb | |||||||
| Forward | Reverse | Forward | Reverse | Forward | Reverse | |||||
| 1 | 0.64 | 0.74 | 0.48 | 0.87 | 0.56 | 0.78 | 0.68 | 52 | 2 | |
| 2 | 0.59 | 0.45 | 0.55 | 0.65 | 0.55 | 0.60 | 0.56 | 57 | 1 | |
| 3 | 0.74 | 0.45 | 0.61 | 0.65 | 0.70 | 0.60 | 0.62 | 58 | 3 | |
| 4 | 0.81 | 0.55 | 0.86 | 0.75 | 0.71 | 0.70 | 0.73 | 68 | 5 | |
| 5 | 0.82 | 0.55 | 0.82 | 0.77 | 0.73 | 0.73 | 0.74 | 64 | 6 | |
| 6 | 0.91 | 0.55 | 0.86 | 0.75 | 0.77 | 0.70 | 0.76 | 64 | 7 | |
| 7 | 0.91 | 0.55 | 0.86 | 0.77 | 0.77 | 0.73 | 0.76 | 65 | 9 | |
| 8 | 0.68 | 0.45 | 0.60 | 0.68 | 0.68 | 0.64 | 0.62 | 66 | 4 | |
| 9 | 0.64 | 0.86 | 0.64 | 0.95 | 0.72 | 0.91 | 0.79 | 67 | 12 | |
| 10 | 0.91 | 0.62 | 0.86 | 0.81 | 0.77 | 0.71 | 0.78 | 67 | 10 | |
| 11 | 0.87 | 0.64 | 0.83 | 0.82 | 0.74 | 0.73 | 0.77 | 68 | 11 | |
| 12 | 0.88 | 0.45 | 0.84 | 0.65 | 0.80 | 0.70 | 0.72 | 68 | 8 | |
| 13 | 0.67 | 0.85 | 0.67 | 0.95 | 0.71 | 0.90 | 0.79 | 68 | 13 | |
Identity score = no. of oligonucleotide bases identical to aligned template/total no. of oligonucleotide bases.
Mean identity score for both primers of each set to all three E. chaffeensis groups.
Determined by the GCG (version 10.2) Prime program. The sum of annealing scores for primer secondary structure, nonspecific primer binding to the template sequence and self-complementarity of individual primers and complementarity between primer pairs is shown. The lowest scores indicate the primer sets with least complementarity to sequences other than the target binding site.
Rank was determined by the product of the annealing score and the mean identity score: lower values have higher rank.
Optimization of the p30-based PCR.
Initial optimization experiments with primer set 2 resulted in the amplification of multiple bands; thus, this primer set was omitted, and the remaining trials were focused on sets 1 and 12. For the primary reaction with set 12, the optimum PCR conditions were determined to be 1.5 mM MgCl2, 0.2 μM each primer, 0.04 U of Amplitaq-Gold per μl, and, after enzyme activation at 95°C for 10 min, 55 cycles at denaturation, annealing, and extension temperatures of 94, 65, and 72°C, respectively, for 30 s each.
For the secondary or nested PCR with internal primer set 1, optimum reaction conditions were determined to be 2.5 mM MgCl2, 0.5 μM each primer, 0.03 U of Amplitaq-Gold per μl, 10% (vol/vol) of the appropriate primary reaction with primer set 12, and 40 cycles at denaturation, annealing, and extension temperatures of 94, 60, and 72°C, respectively, for 30 s each.
Amplification of the 200-bp target sequence by nested PCR of E. canis-infected peripheral blood from dogs with acute experimental CME.
As expected, a 200-bp band was amplified from DNA prepared from blood samples collected from three dogs during acute experimental CME (Fig. 1). This band was not observed from blood collected prior to infection of the two dogs assayed during experimentally induced acute CME. The DNA sequence of this amplicon was found to be identical to the 200-bp p30 target sequence and the reverse complement of ECAR1583A (Fig. 2).
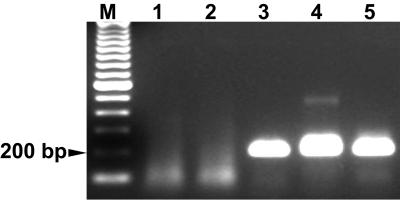
FIG. 1.
PCR assay of peripheral blood from dogs during acute CME. Three dogs were tested with the nested p30-based PCR described in the text. Buffy coat DNA preparations were assayed from two dogs prior to (lanes 1 and 2) and 21 days after (lanes 3 and 4) inoculation with E. canis Ebony isolate-infected blood from an experimentally infected dog (lane 5). The molecular size standard (M) is a 100-bp ladder.
FIG. 2.
Confirmation of the E. canis nested target amplicon sequence. Multiple sequence alignment of the amplicon derived from nested p30-based PCR of DNA from E. canis (Ebony isolate), the E. canis p30 target DNA sequence (Jake isolate), and primer set 1 (ECA30-384S and ECA30-583A). The DNA sequence for ECA30 -583A is the reverse complement of the oligonucleotide shown. The sequences were aligned and compared by using the Pileup (GCG, version 10.2) and Boxshade programs. White letters surrounded by a black box represent bases that are identical in each sequence of the alignment.
Sensitivity and specificity of the p30-based PCR assays for E. canis.
The relative sensitivities of the p30-based and 16S rDNA-based PCR assays were compared through amplification of a 10-fold dilution series of DNA isolated from buffy coats collected from an experimentally infected dog during acute CME (Fig. 3). The primary and nested p30-based assays amplified target template diluted as much as 1 × 10−2 and 1 × 10−3, respectively, while the 16S rDNA-based assay only amplified undiluted template. Thus, in our hands, the single-step and nested p30-based PCR assays for E. canis were more sensitive than the nested 16S rDNA-based assay by factors of 100 and 1,000, respectively.
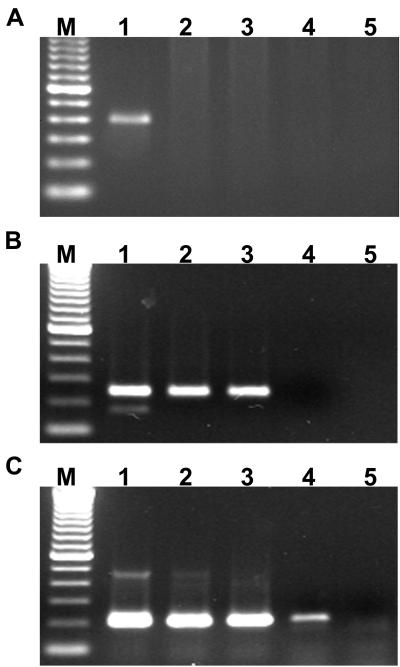
FIG. 3.
Sensitivity of the p30-based nested PCR assay. The nested 16S rDNA (A) and primary (B) and nested (C) p30 PCR assays were compared to determine relative sensitivities. Buffy coat DNA was isolated from an E. canis carrier dog and subjected to a dilution series for simultaneous amplification with the two assays. Lanes 1 to 5, respectively, represent assays of the same template dilutions of 1 × 100, 1 × 10−1, 1 × 10−2, 1 × 10−3, and 1 × 10−4 for each panel.
The specificity of the E. canis p30-based assay was tested with a DNA template isolated from experimentally cultivated E. chaffeensis, HGE agent, and E. muris. Among the isolates tested, a robust 200-bp target amplicon was only amplified from E. canis, indicating that this p30-based PCR assay is species specific (Fig. 4).
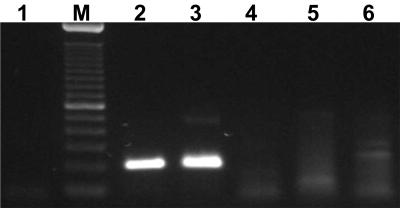
FIG. 4.
Specificity of the p30-based nested PCR assay. Lane 1 is the template-free control, lanes 2 and 3 represent amplification of 50 ng of E. canis Ebony and Oklahoma isolates, respectively, and lanes 4 to 6 represent assays of 50 ng of DNA isolated from E. chaffeensis, HGE agent, and E. muris, respectively. The 100-bp ladder (M) serves as the molecular size standard.
Detection of E. canis in canine blood.
One of our goals is to better understand the interactions between ehrlichial organisms and their vertebrate and invertebrate hosts. The ability to detect rickettsiae directly within vertebrate blood and tissue samples as a test for the presence of infection is part of this goal. First, we determined the best procedure for blood preparation for the p30-based PCR assay for E. canis. Venous blood was collected with heparin from an experimentally infected E. canis carrier, and equal aliquots were subjected to different protocols for the preparation of template for PCR. Inhibition of PCR by hemoglobin is well known; thus, removal of hemoglobin from the blood sample is a major concern of any procedure in which PCR is used to assay samples. Although commercially available chemical and spin column kits designed for DNA isolation provided some positive results with buffy coat and whole-blood samples, these results were inconsistent in our hands. The only procedure that resulted in consistent, reliable amplification of the 200-bp target amplicon was buffy coat isolation followed in sequence by erythrolysis, hemoglobin removal, protein digestion, and phenol-chloroform protein extraction and ethanol precipitation of DNA.
The utility of this buffy coat assay was also tested for detection of E. canis in canine blood during the persistent, cyclic rickettsemia associated with postacute and carrier-stage CME. Blood samples were randomly collected for over 2 years from dog A72, which was infected with E. canis by tick transmission. The buffy coat DNA isolation method was then used in combination with the p30-based PCR assay with samples from days 33 through 867 post-tick attachment (Fig. 5). The single-step PCR assay detected E. canis in samples from post-tick attachment days 33, 142, and 867, but E. canis was only detected in all of the samples when the nested PCR assay was used. Amplicons were not observed in the preinfection buffy coat sample or the template-free negative controls for either PCR assay.
FIG. 5.
Time course PCR assays of peripheral blood from a dog infected by tick transmission. Dog A72 was experimentally infected by transmission feeding of adult-stage R. sanguineus ticks that were infected with E. canis when they fed as nymphs. Buffy coats were prepared from peripheral blood collected and stored at −80°C until DNA isolations were performed as described in the text. PCR assays with the primary primer set (A) and the nested PCR assay (B) are shown. For each panel, lane 1 represents a buffy coat collected prior to exposure, and lanes 2 to 8 represent samples collected at 33, 142, 295, 427, 617, 733, and 867 days after tick attachment, respectively. The molecular size standard (M) is a 100-bp ladder.
Detection of E. canis in R. sanguineus ticks.
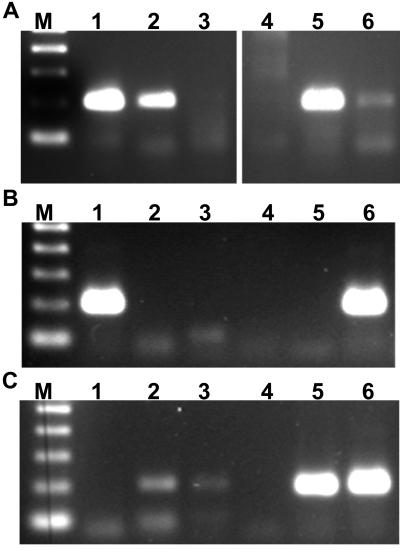
Reliable detection of Ehrlichia spp. in individual ticks is another objective that must be achieved for further characterization of the transmission cycle of ehrlichial parasites. The purpose of this experiment was to compare the efficacies of different template preparation protocols for the p30-based assay for E. canis in R. sanguineus. Different template preparation methods were compared on pooled tick samples that had been incubated for 80 h at room temperature or 37°C prior to bisection. One-half of each bisected tick was subjected to protein digestion in the presence of nonionic detergents, and then the individual samples were pooled, and digestion with proteinase K alone was compared to subsequent phenol extraction of proteins followed by ethanol precipitation of DNA. Protein digestion alone appeared to be the best method for assaying pools of tick tissues (Fig. 6A). Furthermore, assay of both individual and pooled samples indicated that incubation of ticks at 37°C resulted in more sensitive detection of E. canis. Pooled samples of ticks incubated at the higher temperature resulted in a brighter PCR product. In addition, E. canis was detected in four of five individual experimentally infected ticks after incubation for 80 h at 37°C, compared to one of five ticks incubated at room temperature (Fig. 6B). Companion ticks successfully transmitted E. canis to dog A72, which tested PCR positive for infection (Fig. 5).
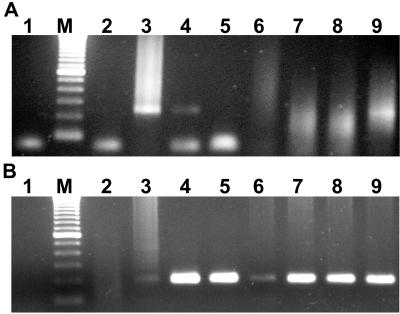
FIG. 6.
Detection of E. canis in experimentally infected ticks. R. sanguineus adult male ticks were infected as nymphs and incubated at RT or 37°C prior to aseptic bisection, followed by digestion of individual tick halves with proteinase K in the presence of nonionic detergents. (A) Pooled digests of ticks subjected to proteinase K digestion alone (lanes 2 and 5) or to DNA purification (lanes 3 and 6). Lanes 1 and 4 represent infected canine buffy coat DNA and a template-free control, respectively. (B) Individual ticks incubated at RT (lanes 2 to 6) Lane 1 represents infected canine buffy coat DNA as a positive control. (C) Individual ticks (lanes 2 to 6) incubated at 37°C prior to bisection and proteinase K digestion. Lane 1 represents a template-free control.
DISCUSSION
Detection of pathogens in invertebrate and vertebrate hosts is an important tool for experimental study of arthropod-borne diseases and is critical to understanding the transmission of these pathogens in nature. The results of this study demonstrate the utility of basing the design of PCR assays on genes that are unique to the target organism rather than those that are widely conserved among bacterial species. This work represents, to our knowledge, the first demonstration of an Ehrlichia species in individual experimentally infected ticks with a PCR assay.
A nested PCR assay for E. canis based on p30 was developed, and the 200-bp amplicon was confirmed as the target by cycle sequencing and its amplification only from blood of experimentally infected dogs. The optimum numbers of cycles for both reactions, particularly the primary reaction, were greater than expected. One explanation for this finding may be the enzyme used, Amplitaq-Gold, which requires heat activation prior to amplification. It is conceivable that a portion of this enzyme remains inactive until the amplification cycles are under way; thus, additional active enzyme becomes available in the course of the reaction, increasing the number of cycles required for optimal PCR. The sensitivity and specificity of this assay were also analyzed to determine its potential value under experimental as well as field conditions.
To determine the relative sensitivity of the p30-based PCR assay, both the p30-based and a previously reported 16S rDNA-based PCR assay were used to amplify a 10-fold dilution series of DNA isolated from buffy coats collected from an experimentally infected dog during acute CME. The diploid mammalian genome is approximately 6,000-fold greater in size than the ehrlichial genome (30), and potential contamination with host cell DNA makes estimation of ehrlichial DNA quantities in canine buffy coat samples impractical for our purposes. Therefore, the relative sensitivities of the primary and nested p30-based assays were determined by amplifying equal amounts of the same template dilutions. Both the primary and nested p30-based assays were more sensitive than the nested 16S rDNA-based assay, but in our hands, only the nested PCR consistently detected E. canis in carrier blood. A single-step PCR assay would be preferred as a diagnostic test, because it would be more convenient and would be less apt to provide false-positive results due to carryover contamination. However, we found that the greater sensitivity of the nested PCR assay is still needed for experimental studies. Addition of a more sensitive means of detecting amplicons, such as inclusion of biotinylated primers or a probe hybridization step (19, 20), may improve the sensitivity of a single-step p30-based PCR assay to levels that are comparable to those of nested PCR. More recently, reverse transcription (RT)-PCR amplification of 16S rRNA was found to be 100-fold more sensitive for detection of E. chaffeensis than the 16S rDNA-based PCR with the same primers (13). A similar approach could prove useful for increasing the sensitivity of the p30- or 16S rDNA-based assays for E. canis and may circumvent the need for a nested PCR assay. Primer sets 1 and 12 occur only once in the p30 multigene clusters. Thus, the 100- and 1,000-fold greater sensitivities of the p30-based primary and nested assays, respectively, probably resulted from selection of optimal primer and/or amplicon sequences from the p30 target sequence, which is also divergent from the homologous p28 sequence of E. chaffeensis. Interestingly, all of the optimal forward and reverse primer sequences were located within a region of 135 bp or less, flanking a common amplicon target. These observations indicate that development of PCR assays based on species-specific target sequences is a valid alternative to the design of unique primers for widely conserved sequences. Sensitivity was the primary concern for a PCR assay being applied under experimental conditions, but specificity is also vitally important if this assay is to be useful under field conditions or under experimental conditions that involve mixed infections.
It was expected that primers divergent from E. chaffeensis were likely to be specific for E. canis, but the potential for amplification of other copies of the omp-1 gene family in other Ehrlichia spp. could not be ignored. Thus, DNA extracted from E. chaffeensis and E. muris, the two known species most closely related to E. canis (based on 16S rDNA sequence homology), in addition to the HGE agent were used to test the specificity of this assay. The robust 200-bp band was only observed with the E. canis template. However, specificity trials with additional hemoparasite species, as well as adaptability of these primers to more geographically diverse E. canis isolates, would be advisable prior to the application of this test to field studies.
Detection of E. canis in ticks with this newly developed assay will be useful for monitoring experimental transmission efforts, conducting epidemiological investigations, identifying infected vectors, and possibly identifying vertebrate hosts through xenodiagnosis. Several methods have been developed for detecting pathogens within vectors, but the advantages of PCR and RT-PCR include improved sensitivity, adjustable specificity, reduction of sample size, and often relatively simple sample preparation procedures. In addition, there are technical advantages of assaying tick vectors for rickettsiae. First, hemoglobin from the host bloodmeal does not appear to be a major problem with regard to inhibition of the PCR assay (33-35). Also, because invertebrate vectors usually are biological hosts for these pathogens, ticks can be used to biologically amplify the assay target organism prior to attempting PCR. The latter advantage also allows manipulation (e.g., incubation of suspected tick hosts at 37°C to stimulate proliferation of the pathogens) for greater sensitivity and enhances the potential for xenodiagnosis of suspected vertebrate reservoirs. The p30-based PCR assay is expected to be a valuable tool for experimental and field studies of E. canis in both vertebrate and invertebrate hosts. Similar approaches to design of PCR assays for other Ehrlichia spp. are under development.
Acknowledgments
This work was supported by a grant from College of Veterinary Medicine Canine Research Fund and the Department of Veterinary Preventive Medicine at The Ohio State University.
We thank Robert Hamlin of The Ohio State University Department of Veterinary Biosciences for support.
REFERENCES
- 1.Allsopp, B. A., M. T. Allsopp, J. H. Du Plessis, and E. S. Visser. 1996. Uncharacterized Ehrlichia spp. may contribute to clinical heartwater. Ann. N. Y. Acad. Sci. 791:17-23. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, B. E., J. E. Dawson, D. C. Jones, and K. H. Wilson. 1991. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J. Clin. Microbiol. 29:2838-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belongia, E. A., K. D. Reed, P. D. Mitchell, C. P. Kolbert, D. H. Persing, J. S. Gill, and J. J. Kazmierczak. 1997. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J. Clin. Microbiol. 35:1465-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J. Clin. Microbiol. 36:2645-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buller, R. S., M. Arens, S. P. Hmiel, C. D. Paddock, J. W. Sumner, Y. Rikhisa, A. Unver, M. Gaudreault-Keener, F. A. Manian, A. M. Liddell, N. Schmulewitz, and G. A. Storch. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341:148-155. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, J. E., and S. A. Ewing. 1992. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am. J. Vet. Res. 53:1322-1327. [PubMed] [Google Scholar]
- 7.Dawson, J. E., D. E. Stallknecht, E. W. Howerth, C. Warner, K. Biggie, W. R. Davidson, J. M. Lockhart, V. F. Nettles, J. G. Olson, and J. E. Childs. 1994. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J. Clin. Microbiol. 32:2725-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson, J. E., C. K. Warner, V. Baker, S. A. Ewing, D. E. Stallknecht, W. R. Davidson, A. A. Kocan, J. M. Lockhart, and J. G. Olson. 1996. Ehrlichia-like 16S rDNA sequence from wild white-tailed deer (Odocoileus virginianus). J. Parasitol. 82:52-58. [PubMed] [Google Scholar]
- 9.Dumler, J. S., and J. S. Bakken. 1995. Ehrlichial diseases of humans: emerging tick-borne infections. Clin. Infect. Dis. 20:1102-1110. [DOI] [PubMed] [Google Scholar]
- 10.Ewing, S. A. 1969. Canine ehrlichiosis. Adv. Vet. Sci. Comp. Med. 13:331-353. [PubMed] [Google Scholar]
- 11.Ewing, S. A., J. E. Dawson, A. A. Kocan, R. W. Barker, C. K. Warner, R. J. Panciera, J. C. Fox, K. M. Kocan, and E. F. Blouin. 1995. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 32:368-374. [DOI] [PubMed] [Google Scholar]
- 12.Ewing, S. A., J. E. Dawson, R. J. Panciera, J. S. Mathew, K. W. Pratt, P. Katavolos, and S. R. Telford III. 1997. Dogs infected with a human granulocytotropic Ehrlichia spp. (Rickettsiales: Ehrlichieae). J. Med. Entomol. 34:710-718. [DOI] [PubMed] [Google Scholar]
- 13.Felek, S., A. Unver, R. W. Stich, and Y. Rikihisa. 2001. Sensitive detection of Ehrlichia chaffeensis in cell culture, blood, and tick specimens by reverse transcription-PCR. J. Clin. Microbiol. 39:460-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French, D. M., T. F. McElwain, T. C. McGuire, and G. H. Palmer. 1998. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 66:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman, E. E., E. B. Breitschwerdt, C. B. Grindem, B. C. Hegarty, J. J. Walls, and J. S. Dumler. 1998. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J. Vet. Intern. Med. 12:61-70. [DOI] [PubMed] [Google Scholar]
- 16.Groves, M. G., G. L. Dennis, H. L. Amyx, and D. L. Huxsoll. 1975. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am. J. Vet. Res. 36:937-940. [PubMed] [Google Scholar]
- 17.Lewis, G. E., Jr., M. Ristic, R. D. Smith, T. Lincoln, and E. H. Stephenson. 1977. The brown dog tick Rhipicephalus sanguineus and the dog as experimental hosts of Ehrlichia canis. Am. J. Vet. Res. 38:1953-1955. [PubMed] [Google Scholar]
- 18.Mathew, J. S., S. A. Ewing, R. W. Barker, J. C. Fox, J. E. Dawson, C. K. Warner, G. L. Murphy, and K. M. Kocan. 1996. Attempted transmission of Ehrlichia canis by Rhipicephalus sanguineus after passage in cell culture. Am. J. Vet. Res. 57:1594-1598. [PubMed] [Google Scholar]
- 19.Mathew, J. S., S. A. Ewing, J. R. Malayer, J. C. Fox, and K. M. Kocan. 2000. Efficacy of a modified polymerase chain reaction assay for detection of Ehrlichia canis infection. J. Vet. Diagn. Investig. 12:456-459. [DOI] [PubMed] [Google Scholar]
- 20.McBride, J. W., R. E. Corstvet, S. D. Gaunt, J. Chinsangaram, G. Y. Akita, and B. I. Osburn. 1996. PCR detection of acute Ehrlichia canis infection in dogs. J. Vet. Diagn. Investig. 8:441-447. [DOI] [PubMed] [Google Scholar]
- 21.McBride, J. W., X. Yu, and D. H. Walker. 1999. Molecular cloning of the gene for a conserved major immunoreactive 28-kilodalton protein of Ehrlichia canis: a potential serodiagnostic antigen. Clin. Diagn. Lab. Immunol. 6:392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBride, J. W., X. J. Yu, and D. H. Walker. 2000. A conserved, transcriptionally active p28 multigene locus of Ehrlichia canis. Gene 254:245-252. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohashi, N., A. Unver, N. Zhi, and Y. Rikihisa. 1998. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J. Clin. Microbiol. 36:2671-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez, M., Y. Rikihisa, and B. Wen. 1996. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J. Clin. Microbiol. 34:2133-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy, G. R., C. R. Sulsona, A. F. Barbet, S. M. Mahan, M. J. Burridge, and A. R. Alleman. 1998. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem. Biophys. Res. Commun. 247:636-643. [DOI] [PubMed] [Google Scholar]
- 28.Rikihisa, Y., S. A. Ewing, and J. C. Fox. 1994. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J. Clin. Microbiol. 32:2107-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rikihisa, Y., N. Zhi, G. P. Wormser, B. Wen, H. W. Horowitz, and K. E. Hechemy. 1997. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York state. J. Infect. Dis. 175:210-213. [DOI] [PubMed] [Google Scholar]
- 30.Rydkina, E., V. Roux, and D. Raoult. 1999. Determination of the genome size of Ehrlichia spp., using pulsed field gel electrophoresis. FEMS Microbiol. Lett. 176:73-78. [DOI] [PubMed] [Google Scholar]
- 31.Smith, R. D., D. M. Sells, E. H. Stephenson, M. R. Ristic, and D. L. Huxsoll. 1976. Development of Ehrlichia canis, causative agent of canine ehrlichiosis, in the tick Rhipicephalus sanguineus and its differentiation from a symbiotic rickettsia. Am. J. Vet. Res. 37:119-126. [PubMed] [Google Scholar]
- 32.Sparagano, O. A., M. T. Allsopp, R. A. Mank, S. G. Rijpkema, J. V. Figueroa, and F. Jongejan. 1999. Molecular detection of pathogen DNA in ticks (Acari: Ixodidae): a review. Exp. Appl. Acarol. 23:929-960. [DOI] [PubMed] [Google Scholar]
- 33.Stich, R. W., J. A. Bantle, K. M. Kocan, I. S. Eriks, and P. H. Palmer. 1991. Preliminary development of a polymerase chain reaction assay for Anaplasma marginale in ticks. Biotechnol. Tech. 5:269-274. [Google Scholar]
- 34.Stich, R. W., J. A. Bantle, K. M. Kocan, and A. Fekete. 1993. Detection of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in hemolymph of Dermacentor andersoni (Acari: Ixodidae) with the polymerase chain reaction. J. Med. Entomol. 30:781-788. [DOI] [PubMed] [Google Scholar]
- 35.Stich, R. W., J. R. Sauer, J. A. Bantle, and K. M. Kocan. 1993. Detection of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in secretagogue-induced oral secretions of Dermacentor andersoni (Acari: Ixodidae) with the polymerase chain reaction. J. Med. Entomol. 30:789-794. [DOI] [PubMed] [Google Scholar]
- 36.Wen, B., Y. Rikihisa, J. M. Mott, R. Greene, H. Y. Kim, N. Zhi, G. C. Couto, A. Unver, and R. Bartsch. 1997. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J. Clin. Microbiol. 35:1852-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu, X. J., J. W. McBride, and D. H. Walker. 1999. Genetic diversity of the 28-kilodalton outer membrane protein gene in human isolates of Ehrlichia chaffeensis. J. Clin. Microbiol. 37:1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]